Abstract
Congenital arteriovenous fistulae (AVF) of the internal maxillary artery (IMA) are rare. We present the angiographic findings and management of six AVF of the IMA, selected from 147 patients with facial vascular malformations. The fistula was thought to be congenital in all six in view of a life-long history, with no recorded trauma. Our analysis included angioarchitecture, treatment modality, embolic material, treatment results and follow-up. All patients had angiography showing an AVF originating from the IMA and draining to the jugular vein. Five patients underwent endovascular treatment with detachable balloons; a combination of Guglielmi detachable coils and N-acetyl-2-cyanoacrylate (NBCA) was used in one child. We successfully closed the AVF in all cases, without procedure-related complications, except for delayed transient facial numbness in one patient. No recurrence was observed on follow-up of 5 months to 7 years (mean 44 months).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Facial arteriovenous fistulae (AVF) on the external carotid artery (ECA) are rare, and mainly traumatic [1, 2, 3]. Congenital fistula on the internal maxillary artery (IMA) are even less common, a review of the literature revealing only 15 cases [3, 4, 5, 6, 7, 8, 9]. They are usually single-hole fistulae, and have been treated by surgery or balloon embolization. We present our experience in the management of six congenital AVF of the IMA, and review the literature.
Materials and methods
We identified six patients with congenital AVF of the IMA from our facial vascular malformation database of 147 patients, and reviewed the clinical and imaging data. There were two males and four females with ages ranging from 6 to 40 years (mean 22 years); two were 6-year-old boys. They were treated at our institution between 1995 and 2002. All had a life-long history of symptoms related to the AVF. Common presentations were a bruit in four and a pulsatile mass, also in four. One child had high-output congestive heart failure. One patient had headache without neurologic symptoms and one facial pain. No patient had a history of trauma or evidence of a connective tissue disorder.
Our analysis included angioarchitecture, treatment modality, embolic materials, treatment results and follow-up. Angiography was available in all patients, who then underwent endovascular treatment. Embolization was transarterial, under general anesthesia, with puncture of the femoral arteries. We used coaxial catheter systems with a variety of guiding catheters and microcatheters. A selective IMA angiogram was performed to localize the fistula precisely. In five patients we used detachable balloons as the only material. We combined fibered Guglielmi detachable coils (GDC) and liquid embolic material, N-acetyl-2-cyanoacrylate (NBCA) in one patient.
Results
The angioarchitecture and treatment results are presented in Table 1. All patients had complete cerebral angiography as well as selective ECA and IMA injections. The fistula lay at an inferior branch of the IMA near the origin of the middle meningeal artery in all patients. The fistulous segment was U-shaped in four, the inferiorly originating branch giving off the fistula, which drained upward into the maxillary and then the jugular veins (Fig. 1). The site of the fistula was shown as a focal narrowing, connected to a dilated draining vein. In one child the fistula was short and just drained inferiorly into the internal jugular vein (Fig. 2), but again focal narrowing was seen at the abnormal arteriovenous communication. In another patient the fistula drained directly into a large venous sac in the parotid region, with focal narrowing at the fistula site. This patient also had an ipsilateral facial vascular malformation, fed mainly by the occipital, facial, and posterior auricular arteries.
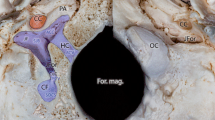
Case 6. A 30-year-old woman presented with a thrill and a bruit. An arteriovenous fistula (AVF) on the right internal maxillary artery (IMA) was occluded by detachable balloons. Lateral projections of IMA in a early and b late phases show a high-flow AVF on an inferior branch. Note focal narrowing at the U-shaped AV connection, suggesting that this is the site of the fistula. Antegrade flow to the distal IMA is reduced. Venous drainage is into the internal jugular vein via maxillary vein. c Lateral plain film shows two balloons in the fistula. d Lateral projection of IMA injection after treatment shows complete obliteration of fistula and increased flow to the distal branches of the IMA
Case 4. A 6-year-old boy presented with a pulsating mass and a thrill. An AVF on the right IMA was embolized with a combination of Guglielmi detachable coils (GDC) and N-acetyl-2-cyanoacrylate (NBCA). a Lateral projection of IMA injection shows a single-hole AVF as focal narrowing. Venous drainage is into the internal jugular vein. A vortex GDC was placed coaxially in the fistula via the microcatheter, and detached after confirmation of proper positioning. b. Embolization was then carried out with 2.5 cc NBCA and 0.5 cc Lipiodol, with tantalum powder. c Lateral postembolization angiogram shows complete closure of the fistula and preservation of the normal vessels
The proximal ECA and IMA were markedly wider than the narrow lumen of the internal carotid artery and the distal branches of the IMA beyond the fistula. The main venous drainage of the fistula was to the internal jugular vein in four patients and the external in two.
In five patients, a detachable balloon was inflated with contrast medium and detached in or just proximal to the fistula. A single balloon was deposited in four patients (Fig. 3), and two were placed in the fistula in one (Fig. 1). In one patient, the proximal ECA was embolized by coils to stabilize the balloon and to obliterate collateral flow via the facial artery. In one child with very short-segment AVF we embolized with fibered vortex GDC followed by a small amount of NBCA (Fig. 2), because a balloon appeared unstable in this fistula.
Case 1. A 19-year-old girl presented with a pulsatile mass, tinnitus and headache. Lateral IMA injections in a early and b late phases show a high-flow AVF draining to a number of large veins in the infratemporal fossa, some of which appear dysplastic, thence to the right internal jugular and facial veins, as well as to the superior ophthalmic vein. c Anteroposterior plain film after detachment of a balloon in the fistula the latter to be medial to the mandibular condyle. d Postembolization anteroposterior external carotid angiogram shows complete closure of the fistula
All AVF were all angiographically occluded immediately after the embolization and the presenting symptoms were ameliorated in all patients. No immediate procedure-related complications were observed, but the patient with two balloons in the fistula developed transient facial numbness 1 day after the procedure which had resolved 3 weeks later.
No clinical evidence of recurrent fistula was documented on follow-up ranging from 5 to 84 months (mean 44 months) after treatment. Angiographic follow-up in three patients from 3–50 months after treatment also showed no recurrence.
Discussion
Congenital facial AVF represent less than 1.5% of vascular lesions of the face [10]. There are 35 patients reported to have congenital AVF involving the ECA, 15 whom were described or illustrated as having IMA fistulae [3, 4, 5, 6, 7, 8, 9]. The clinical features, treatment and results in these cases are summarized in Table 2.
Common presentations of our cases were a bruit and a pulsating mass, both in two thirds of the patients. This is similar to reported cases [7], in which a bruit (83%) and a pulsatile mass (67%) were common. Neurologic symptoms are rare with these AVF. One patient is reported as presenting with a diminished level of consciousness and an ataxic gait [9], and as showing reversible abnormal signal in the brain stem and cerebellum on MRI. It was suggested that these deficits were due to venous congestion of the inferior petrosal sinus and basilar plexus and increased pressure in the jugular vein. Unilateral pulsatile headache has been reported in a patient with an enlarged middle meningeal artery supplying collateral flow to the fistula after incomplete surgical ligation of the proximal ECA [7]. One of our patients presented with headache, and ECA injections showed an AVF with drainage toward the cavernous sinus via facial and ophthalmic veins, as well as into the internal jugular vein (Fig. 3). It is not certain, however, whether the headache was related to increased pressure in the cavernous sinus.
We treated four of our patients as adults, although their fistula had been diagnosed earlier. While the natural history of congenital AVF of the IMA is not known, our experience suggests that its is usually benign, so treatment can be delayed if the fistula is well tolerated. However, we treated one child with congestive heart failure due to volume overload, which is rare with congenital ECA-jugular AVF [11]. There are no reports of, and we have not seen spontaneous thrombosis of a facial AVF.
We thought all the AVF reported here to be congenital, in view of a life-long history and absence of trauma or connective tissue disorders. All the patients showed a very large common carotid artery and proximal IMA and very narrow proximal internal carotid artery, findings which support the suggested congenital or at least long-standing nature of the AVF.
The fistulae shared several common features: an origin inferiorly from the proximal IMA, medial to the mandible near the parotid gland, and drainage into the jugular vein via the maxillary vein. These are similar to the observations in other reports [5]. ECA fistulae occur where the branches of the artery are close to adjacent veins [12]. Persistence of embryonic communications during the development of arteries and veins from the common capillary plexus in the human fetus might be the basis for congenital AVF, and the head and neck are thought to be a common site for persistence of these abnormal arteriovenous connections [13, 14]. A concept of segmental identity and vulnerability has been suggested to explain why certain conditions involve specific areas of the vascular tree and spare others [15]. The memory of the evolutionary steps and their chronology is imprinted on the arterial anatomy and thus potentially readable. One can thus postulate that, since the age of each arterial segment is different, its resistance to time and stimuli can be variable. The IMA has a complex embryonic origin from the hyostapedial and ventral pharyngeal arteries, and various annexations and regressions are involved during its development. Thus, various anatomical variants of the ECA system can develop from deviations from this program [16].
Halbach et al. [7] described absent angiographic demonstration of the branches of the IMA distal to the fistula before treatment, and restoration of antegrade flow after occlusion of the fistula. Gabrielsen et al. [5], however, observed antegrade flow to the distal IMA before treatment in their four cases. We also showed narrow vessels with maintained antegrade flow to the distal IMA on IMA injection in all six patients. Selective catheterization of the IMA with improved digital subtraction angiography may explain this discrepancy. Selective angiography is mandatory for complete assessment of the vascular anatomy and angioarchitecture of the lesion before endovascular treatment.
Endovascular treatment is considered the first-line approach to AVF in the head and neck [5, 7, 17, 18]. It has several advantages over surgery: precise localization and selective occlusion of the fistula, sparing the surrounding arteries, veins, parotid gland and the facial nerve, and no facial scars or risk of bleeding during surgery. In the literature, 12 (ten adults and two children) of 15 patients with AVF of the IMA have undergone endovascular treatment [3, 4, 5, 7, 8]. The others, all children, 22 months–5 years of age, were treated surgically [5, 6]. Although direct surgical ligation of the fistula may be possible when it is superficial, advances in embolic materials and catheter technology make it possible to treat childhood AVF safely and efficiently [19, 20]. We successfully treated two children by the endovascular approach, with no procedure-related morbidity. We believe it to be the first-line treatment for congenital AVF on the IMA in both children and adults.
The goal of treatment is occlusion of fistula the itself, and we should avoid proximal ligation or distal embolization. A patent AVF with occluded proximal vessels can recruit retrograde collateral flow. We preferred detachable balloons as the primary embolic material, as in 11 (92%) of 12 published cases. They are usually the best choice in large AVF since they can be positioned with precision [12, 18]. The high-flow aids in guiding the balloon to the site of the fistula. Balloons can be placed directly at the site of the abnormal arteriovenous communication, and be deflated and repositioned before detachment when thought to be improperly placed [12]. In cases of incomplete occlusion by balloon, other embolic material can be used to supplement closure. One with a recurrent AVF on the IMA after migration of a detached balloon was then treated by coils [7]. These can be also used when the fistula cannot be reached with a flow-directed balloon. The electrolytic detachable system enables use of coils in treatment of AVF. They can be placed and repositioned several times until an ideal position has been achieved, and there is a wide variety of sizes to fit the fistula. The risk of coil displacement or migration can be avoided or at least reduced by using a detachable system. However, the high flow of the AVF and the large size of the fistulous channel may preclude safe positioning of coils. Coils also have less space-filling capacity than balloons, which may result in incomplete occlusion if the fistula cannot be densely packed [21]. For this situation, NBCA can be used in combination with coils. We embolized combining a GDC and NBCA in one child. Although NBCA is not usually the primary embolic material for wide-channel fistulae, it can be used to occlude the proximal ECA for protection of a balloon detached in the fistula [17].
No procedure-related morbidity or mortality developed with endovascular treatment of 18 AVF of the IMA, including our cases. One of our patients had transient facial numbness after double-balloon; we thought this transient cranial nerve deficit might have resulted from the mass effect of the balloon or from ongoing venous thrombosis.
Including our cases, clinical follow-up (2 months–10 years, mean 41 months) after endovascular treatment is reported in 15 of 18 cases. One AVF recurred within weeks of balloon embolization, but no late recurrence was observed. Balloon migration within weeks of embolization is generally due to premature deflation or trauma [22, 23, 24, 25]. The AVF on the IMA have a unique site near the lateral pterygoid muscle and deep to the mandibular ramus, so that motion of the mandible could be a reason for balloon migration. However, the absence of late recurrences indicates stability of occlusion once the fistula has been obliterated by thrombosis.
References
Mehringer CM, Hieshima GB, Grinnell VS, et al (1983) Therapeutic embolization for vascular trauma of the head and neck. AJNR 4: 137–142
Luo CB, Lirng JF, Teng MM, Chen SS, Guo WY, Chang T (1998) Endovascular embolization of arteriovenous fistulas of the external carotid artery. Zhonghua Yi Xue Za Zhi (Taipei) 61: 260–266
Berenstein A, Scott J, Choi IS, Persky M (1986) Percutaneous embolization of arteriovenous fistulas of the external carotid artery. AJNR 7: 937–942
Cluzel P, Pierot L, Jason M, Rose M, Kieffer E, Chiras J (1992) Arteriovenous fistula of the internal maxillary artery in a child: case report. Neuroradiology 34: 460–461
Gabrielsen TO, Deveikis JP, Introcaso JH, Coran AG (1994) Congenital arteriovenous fistulas supplied by a single branch of the maxillary artery. AJNR 15: 653–657
Girevendulis AK, Lipper MH, Kishore PR, Vines FS, Greenfield LJ (1980) Congenital arteriovenous fistula of the external carotid artery. South Med J 73: 1549–1550
Halbach VV, Higashida RT, Hieshima GB, Hardin CW (1988) Arteriovenous fistula of the internal maxillary artery: treatment with transarterial embolization. Radiology 168: 443–445
Kawakami K, Takahashi A, Sugawara T, Nakamura N, Yoshimoto T, Suzuki J (1987) [Spontaneous arteriovenous fistula of the external carotid artery treated by a detachable balloon—a case report]. No Shinkei Geka 15: 549–553
Horiuchi M, Kamo T, Sugihara H, et al (2001) An adult case of congenital external carotid-jugular arteriovenous fistula with reversible circulatory insufficiency in the cerebellum and lower brain stem. AJNR 22: 273–276
Reizine D, Merland JJ, Birkui P, Leban M, Riche MC (1985) Treatment of spontaneous intraparotid direct arteriovenous fistulae using a detachable balloon technique. J Neuroradiol 12: 35–43
Kuss JJ, Karli A, Fischbach M, et al (1979) [Congenital carotid to jugular aneurysm]. Arch Fr Pediatr 36: 502–507
Lasjaunias P, Berenstein A (1987) Surgical neuroangiography. 2. Endovascular treatment of craniofacial lesions. Springer-Verlag, Berlin, pp 175–233
Samuels SS (1956) Diagnosis and treatment of vascular disorders (angiology). Williams & Wilkins, Baltimore, pp 430–431
Pemberton JJ, Saint JH (1928) Congenital arteriovenous communications. Surg Gynecol Obstet 46: 470–483
Lasjaunias P (2000) Segmental identity and vulnerability in cerebral arteries. Intervent Neuroradiol 6: 113–124
Lasjaunias P, Berenstein A, TerBrugge K (2002) Surgical neuroangiography. Springer-Verlag, Berlin Heidelberg, pp 262–324
Gobin YP, García de la Fuente JA, Herbreteau D, Houdart E, Merland JJ (1993) Endovascular treatment of external carotid-jugular fistulae in the parotid region. Neurosurgery 33: 812–816
Goyal M, Willinsky R, Montanera W, TerBrugge K (1999) Spontaneous vertebrovertebral arteriovenous fistulae: clinical features, angioarchitecture and management of twelve patients. Interventional Neuroradiology 5: 219–224
Burrows PE, Lasjaunias P, TerBrugge KG (1989) A 4-F coaxial catheter system for pediatric vascular occlusion with detachable balloons. Radiology 170: 1091–1094
Kincaid PK, Duckwiler GR, Gobin YP, Viñuela F (2001) Dural arteriovenous fistula in children: endovascular treatment and outcomes in seven cases. AJNR 22: 1217–1225
Guglielmi G, Viñuela F, Duckwiler G, Dion J, Stocker A (1995) High-flow, small-hole arteriovenous fistulas: treatment with electrodetachable coils. AJNR 16: 325–328
Debrun GM (1983) Treatment of traumatic carotid-cavernous fistula using detachable balloon catheters. AJNR 4: 355–356
Tsai FY, Hieshima GB, Mehringer CM, Grinnell V, Pribram HW (1983) Delayed effects in the treatment of carotid-cavernous fistulas. AJNR 4: 357–361
Leipzig TJ, Mullan SF (1983) Deflation of metrizamide-filled balloon used to occlude a carotid- cavernous fistula. Case report. J Neurosurg 59: 524–528
Lewis AI, Tomsick TA, Tew JM Jr (1995) Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery 36: 239–244
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, B.S., Lee, S.K. & terBrugge, K.G. Endovascular treatment of congenital arteriovenous fistulae of the internal maxillary artery. Neuroradiology 45, 445–450 (2003). https://doi.org/10.1007/s00234-003-0967-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-003-0967-3