Abstract
Aims
Several trials have assessed the antihyperglycemic effects of sodium-glucose cotransporter 2 inhibitors (SGLT2Is) in patients with type 2 diabetes mellitus (T2DM). We conducted a quantitative analysis to assess the effects of SGLT2Is on renal risk factors in patients with abnormal glucose metabolism.
Materials and methods
Randomized controlled trials (RCTs) were identified by searching the PubMed, Embase, Scopus, and Web of Science databases published before September 30, 2022. The intervention group received SGLT2Is as monotherapy or add-on treatment, and the control group received placebos, standard care, or active control. Risk of bias assessment was performed using the Cochrane risk of bias assessment tool. Meta-analysis was performed on studies with abnormal glucose metabolism populations and studies using the weighted mean differences (WMDs) as the measure of the effect size. Clinical trials providing changes in serum uric acid (SUA) were included. The mean change of SUA, glycated hemoglobin (HbA1c), body mass index (BMI), and estimated glomerular filtration rate (eGFR) were calculated.
Results
After a literature search and detailed evaluation, a total of 11 RCTs were included for quantitative analysis to analyze the differences between the SGLT2I group and the control group. The results showed that SGLT2I significantly reduced SUA (MD = −0.56, 95% CI = −0.66 ~ −0.46, I2 = 0%, P < 0.00001), HbA1c (MD = −0.20, 95% CI = −0.26 ~ −0.13, I2 = 0%, P < 0.00001), and BMI (MD = −1.19, 95% CI = −1.84 ~ −0.55, I2 = 0%, P = 0.0003). There was no significant difference in the reduction of eGFR observed in the SGLT2I group (MD = −1.60, 95% CI = −3.82 ~ 0.63, I2 = 13%, P = 0.16).
Conclusions
These results showed that the SGLT2I group caused greater reductions in SUA, HbA1c, and BMI but had no effect on eGFR. These data suggested that SGLT2Is may have numerous potentially beneficial clinical effects in patients with abnormal glucose metabolism. However, these results need to be consolidated by further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global prevalence of diabetes mellitus is increasing rapidly, affecting human health and life span. Diabetic nephropathy is one of the most common complications and the most common cause of end-stage renal disease. About 35%–40% of people with diabetes will develop diabetic nephropathy [1]. Therefore, we should consider whether hypoglycemic therapy can reduce the risk of diabetic nephropathy and improve its prognosis. Studies have shown that SGLT2Is can reduce blood glucose by inhibiting the reabsorption of sodium and glucose in the proximal renal tubule and increasing the excretion of urinary glucose [2]. In addition to the hypoglycemic effect, the renal protective effect of SGLT2Is runs through the whole process of diabetic nephropathy. The significant renal protective features of SGLT2Is have led to their widespread use as a monotherapy or add-on therapy to other hypoglycemic agents in clinical practice [3]. The change in renal hemodynamics is one of the most important mechanisms of renal protection by these drugs [4]. At the same time, some studies have shown that SGLT2Is can reduce the risk factors of diabetic nephropathy, such as HbA1c, eGFR, body weight, and SUA. These may be an intermediate role of SGLT2Is in renal protection. Therefore, in this study, we aim to explore this association through a systematic review of the existing literature. In addition, we provide insight into the mechanisms underlying this association.
Recently, a meta-analysis based on randomized, placebo-controlled trials showed that SGLT2Is could reduce SUA, fasting plasma glucose, and HbA1c in diabetic patients [5]. However, there is a lack of sufficient data to indicate the role of SGLT2Is on renal risk factors. We need to provide more data to prove it. In addition, some previous meta-analyses have also evaluated the effect and safety of SGLT2Is on SUA [6,7,8,9]. However, these manuscripts were all published before 2017. Several recently published RCTs of SGLT2Is on renal risk factors need to be evaluated in new meta-analyses. Therefore, we collected data from nearly 5 years of randomized controlled trials and performed this meta-analysis to evaluate the effects of SGLT2Is on renal risk factors, including SUA, eGFR, HbA1c, and BMI in patients with abnormal glucose metabolism.
Materials and methods
Data sources and searches
The electronic databases of PubMed, Embase, Web of Science, and Scopus were searched to identify eligible RCTs using relevant search terms described in Table S1. Our search in all databases was restricted to the use of these terms in the title, abstract, and keyword. We identified articles published up to September 30, 2022. Trials that were published between 2018 and 2022 were manually searched. An English language restriction was imposed. We did a further manual search of the reference lists of all selected papers, previous similar reviews, and pooled analysis studies to look for possible missing papers.
Study selection
Studies meeting the following criteria were included according to the PICOS scheme: (1) population, patients with abnormal glucose metabolism; (2) intervention, SGLT2Is as monotherapy or add-on treatment; (3) comparison, placebo, active control, or standard care; (4) primary outcomes, change in SUA from baseline; secondary outcomes, changes in HbA1c, BMI, and eGFR from baseline; (5) design, randomized controlled trials. However, single-arm trials, trials with self-control and historical controls, and crossover trials were excluded.
Data extraction
The two investigators (Mengnan Li and Yunfeng Liu) independently extracted the following data: first author, year of publication, patient characteristics, comparison (placebo, active control, or standard care), intervention (type of SGLT2Is and dose regimen), duration of SGLT2I treatment, duration of diabetes, baseline SUA, BMI, HbA1c, eGFR, and outcomes (changes in SUA, HbA1c, BMI, and eGFR from baseline).
Quality assessment
Two independent reviewers (Mengnan Li and Yunfeng Liu) evaluated the studies according to the inclusion and exclusion criteria and assessed the risk of bias according to the Cochrane risk of bias tool [10]. The following domains were considered: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Disagreements were resolved in discussions.
Publication bias and statistical analysis
Publication bias was examined using funnel plot asymmetry. The effect of SGLT2Is on renal function was evaluated according to the changes in HbA1c, BMI, SUA, and eGFR. All four outcomes were assessed as continuous variables. We calculated pooled outcomes for the weighted mean differences (WMDs) and 95% confidence intervals (CIs) using a random-effects model. Heterogeneity was assessed using the I2 statistic. Values > 50% were viewed as indicative of moderate-to-high heterogeneity [11]. We also carried out subgroup and sensitivity analyses to explore the causes of heterogeneity. Random-effects meta-regression analyses were used to evaluate the association between changes in some outcome measures and baseline characteristics. We used Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata/SE 16.0 (Stata Corp., College Station, TX, USA) for all statistical analyses. The statistical level of significance was set at a P-value < 0.05.
Results
Study selection and characteristics
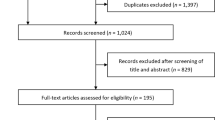
A total of 4490 articles were collected by searching the various databases including PubMed (n = 289), Scopus (n = 987), Embase (n = 1288), and Web of Science (n = 1926) databases. Of these, 1637 were duplicates, leaving 2853 to be further evaluated. Most citations (n = 1264) were excluded due to unrelated trial design, article type, and non-English language. The remaining 1589 articles were carefully checked. A total of 1050 records were excluded by inspecting titles and abstracts. The remaining 539 articles were carefully assessed for data extraction in full text, and an additional 528 articles were excluded by reason of unrelated topic (n = 400), incomplete data (n = 96), patients selection (n = 20), and uninterested outcomes (n = 12). Finally, 11 studies met our criteria for systematic review, which was followed up with a meta-analysis (Fig. 1). No additional study was identified by manual search.
Characteristics of the included trials are summarized in Table 1. The included trials (n = 11) were RCTs from 2018 to 2022. A total of 489 patients were in the SGLT2I group, and 492 were in the placebo group. Most trials used a placebo (n = 4) and standardized hypoglycemic therapy (n = 5) as controls. We also included two trials with valsartan (80 mg bid) and liraglutide (0.9 mg qd) as controls respectively. Three types of SGLT2Is were mentioned in the studies, most of the trials were on dapagliflozin (n = 5) and empagliflozin (n = 4), which, respectively, used 5–10 mg of dapagliflozin and 10 mg of empagliflozin. We also included one study on ipragliflozin (5 mg qd) and one on dapagliflozin (5–10 mg qd) and empagliflozin (10 ~ 25 mg qd) in combination treatment. Participants were usually middle-aged. Two of these trials involved patients with diabetic nephropathy and prediabetic patients, respectively. The remaining experiments involved patients with type 2 diabetes. The duration of intervention in most trials was 12 or 24 weeks. The study by Hussain et al. had the shortest time of intervention (4 weeks). However, Hao et al. did not report the duration of the intervention. The changes in SUA, HbA1c, BMI, and eGFR of each study are reported in Table S2.
Quality assessment
For all of the included studies, a risk of bias assessment was carried out using The Cochrane Collaboration risk of bias tool (Fig. 2).
Effect of SGLT2Is on SUA
In this meta-analysis, 11 studies were a two-group analysis between SGLT2Is and control groups to evaluate the association between SUA levels and SGLT2Is in a total of 981 patients. The results of the pooled analysis of 11 studies showed that patients in the SGLT2I group had a greater reduction in SUA than those in the control group (MD = −0.56, 95% CI = −0.66 ~ −0.46, I2 = 0%, P < 0.00001). There was little or no heterogeneity in the meta-analysis (I2 = 0%), suggesting a consistent drug effect (Fig. 3).
A subgroup analysis was performed according to the different control groups and baseline characteristics (Fig. 4). Compared with the placebo group (n = 4), the SGLT2I group reduced SUA concentration by 0.58 mg/dl more than the placebo group, and the difference was statistically significant (95% CI = −0.93 ~ −0.23, I2 = 51%, P = 0.001). Similarly, the SGLT2I group significantly reduced SUA concentration more than the active control group (n = 7, including metformin, insulin, and sulphonylureas) (MD = −0.59, 95% CI = −0.82 ~ −0.35, I2 = 0%, P < 0.00001) (Fig. 4A).
There were ten studies that reported treatment time. We analyzed the indicator in the short-term (n = 7; ≤ 12 weeks) and long-term (n = 3; 24 weeks) subgroups, separately. The MD of reduction in SUA was greater in the short-term subgroup (MD = −0.57, 95% CI = −0.68 ~ −0.46, I2 = 0%, P < 0.00001) than that in the long-term subgroup (MD = −0.44, 95% CI = −1.00 ~ 0.12, I2 = 65%, P = 0.12), albeit not statistically significant in the long-term subgroup (Fig. 4B).
Ten studies have reported the effects of one type of SGLT2Is. We found that empagliflozin (n = 4) reduced the SUA more than dapagliflozin (n = 5) and ipragliflozin (n = 1). There were significant differences in the decrease of SUA among different types (MD = −0.56, 95% CI = −0.68 ~ −0.43, I2 = 4%, P < 0.00001) (Fig. 4C).
HbA1c was reported in 11 studies. According to HbA1c, we found that the MD of reduction in SUA was greater in the lower HbA1c subgroup (n = 5; HbA1c < 8) (MD = −0.63, 95% CI = −0.78 ~ −0.48, I2 = 5%, P < 0.00001) than that in the higher HbA1c subgroup (n = 6; HbA1c > = 8) (MD = −0.38, 95% CI = −0.63 ~ −0.13, I2 = 0%, P = 0.003) (Fig. 4D).
Seven studies reported the duration of diabetes. We found that the MD of reduction in SUA was greater in the short-term subgroup (n = 3; ≤ 5 years) than that in the long-term subgroup (n = 4; > 5 years or 10 years). There were significant differences in the decrease of SUA among different duration of diabetes (MD = −0.63, 95% CI = −0.86 ~ −0.40, I2 = 0%, P < 0.00001) (Fig. 4E).
Random-effects meta-regression was performed to assess whether the reduction in SUA levels is dependent on the duration of diabetes, different types, the duration of SGLT2I treatment, and glycated hemoglobin levels. The results demonstrated that SUA change was not significantly correlated with the duration of diabetes, different types, duration of SGLT2I treatment, and glycated hemoglobin levels (Fig. S1) (Table 2).
No publication bias was found using the funnel plots (Fig. S2). There was little or no heterogeneity in the meta-analysis (I2 = 0%), suggesting a consistent drug effect.
Effect of SGLT2Is on BMI
Five studies reported the effect of SGLT2Is on BMI. Compared with the control group, SGLT2I significantly reduced BMI (MD = −1.19, 95% CI = −1.84 ~ −0.55, I2 = 0%, P = 0.0003). Publication bias cannot be assessed due to the small number of included studies. There was little or no heterogeneity in the meta-analysis (I2 = 0%), suggesting a consistent drug effect (Fig. 5).
Effect of SGLT2Is on eGFR
Six studies reported the effect of SGLT2Is on eGFR. There was no significant difference in the reduction of eGFR observed in the SGLT2I group compared with that in the control group (MD = −1.60, 95% CI = −3.82 ~ 0.63, I2 = 13%, P = 0.16) (Fig. 6). Considering the impact of the variation in the follow-up period, we analyzed the indicator in the short-term (n = 4; ≤ 12 weeks) and long-term (n = 2; ≥ 24 weeks) subgroups, separately. In the short-term subgroup, SGLT2Is could significantly reduce eGFR (MD = −2.79, 95% CI = −3.74 ~ −1.84, I2 = 0%, P < 0.00001). In the long-term subgroup, SGLT2Is could increase eGFR (MD = 1.86, 95% CI = −3.55 ~ 7.28, I2 = 0%, P = 0.50), but the results were not significant (Fig. 7). Publication bias cannot be assessed due to the small number of included studies. There was little or no heterogeneity in the meta-analysis (I2 = 13%), suggesting a consistent drug effect.
Effect of SGLT2Is on HbA1c
Eight studies reported the effect of SGLT2Is on HbA1c. There was a significant difference in the reduction of HbA1c observed in the SGLT2I group compared with that in the control group in seven studies (MD = −0.20, 95% CI = −0.26 ~ −0.13, I2 = 0%, P < 0.00001) (Fig. 8). When we excluded one study at a time to assess the stability of the results, there was a significant change in the pooled MD or 95% CI when Nakaguchi et al.’s study was excluded (Fig. S3). The MD of HbA1c changed from −0.03 (95% CI = −0.34 ~ 0.28, I2 = 89%, P = 0.85) to −0.20 (95% CI = −0.26 ~ −0.13, I2 = 0%, P < 0.00001). Nakaguchi et al.’s study showed that the liraglutide group reduced SUA concentration by 0.89 mg/dl more than the empagliflozin group [12]. The result of the original study resulted in a lack of robustness. Therefore, we removed this paper from the meta-analysis.
Finally, to assess the publication bias of the results in the present meta-analysis, we constructed funnel plots using Review Manager. The symmetry of the HbA1c funnel plot shows that there was a low risk of publication bias (Fig. S4).
Discussion
In this meta-analysis, we provide evidence that SGLT2I significantly reduces SUA, HbA1c, and BMI. While there were no significant changes in eGFR after the use of SGLT2Is, these results suggested the beneficial effects of SGLT2Is on diabetic nephropathy risk factors. It is therefore vital to study SGLT2I protective effects against diabetic nephropathy in patients with abnormal glucose metabolism.
SUA level is closely related to early renal disease in T2DM patients, which can lead to the progression and deterioration of renal disease in T2DM patients [13,14,15]. Many studies have shown that SUA can affect renal function through a variety of mechanisms, including the induction of inflammatory pathways [16], ischemia [17], or lower eGFR [18]. The subsequent appearance of high blood uric acid and low urinary uric acid excretion (UUAE) levels further increases the risk of diabetic nephropathy [19]. There is controversy in the current study as to whether the effect of SUA on renal function decline depends on the baseline blood uric acid level or the ΔUA [20, 21]. Allopurinol or febuxostat is known to significantly reduce SUA, but further studies have shown that these drugs did not result in a clinically meaningful improvement in kidney outcome [22, 23]. If the glucose-lowering drugs themselves could lower SUA, then we could avoid the use of additional drugs. SGLT2Is have been found to significantly reduce SUA. This meta-analysis reached a similar conclusion. The results of the present study show that SUA levels decreased by 0.56 mg/dl more in the SGLT2I group than in the control group. The underlying mechanism involves the renal SLC2A9 (GLUT9) transporter, which transports uric acid and d-glucose. SGLT2Is lead to increased urinary glucose excretion, accompanied by increased uric acid exchange at the apical membrane of renal tubular cells. This subsequently causes increased excretion of uric acid and hypouricemia [24].
Subgroup analyses of baseline characteristics revealed that SUA reduction decreased with higher HbA1c and longer duration of disease or intervention. In a meta-analysis [5], it was found that empagliflozin had the highest rate of SUA reduction, which was consistent with our subgroup analysis results. The meta-regression of Zhao et al. [9] showed that the reduction of SUA was associated with the duration of SGLT2I treatment. However, another meta-analysis showed that the reduction of SUA was not related to the dose and duration of SGLT2I treatment but was related to the duration of diabetes [5]. In contrast, the decrease in SUA was not associated with the duration of diabetes, duration of treatment, and type of SGLT2Is in our study. The differences may be due to the mean treatment period, the number of patients, and different analysis tools. Several similar meta-analyses have been published previously [6,7,8, 25]. Although they all reached similar conclusions on the outcome of SUA, this paper conducted subgroup analysis and regression analysis from different perspectives rather than repeating the existing meta-analysis. In addition, this article mainly included RCT studies in the past 5 years, which provided updated and more comprehensive data support for the effect of SGLT2Is on uric acid reduction.
Weight loss is essential for patients with type 2 diabetes. In addition to the potential benefits of lowering blood glucose levels, there are also some renal benefits. There is evidence of the benefit of weight loss in reducing proteinuria in overweight and obese patients [26]. And another study noted that weight loss can lead to a reduction in blood pressure [27]. Interestingly, weight loss also has the effect of reducing uric acid. These results will reduce the incidence of diabetic nephropathy complications. The results of this meta-analysis indicated that compared with the control group, SGLT2I significantly reduced BMI (MD = −1.19, 95% CI = −1.84 ~ −0.55, I2 = 0%, P < 0.001), which is in line with previous findings. The mechanism of weight loss has been partially understood. First, the SGLT2I-mediated increase in urine glucose can reduce fat mass [28], thereby reducing chronic inflammation [29] and body weight. Second, brown fat is known to fight obesity, and SGLT2Is can induce the browning of white adipose tissue to reduce body weight [30]. Third, the insulin-sparing effect of SGLT2Is may also have contributed to weight loss in patients who used insulin [31]. In addition, some studies have further found that SGLT2Is have a better weight loss effect in patients with normal renal function than in patients with reduced renal function [32].
Studies have shown that hypoglycemic therapy significantly reduces renal deterioration and macroalbuminuria [33]. In this study, SGLT2I therapy was more effective than the control group in the treatment of type 2 diabetes and was more effective in improving glycated hemoglobin control, indicating that SGLT2Is can effectively control glucose and protect the kidney, which is consistent with the results of previous studies. Of note, SGLT2I therapy should be used with caution in patients with very high levels of HbA1c and is contraindicated in patients with a history of diabetic ketoacidosis [34]. In addition, it has been reported that in patients with stage 2 or 3 diabetic nephropathy, the addition of SGLT2I significantly reduced HbA1c. However, no reduction in HbA1c was observed in stage 4 patients with chronic kidney disease. Therefore, the use of SGLT2Is is contraindicated in patients with severe chronic kidney disease [35]. In addition, studies have shown that in patients 75 years and older treated with SGLT2Is, the reduction of HbA1c is diminished and may increase the risk of decreased blood volume, which may affect renal function. Therefore, SGLT2Is should be used with caution in the elderly [34].
Interestingly, existing studies suggest that the effect of SGLT2Is on eGFR may depend on the duration of treatment. Early treatment decreases eGFR, which remains stable or increases after long-term treatment [36, 37]. Consistent with these findings, we found that within 12 weeks of treatment, eGFR decreased more in SGLT2Is than in the control group. In contrast, after 24 weeks of treatment, there was an increase in eGFR in the SGLT2I group, although the result was not significant. These findings may suggest a renal protective effect of SGLT2Is in the long run. Furthermore, although an acute, modest decrease in eGFR occurs initially, it has been shown not to affect the progression of kidney disease [38, 39]. The mechanism of early reduction of eGFR by SGLT2Is has been partially investigated. There have been several studies showing that SGLT2Is activate tubule-glomerular feedback and increase renal tubular back pressure by increasing fluid and electrolyte delivery to the macula densa. This results in a decrease in intraglomerular pressure and eGFR [37, 40].
Limitation
Some limitations of this meta-analysis should be noted. (1) We did not exclude patients with diabetic nephropathy. Their uric acid levels may have been elevated as the disease progressed, which could have affected the results. (2) We included a small number of studies with inconsistent follow-up times and different background treatments. (3) All the included original data were not accessed, and most experiments did not emphasize the measurement methods of each indicator. These factors may have a potential impact on the results. (4) Because of the limited data in the included literature, we were limited to performing subgroup analyses on the basis of baseline. For example, we were unable to perform subgroup analyses of doses, and there were few types of SGLT2Is involved.
Conclusion
In conclusion, this meta-analysis provides evidence of the effect of SGLT2Is on SUA, BMI, HbA1c, and eGFR. These findings suggest a beneficial effect of SGLT2Is on renal risk factors in patients with abnormal glucose metabolism.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Huang W, Chen YY, Li ZQ, He FF, Zhang C (2022) Recent advances in the emerging therapeutic strategies for diabetic kidney diseases. Int J Mol Sci 23(18). https://doi.org/10.3390/ijms231810882
Bakris GL, Fonseca VA, Sharma K, Wright EM (2009) Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 75(12):1272–1277. https://doi.org/10.1038/ki.2009.87
Tamez-Perez HE, Delgadillo-Esteban E, Soni-Duque D, Hernández-Coria MI, Tamez-Peña AL (2017) SGLT2 inhibitors as add on therapy in type 2 diabetes: a real world study. J Diabetes Metab Disord 16:27. https://doi.org/10.1186/s40200-017-0308-4
Kim NH, Kim NH (2022) Renoprotective Mechanism of sodium-glucose cotransporter 2 inhibitors: focusing on renal hemodynamics. Diabetes Metab J 46(4):543–551. https://doi.org/10.4093/dmj.2022.0209
Akbari A, Rafiee M, Sathyapalan T, Sahebkar A (2022) Impacts of sodium/glucose cotransporter-2 inhibitors on circulating uric acid concentrations: a systematic review and meta-analysis. J Diabetes Res 2022:7520632. https://doi.org/10.1155/2022/7520632
Wu B, Zheng H, Gu J, Guo Y, Liu Y, Wang Y, Chen F, Yang A, Wang J, Wang H, Liu Y, Wang D (2019) Effects of sodium-glucose cotransporter 2 inhibitors in addition to insulin therapy on cardiovascular risk factors in type 2 diabetes patients: a meta-analysis of randomized controlled trials. J Diabetes Investig 10(2):446–457. https://doi.org/10.1111/jdi.12876
Zhang M, Zhang L, Wu B, Song H, An Z, Li S (2014) Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 30(3):204–221. https://doi.org/10.1002/dmrr.2479
Zhao D, Liu H, Dong P (2019) Empagliflozin reduces blood pressure and uric acid in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Hum Hypertens 33(4):327–339. https://doi.org/10.1038/s41371-018-0134-2
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L (2018) Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 20(2):458–462. https://doi.org/10.1111/dom.13101
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
Nakaguchi H, Kondo Y, Kyohara M, Konishi H, Oiwa K, Terauchi Y (2020) Effects of liraglutide and empagliflozin added to insulin therapy in patients with type 2 diabetes: a randomized controlled study. J Diabetes Investig 11(6):1542–1550. https://doi.org/10.1111/jdi.13270
Ji P, Zhu J, Feng J, Li H, Yu Q, Qin H, Wei L, Zhang J (2022) Serum uric acid levels and diabetic kidney disease in patients with type 2 diabetes mellitus: a dose-response meta-analysis. Prim Care Diabetes 16(3):457–465. https://doi.org/10.1016/j.pcd.2022.03.003
Kocak MZ, Aktas G, Duman TT, Atak BM, Savli H (2019) Is uric acid elevation a random finding or a causative agent of diabetic nephropathy? Rev Assoc Med Bras (1992) 65(9):1155–1160. https://doi.org/10.1590/1806-9282.65.9.1156
Li GX, Jiao XH, Cheng XB (2018) Correlations between blood uric acid and the incidence and progression of type 2 diabetes nephropathy. Eur Rev Med Pharmacol Sci 22(2):506–511. https://doi.org/10.26355/eurrev_201801_14202
Guarda NS, Bollick YS, de Carvalho JAM, Premaor MO, Comim FV, Moresco RN (2019) High serum uric acid is associated with tubular damage and kidney inflammation in patients with type 2 diabetes. Dis Markers 2019:6025804. https://doi.org/10.1155/2019/6025804
Suijk DL, Smits MM, Muskiet MH, Tonneijck L, Kramer MH, Joles JA, van Raalte DH (2020) Plasma uric acid and renal haemodynamics in type 2 diabetes patients. Nephrology (Carlton) 25(4):290–297. https://doi.org/10.1111/nep.13645
Xia Q, Zhang SH, Yang SM, Zhu XL, Su S, Hu AP, Zhu J, Li DM (2020) Serum uric acid is independently associated with diabetic nephropathy but not diabetic retinopathy in patients with type 2 diabetes mellitus. J Chin Med Assoc 83(4):350–356. https://doi.org/10.1097/jcma.0000000000000285
Chen MY, Wang AP, Wang JW, Ke JF, Yu TP, Li LX, Jia WP (2019) Coexistence of hyper-uricaemia and low urinary uric acid excretion further increases risk of chronic kidney disease in type 2 diabetes. Diabetes Metab 45(6):557–563. https://doi.org/10.1016/j.diabet.2019.03.001
Hanai K, Tauchi E, Nishiwaki Y, Mori T, Yokoyama Y, Uchigata Y, Babazono T (2019) Effects of uric acid on kidney function decline differ depending on baseline kidney function in type 2 diabetic patients. Nephrol Dial Transplant 34(8):1328–1335. https://doi.org/10.1093/ndt/gfy138
Shibata Y, Yamazaki M, Kitahara J, Okubo Y, Oiwa A, Sato A, Komatsu M (2021) Changes in serum uric acid levels as a predictor of future decline in renal function in older adults with type 2 diabetes. Medicine (Baltimore) 100(40):e27420. https://doi.org/10.1097/md.0000000000027420
Tiku A, Johnson DW, Badve SV (2021) Recent evidence on the effect of urate-lowering treatment on the progression of kidney disease. Curr Opin Nephrol Hypertens 30(3):346–352. https://doi.org/10.1097/mnh.0000000000000699
Watanabe K, Nakayama M, Yamamoto T, Yamada G, Sato H, Miyazaki M, Ito S (2021) Different clinical impact of hyperuricemia according to etiologies of chronic kidney disease: Gonryo Study. PLoS One 16(3):e0249240. https://doi.org/10.1371/journal.pone.0249240
Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, Bandinelli S, Schlessinger D, Lakatta E, Scuteri A, Najjar SS, Guralnik J, Naitza S, Crisponi L, Cao A, Abecasis G, Ferrucci L, Uda M, Chen WM, Nagaraja R (2007) The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3(11):e194. https://doi.org/10.1371/journal.pgen.0030194
Musso G, Gambino R, Cassader M, Pagano G (2012) A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 44(4):375–393. https://doi.org/10.3109/07853890.2011.560181
Kittiskulnam P, Kanjanabuch T, Tangmanjitjaroen K, Chancharoenthana W, Praditpornsilpa K, Eiam-Ong S (2014) The beneficial effects of weight reduction in overweight patients with chronic proteinuric immunoglobulin a nephropathy: a randomized controlled trial. J Ren Nutr 24(3):200–207. https://doi.org/10.1053/j.jrn.2014.01.016
Sjöström CD, Hashemi M, Sugg J, Ptaszynska A, Johnsson E (2015) Dapagliflozin-induced weight loss affects 24-week glycated haemoglobin and blood pressure levels. Diabetes Obes Metab 17(8):809–812. https://doi.org/10.1111/dom.12500
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S (2012) Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97(3):1020–1031. https://doi.org/10.1210/jc.2011-2260
Kassi E, Dalamaga M, Hroussalas G, Kazanis K, Merantzi G, Zachari A, Giamarellos-Bourboulis EJ, Dionyssiou-Asteriou A (2010) Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas 67(1):72–77. https://doi.org/10.1016/j.maturitas.2010.05.004
Arany Z (2019) Taking a BAT to the chains of diabetes. N Engl J Med 381(23):2270–2272. https://doi.org/10.1056/NEJMcibr1911353
Zhang L, Feng Y, List J, Kasichayanula S, Pfister M (2010) Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 12(6):510–516. https://doi.org/10.1111/j.1463-1326.2010.01216.x
Huh Y, Kim YS (2022) Predictors for successful weight reduction during treatment with dapagliflozin among patients with type 2 diabetes mellitus in primary care. BMC Prim Care 23(1):134. https://doi.org/10.1186/s12875-022-01748-1
Chalmoukou K, Polyzos D, Manta E, Tatakis F, Konstantinidis D, Thomopoulos C, Costas T (2022) Renal outcomes associated with glucose-lowering agents: systematic review and meta-analysis of randomized outcome trials. Eur J Intern Med 97:78–85. https://doi.org/10.1016/j.ejim.2021.12.018
Wilding J, Fernando K, Milne N, Evans M, Ali A, Bain S, Hicks D, James J, Newland-Jones P, Patel D, Viljoen A (2018) SGLT2 inhibitors in type 2 diabetes management: key evidence and implications for clinical practice. Diabetes Ther 9(5):1757–1773. https://doi.org/10.1007/s13300-018-0471-8
Scheen AJ (2015) Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet 54(7):691–708. https://doi.org/10.1007/s40262-015-0264-4
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375(4):323–334. https://doi.org/10.1056/NEJMoa1515920
Jongs N, Chertow GM, Greene T, McMurray JJV, Langkilde AM, Correa-Rotter R, Kashihara N, Rossing P, Sjöström CD, Stefánsson BV, Toto RD, Wheeler DC, Heerspink HJL (2022) Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor Dapagliflozin in patients with CKD. J Am Soc Nephrol 33(11):2094–2107. https://doi.org/10.1681/asn.2022030306
Meraz-Muñoz AY, Weinstein J, Wald R (2021) eGFR Decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360 2(6):1042–1047. https://doi.org/10.34067/kid.0001172021
Zannad F, Ferreira JP, Gregson J, Kraus BJ, Mattheus M, Hauske SJ, Butler J, Filippatos G, Wanner C, Anker SD, Pocock SJ, Packer M (2022) Early changes in estimated glomerular filtration rate post-initiation of empagliflozin in EMPEROR-Reduced. Eur J Heart Fail 24(10):1829–1839. https://doi.org/10.1002/ejhf.2578
Vallon V, Thomson SC (2017) Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 60(2):215–225. https://doi.org/10.1007/s00125-016-4157-3
Hao Z, Huang X, Shao H, Tian F (2018) Effects of dapagliflozin on serum uric acid levels in hospitalized type 2 diabetic patients with inadequate glycemic control: a randomized controlled trial. Ther Clin Risk Manag 14:2407–2413. https://doi.org/10.2147/tcrm.S186347
Huang Y, Lu W, Lu H (2022) The clinical efficacy and safety of dapagliflozin in patients with diabetic nephropathy. Diabetol Metab Syndr 14(1):47. https://doi.org/10.1186/s13098-022-00815-y
Hussain M, Elahi A, Hussain A, Iqbal J, Akhtar L, Majid A (2021) Sodium-glucose cotransporter-2 (SGLT-2) attenuates serum uric acid (SUA) level in patients with type 2 diabetes. J Diabetes Res 2021:9973862. https://doi.org/10.1155/2021/9973862
Tanaka M, Yamakage H, Inoue T, Odori S, Kusakabe T, Shimatsu A, Satoh-Asahara N (2020) Beneficial effects of ipragliflozin on the renal function and serum uric acid levels in Japanese patients with type 2 diabetes: a randomized, 12-week, open-label, active-controlled trial. Intern Med 59(5):601–609. https://doi.org/10.2169/internalmedicine.3473-19
Okada K, Hoshide S, Kato M, Kanegae H, Ishibashi S, Kario K (2021) Safety and efficacy of empagliflozin in elderly Japanese patients with type 2 diabetes mellitus: a post hoc analysis of data from the SACRA study. J Clin Hypertens (Greenwich) 23(4):860–869. https://doi.org/10.1111/jch.14131
Satirapoj B, Korkiatpitak P, Supasyndh O (2019) Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 12(3):326–332. https://doi.org/10.1093/ckj/sfy122
Ramirez-Rodriguez AM, Gonzalez-Ortiz M, Martinez-Abundis E (2020) Effect of dapagliflozin on insulin secretion and insulin sensitivity in patients with prediabetes. Exp Clin Endocrinol Diabetes 128(8):506–511. https://doi.org/10.1055/a-0664-7583
Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Ishikawa M, Maruyama M, Ogano M, Tanabe J, investigators Et (2020) Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 19(1):148. https://doi.org/10.1186/s12933-020-01127-z
Hiruma S, Shigiyama F, Hisatake S, Mizumura S, Shiraga N, Hori M, Ikeda T, Hirose T, Kumashiro N (2021) A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol 20(1):32. https://doi.org/10.1186/s12933-021-01228-3
Pollock C, Stefansson B, Reyner D, Rossing P, Sjostrom CD, Wheeler DC, Langkilde AM, Heerspink HJL (2019) Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 7(6):429–441. https://doi.org/10.1016/S2213-8587(19)30086-5
Acknowledgements
The coauthors thank the National Natural Science Foundation of China (81973378, 82073909), Research Project Supported by Shanxi Scholarship Council of China (2020-0172), and the Shanxi Provincial Central Leading Local Science and Technology Development Fund Project (YDZJSX2022A059) for their support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82073909 and 81973378), the Research Project Supported by Shanxi Scholarship Council of China (No. 2020–172), and the Shanxi Provincial Central Leading Local Science and Technology Development Fund Project (YDZJSX2022A059).
Author information
Authors and Affiliations
Contributions
Mengnan Li and Jian Zhang searched and reviewed studies, extracted and analyzed the data, and drafted and proofed the manuscript. Mengnan Li contributed to data collection and statistical analyses and reviewed the manuscript. Mengnan Li reviewed and edited the manuscript. Yunfeng Liu, Yi Zhang, Minmin Han, Jiaxin Zhang, and Guimei Yang directed the project and contributed to the discussion as well as reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Zhang, J., Yang, G. et al. Effects of sodium-glucose cotransporter 2 inhibitors on renal risk factors in patients with abnormal glucose metabolism: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 79, 859–871 (2023). https://doi.org/10.1007/s00228-023-03490-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03490-8