Abstract
Background
Diabetes is associated with a high rate of events after acute coronary syndrome. It was recently reported that once-daily aspirin might not provide stable biological efficacy in patients with diabetes.
Aims
We sought to compare the biological efficacy of aspirin given once a day versus aspirin divided twice per day in a population of diabetic patients with non-ST elevation acute coronary syndrome (NSTE-ACS) as assessed by the thrombin generation test.
Methods
We performed an open-label single-blind randomized study including 59 consecutive diabetic patients admitted for NSTE-ACS. Patients were randomly treated with aspirin 100 mg once a day (GA100; n = 20), aspirin 160 mg once a day (GA160; n = 19) or aspirin 100 mg twice a day (G2A100; n = 20). The primary endpoint was endogenous thrombin potential (ETP) at discharge and after 6 months.
Results
The mean age of our patients was 61.5 ± 9 years, and 73% were male. The baseline characteristics were comparable between the three groups. In the GA100 group, there was no significant effect on ETP variation at 6 months (1150.46 ± 504.84 vs. 1087.63 ± 454.18; p = 0.794). An increase in aspirin dose with a second daily administration of 100 mg was associated with a significant reduction in ETP at 6 months (1004.87 ± 196.2 vs. 1233.63 ± 333.5; p = 0.003). A nonsignificant decrease in ETP was seen in the GA160 group (from 1173.8 ± 388.07 to 1053.64 ± 269.93 at 6 months, p = 0.117).
Conclusion
Only the twice-daily aspirin regimen led to better control of hypercoagulability in NSTE-ACS diabetic patients. However, no thrombin generation normalization was reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiplatelet therapy has formed the backbone of acute coronary syndrome (ACS) management for decades. It is generally assumed that the antithrombotic effect of aspirin is exerted through acetylation of the platelet cyclooxygenase, resulting in reduced thromboxane formation and platelet inhibition [1]. Moreover, there are studies indicating that aspirin may have antithrombotic effects through inhibition of thrombin generation (TG) [2], enhancement of fibrinolysis [3] or alterations in fibrin gel porosity [4, 5].
Although the incidence of atherothrombotic recurrence is reduced with dual antiplatelet therapy, it remains high in patients with diabetes mellitus (DM), underscoring the need for more efficacious strategies [6, 7].
Several studies have reported reduced clinical benefit and stable biological efficacy of once-daily low-dose aspirin, especially in diabetic patients [8, 9].
The specific mechanisms of aspirin resistance in DM patients have not been entirely elucidated. Several mechanisms have been put forward: (i) drug interactions (e.g., with proton pump inhibitors or nonsteroidal anti-inflammatory drugs) [10, 11]; (ii) metabolic disorders (insulin resistance, hyperlipidemia) [12]; (iii) chronic kidney disease [13]; and (iv) the new hypothesis of increased platelet reactivity and/or turnover, as the introduction into the bloodstream of newly generated platelets not exposed to aspirin may continue to generate thromboxane A2 [14].
In clinical practice, the efficacy of antiplatelet drugs has traditionally been monitored with methods based on the evaluation of induced platelet aggregation. However, these methods cannot determine the degree of platelet aggregability and thrombus suppression as a whole. In recent years, the TG test has been studied extensively for this purpose, and was considered as a useful tool for investigating residual hypercoagulability and may predict cardiovascular outcomes [15, 16].
The impaCt of Aspirin Regimen on THrombin Generation in diabEtic patients with Acute Coronary Syndrome (CARTHaGE-ACS) trial was designed to determine whether a divided dose of aspirin is more efficient in inhibiting thrombin generation than a standard-dose (100 mg–160 mg) regimen using the calibrated automated thrombogram (CAT) in DM patients with non-ST segment elevation acute coronary syndrome (NSTE-ACS).The main hypothesis of the study was that decreasing platelet activity in a twice-daily aspirin regimen would be accompanied by a decrease in thrombin formation on the platelet surface and could be used for evaluating the efficacy of antiplatelet therapy.
Material and methods
Study population
An open-label single-blind randomized study including 59 consecutive diabetic patients admitted to the cardiology department for NSTE-ACS between January and December 2017 was conducted.
Eligible patients were ≥ 18 years old, with a history of diabetes or with newly discovered diabetes admitted for NSTE-ACS. DM was defined as glycosylated hemoglobin (HbA1c) > 6.5% and fasting glycemia >1.26 g/l.
The exclusion criteria were as follows: patients on oral anticoagulation therapy or with a known allergy to clopidogrel or aspirin, platelet count <100.000/μL, history of gastrointestinal bleeding, active bleeding, history of cerebrovascular accident within the last 3 months, severe or moderate anemia, pregnancy, active inflammatory or malignant disease, or severe renal (eGFR <15 ml/1.73 m2/min) or hepatic insufficiency.
Patients with indication for coronary artery bypass grafting, having defective samples, or hospitalized for acute coronary or bleeding events during the follow-up period were excluded.
Study design
The management and therapeutic strategy were in accordance with the guidelines of the European Society of Cardiology for NSTE-ACS [17]. All patients were pretreated with a 300-mg loading dose of clopidogrel and intravenous administration of a 250-mg loading dose of acetylsalicylic acid (ASA) followed by 75 mg of clopidogrel and 100-mg ASA maintenance dose in the following days (Aspegic®, Sanofi-Aventis, Paris, France). Coronary artery disease (CAD) was documented in the overall study population by coronary angiography. Patients were treated, if appropriate, with percutaneous coronary intervention according to current guidelines at the time of the study [18]. The same statin (rosuvastatin) was prescribed for all patients in order to exclude all potential interference of statins with the TG test as described in prior studies [19,20,21]. The use of other therapies, including antithrombin therapy and glycoprotein IIb/IIIa antagonists, was left to the discretion of the attending physician. At discharge, patients were randomly and consecutively assigned to one of three ASA regimens:
-
(1)
100 mg aspirin given once per day (Aspegic® 100 Sanofi).
-
(2)
160 mg aspirin given once per day (Kardegic® 160 Sanofi).
-
(3)
200 mg aspirin divided into two doses per day consisting of 100 mg in the morning 100 mg in the evening (Aspegic® 100 Sanofi).
TG study was performed after randomization, at discharge (T0) (median: 4 days after the clopidogrel loading dose, range: 2–7 days) and 6 months later (T1) in patients free from recurrent ischemic or bleeding events during the follow-up period. Compliance and occurrence of adverse events were systematically assessed by regular phone calls.
The study complied with the Declaration of Helsinki and was approved by the local ethics committee. All eligible participants were fully informed about the study protocol, agreed to participate in the study, and expressed their understanding and provided written informed consent.
Blood samples
Blood was collected in tubes containing 3.2% sodium citrate diluted in a ratio of 1:9. Platelet-poor plasma (PPP) was obtained by centrifugation at 2500×g for 15 min at room temperature for the TG analysis. All samples were kept frozen at −80 °C for a maximum of 7 months. Blood samples for routine tests were prepared and analyzed with conventional methods.
The calibrated automated thrombogram (CAT) assay
In this study, the TG test was performed and results were analyzed according to the method suggested by Hemker et al. in 2003 [22, 23]. Briefly, 80 μl thawed PPP was mixed with 20 μl of a reagent (PPP-Reagent®; Diagnostica Stago, Asnières, France) containing tissue factor and phospholipids, with final concentrations of 5pM and 4 μM, respectively. The reactions were performed in microtiter wells after the addition of a freshly made starting reagent (PPP-Reagent®; Diagnostica Stago) and a thrombin-specific fluorogenic substrate (FluCa Kit®; Diagnostica Stago). The fluorescence intensity was recorded by the Fluoroskan Ascent fluorometer (Diagnostica Stago). A dedicated software program enabled the calculation of thrombin activity against time. The following TG parameters were evaluated: endogenous thrombin potential (ETP; nM/min), peak thrombin (Peak; nM), lag time (LT; min) and time to peak (TTP; min).
Endpoints
The primary endpoints were as follows:
-
ETP variation at 6 months in each group (intragroup variation)
-
ETP variation at 6 months between the three aspirin regimens (intergroup variation)
Intergroup variation was defined as: Δ ETP = (ETP 6 months − ETP discharge)/ETP discharge.
The secondary objective was to assess changes in the remaining TG parameters (Peak, TTP and LT).
Statistical analysis
Categorical variables were expressed as percentages and frequencies, and continuous variables were presented as mean values ± SD. Comparisons of categorical variables were tested by χ2 or Fisher’s exact test, as appropriate. The nonparametric Kruskal-Wallis test was used for comparison of continuous variables. Changes from baseline within the groups were analyzed by the Wilcoxon matched-pairs test. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 14.0 software.
Sample size
It was difficult to calculate sample size regarding the effect of twice-daily aspirin on TG measurement, as there were no studies definitively showing this association. Thus, based on a previous study analyzing the effect of oral anticoagulants on thrombin generation [39], a 20% reduction in ETP at 6 months in patients on aspirin 100 mg/bid versus aspirin once daily (100 and 160 mg) was assumed, and calculations were based on a power of 95% and a significance level of 0.05. According to these values, we determined that a sample population of 54 patients would be sufficient to demonstrate a decrease in TG in patients on aspirin twice daily versus once daily. Ultimately, 69 patients (+27%) were enrolled in this study to allow for potential missing patients during follow-up.
Results
Population characteristics
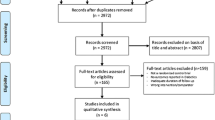
From January to December 2017, a total of 69 DM patients admitted for non-ST elevation myocardial infarction NSTE ACS were enrolled. Of these, 10 were excluded: three patients died, one patient was lost during follow-up and six were excluded for defective samples. Thus, 59 patients completed the study as planned and were divided into three groups according to aspirin dosing (Fig. 1):
-
GA100: Aspirin 100 mg (n = 20)
-
GA160: Aspirin 160 mg (n = 19)
-
G2A100: Aspirin 100 mg×2 (n = 20)
The baseline characteristics of the study population are given in Tables 1 and 2. The mean age was 61.5 ± 9 years (40–80 years). Most were male (72%), with a history of hypertension (68%) and dyslipidemia (48%). The mean level of HbA1c was 8.95 ± 2.057%. Only five patients (G2A100: 3; GA160: 2) had high intra-hospital mortality risk as assessed by the GRACE score (>140). An increased bleeding risk was found (CRUSADE >40) in 11 patients (18%), well balanced among the three aspirin groups. All patients had coronary angiography after a mean duration of 3 ± 1.3 days. During hospitalization, the majority of patients (93%) received anticoagulant treatment with weight-adjusted low molecular weight heparin (enoxaparin). In the remaining patients, unfractionated heparin was used, with a target ACT ratio of 2–3 and with no difference among the three groups (p = 0.733). Most of the patients (n = 51, 86%) underwent percutaneous coronary intervention. Of the eight remaining patients, three (37%) had no clinically significant CAD (either angiographically normal coronary arteries or all lesions with <70% stenosis), and five (63%) were not candidates for any type of revascularization. During percutaneous coronary intervention, all patients had only a weight-adjusted bolus of unfractionated heparin (30–50 UI/kg), with no need for glycoprotein IIb/IIIa antagonists. The patients were optimally treated at discharge, receiving double antiplatelet therapy (clopidogrel + aspirin), statins, beta blockers (88%) and angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blocker (98%). The three treatment groups were comparable with respect to all the listed variables except for the use of ACEI.
Effect of aspirin dosing on TG
Intragroup variation
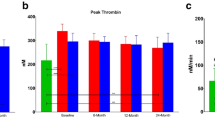
Thrombogram variables measured at baseline and after 6 months of treatment are shown in Table 3 and Fig. 2. No significant differences between the groups were observed at baseline. At follow-up, patients treated with aspirin twice daily (G2A100) had significantly reduced ETP as compared with baseline (baseline: 1233.63 ± 33.49 vs. 6 months: 1004.87 ± 196.20; p = 0.003), whereas aspirin 160 mg once daily (GA160) failed to significantly reduce TG (ETP at baseline: 1173.8 ± 388.07 vs. ETP at 6 months: 1053.64 ± 26,993, p = 0.1). In the 100-mg aspirin group, a slight but nonsignificant increase in ETP and Peak levels was observed at 6 months. In the three aspirin regimens, a trend for reduction in time parameters (TTP/LT) was observed, with no statistical difference except for TTP in G2A100, which was significantly reduced at 6 months (TTP at baseline: 3.92 ± 1.20 vs. TTP at 6 months: 3.03 ± 0.70; p = 0.009).
Intergroup variation
We found significant inhibition of ETP and Peak in G2A100 compared with GA100 (ETP: −15% vs. +5%; p = 0.042) (Fig. 1 in supplementary materials). A similar pattern was observed for ETP variation in G2A100 compared to GA160, but without reaching the significance threshold (ETP: −15% vs. −5%). No significant difference was observed in either ETP or Peak variation at 6 months between GA100 and GA160. The remaining TG variables were similar in the three different aspirin regimens.
Clinical events
Clinical adverse events occurred in six patients during the follow-up period. One patient in each of the three groups experienced cardiac death (two sudden cardiac death, one myocardial infarction). Three other patients on high-dose aspirin (100 mg twice per day or 160 mg) reported minor bleeding (epistaxis/gingival bleeding). No major bleeding episode was reported.
Discussion
The main results of this study can be summarized as follows:
-
No variation in TG was reported in diabetic patients on aspirin 100 mg once per day 6 months after an episode of NSTE-ACS.
-
An intermediate regimen of aspirin 160 mg once per day was associated with a nonsignificant reduction in TG in the study population.
-
A regimen of aspirin 100 mg twice per day succeeded in significantly reducing the ETP (mean reduction of 15%) 6 months after acute coronary syndrome in diabetic patients.
We opted in this trial to use an aspirin dose of 160 mg once daily instead of 200 mg once daily mainly to avoid a potential increase in bleeding and patient noncompliance. We used the CAT assay as the sole laboratory method. Even though it is a reliable and consistent test, it still suffers from lack of standardization. ETP measurement upon admission to the hospital and the use of platelet function tests as “light transmission aggregometry” would have added more information. The other major limitation was the use of PPP for TG analysis. In fact, by excluding the contribution of platelets to thrombin synthesis, the use of PPP may underestimate the effect of aspirin on thrombin generation. Follow-up of bleeding events and major adverse cardiovascular events was conducted for only 6 months. A more prolonged and structured follow-up would have provided more prognostic information to our study. Finally, our results showed that multiple daily dosing rather than a once-daily dose of aspirin may be more beneficial in diabetic patients. However, this is only a hypothesis-generating study due to its sample size, and a large prospective randomized study is needed to confirm whether this approach will be clinically relevant and safe in patients with diabetes.
Thrombus formation after ACS is driven by coordinated platelet and thrombin pathways. Key to thrombus formation is the generation of thrombin, which not only converts fibrinogen to fibrin but also serves as a potent platelet agonist and induces platelet aggregation at the site of vascular injury [24, 25].
Several studies have shown that in vivo thrombin generation as assessed by thrombin–antithrombin complex or prothrombin fragment 1+2 assays was clearly increased in patients with recurrent coronary events and symptomatic CAD [26,27,28], lasting for up to 1 year after myocardial infarction [29, 30] and 6 months after NSTEMI [31, 32]. Ex vivo TG analysis was first reported in CAD patients by Orbe et al. in 2008, showing higher peak thrombin activity in subjects with a history of ACS as compared to patients with stable CAD [33]. Indeed, Smid et al. [34] later confirmed that there was 7% higher ETP and 15% higher peak thrombin in patients with acute myocardial infarction than in controls.
Thus, it can likely be assumed that TG inhibition during ACS can contribute substantially to reducing the recurrence of coronary events, particularly among diabetics.
The optimal dose of aspirin required for secondary prevention of coronary events in diabetic patients is a topic of controversy. In fact, after ACS, patients with DM have a clearly increased risk of death, stent thrombosis and recurrent events despite optimal antiaggregant treatment, suggesting a lower clinical response to aspirin treatment in this population [35]. Possible reasons for the reduced clinical benefit of low-dose aspirin in DM include platelet hyperactivity and a more rapid platelet turnover [36, 37]. In trials using platelet aggregation measurements as an outcome parameter, higher doses of aspirin administered once daily were not superior to lower doses of 75–100 mg [38]. In this study, aspirin 100 mg given once daily for 6 months did not reduce TG, and actually caused a slight increase in TG after 6 months of treatment (baseline ETP: 1087.63 vs. ETP: 6 months: 1150.46; p = 0.794). However, the use of aspirin 160 mg once daily was associated with a reduction in ETP 6 months after the episode of ACS, although this difference was not significant (p = 0.117).
On the other hand, a second daily administration of 100 mg aspirin was able to significantly reduce TG, as evidenced by the reduction in ETP (At 6 months:1004,87 ± 196,2 vs. at baseline:1233.63 ± 333.5; p = 0.003). Additionally, when comparing the reduction in ETP between the groups, G2A100 was associated with a notably greater decrease in TG at 6 months from ACS compared with G100 (−15% vs. +20%; p = 0.043). No significant difference was found between the other groups. Several lines of evidence point to an important role of platelets in TG and thrombus formation by inhibition of thromboxane A2 synthesis. However, studies evaluating the direct effects of aspirin on TG in vitro and ex vivo have shown mixed results [39, 40]. To the best of our knowledge, there are no data concerning the benefit of a twice-daily aspirin regimen on TG in diabetic patients after ACS. Nonetheless, several studies have demonstrated the benefit of a fractionated versus once-daily aspirin regimen on platelet reactivity assessed by platelet function tests. Indeed, Addad et al. showed that 100 mg aspirin twice per day significantly reversed high platelet reactivity in diabetic patients with CAD in comparison with a once-daily dose, as assessed by the platelet function analyzer (PFA)-100 platelet test [41]. These findings were further confirmed by Dillinger et al., who demonstrated that aspirin dosing twice per day (75 mg×2) significantly decreased platelet reactivity to arachidonic acid (0.5 mg/mL), as measured by light transmission aggregometry, when compared to once-daily aspirin (150 mg) in diabetic patients with CAD [42].
Given the fact that platelet activation markers follow a circadian rhythm which peaks between 6 a.m. and noon [43, 44], this advantage of twice-daily dosing might be related to the timing of aspirin administration. In a randomized crossover trial in 14 healthy participants, Bonten et al. [45] demonstrated that bedtime administration of 80 mg aspirin was more effective than morning administration in suppressing COX-1 platelet reactivity during the morning hours. Our hypothesis regarding the benefit of twice-daily versus once-daily aspirin was based on an assumption of the potential effect of multiple daily aspirin dosing on increased platelet turnover in DM. Nevertheless, our results could not completely confirm this hypothesis, since the aspirin doses used in this trial were not equivalent. As a matter of fact, the significant decrease in TG in the G2A100 could be simply due to an aspirin dose-dependent anticoagulant effect, as described in previous studies [46, 47]. The mechanisms by which aspirin attenuates thrombin formation cannot be determined from the present study. However, available data [48] suggest that the effect of aspirin on thrombin production may be partially related to depressed platelet reactivity, but also associated with the properties of the damaged tissues exposed on injury. The decline in ETP could be explained by a change in the assembly of pro- and anticoagulant factors after long-term aspirin treatment. Indeed, Undas et al. [49] reported that 7-day administration of aspirin (75 mg per day) resulted in significantly lower velocity of prothrombin consumption (by 29%), TG (by 27.2%) and prothrombinase formation (by 29%). The post-treatment appearance of both chains of factor Va (FVa) were reduced by 25% (heavy chain) and 29.6% (light chain). Peak levels of light and heavy FVa chains were also decreased in blood collected at the site of vascular injury following aspirin ingestion [49]. Aspirin also delayed FXIII activation by thrombin and decreased the maximum rate of FXIII cleavage [49]. Another explanation for the significant aspirin-induced downregulation of TG might be a decrease in TF expression on monocytes [50] and atherosclerotic plaques [51]. Several other mechanisms of the anticoagulant effect of aspirin have been discussed but remain largely hypothetical. Acetylation of prothrombin in vivo has been suggested [52]; however, there are no fundamental data to support this conjecture. Aspirin has been reported to acetylate antithrombin [53], and this mechanism, if present in vivo, may contribute to the decrease in thrombin markers in plasma after aspirin use. Another potential mechanism of aspirin, independent of the activity of COX-1, might be fibrin acetylation [54]. In fact, it has been speculated that in vivo acetylation of fibrinogen changes its structure, which might result in altered properties of the fibrin clot formed from the modified fibrinogen. [55]
Further, it is not known to what extent aspirin-induced suppression of COX-1 activity, with the subsequent inhibition of thromboxane A2 synthesis, may influence thrombin formation at the platelet with shear surface. At present, the suggestion that impaired platelet function by aspirin translates to lower thrombin production cannot be excluded.
In conclusion, this prospective pilot study in patients with diabetes after NSTE-ACS shows that twice-daily aspirin is more effective in reducing TG than once-daily aspirin, with no episodes of minor or major bleeding events. However, future large-scale clinical outcome trials are needed to confirm this benefit and to assess the clinical efficiency and safety of such a protocol.
References
Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Buda A (2000) Impact of diabetes on long term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 102:1014–1019
Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Heeschen C, Hamm CW, Moliterno DJ, Califf RM, White HD, Kleiman NS, Théroux P, Topol EJ (2001) Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 104:2767–2771
Loll PJ, Picot D, Garavito RM (1995) The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol 2637–43
Szczeklik A, Krzanowski M, Gora P, Radwan J (1992) Antiplatelet drugs and generation of thrombin in clotting blood. Blood 80:2006–2011
Bjornsson TD, Schneider DE, Berger H (1989) Aspirin acetylates fibrinogen and enhances fibrinolysis. Fibrinolytic effect is independent of changes in plasminogen activator levels. J PharmacolExpTher 250:154–161
Undas A, Brummel K, Musial J, Mann KG, Szczeklik A (2001) Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood 2423–31
Fatah K, Beving H, Albage A, Ivert T, Blomback M (1996) Acetylsalicylic acid may protect the patient by increasing fibrin gel porosity. Is withdrawing of treatment harmful to the patient? Eur Heart J 17:1362–1366
Creager MA, Luscher TF, Cosentino F, Beckman JA (2003) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108:1527–1532
Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Wurtz M, Grove EL, Kristensen SD et al (2010) The antiplatelet effect of aspirin is reduced by proton pump inhibitors in patients with coronary artery disease. Heart 96:368–371
Catella-Lawson F, Reilly MP, Kapoor SC et al (2001) Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 345:1809–1817
Davì G, Catalano I, Averna M et al (1990) Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med 322:1769–1774
Angiolillo DJ, Bernardo E, Capodanno D et al (2010) Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease on dual antiplatelet therapy. J Am Coll Cardiol 55:1139–1146
Guthikonda S, Lev EI, Patel R et al (2007) Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J ThrombHaemost. 5:490–496
Kuliczkowski W, Szewczyk M, Kaczmarski J, Sztohryn E, Greif M, Pres D, Fortmann SD, Polonski L, Serebruany V (2014) Thrombin generation and platelet reactivity at hospital discharge and 6-month outcome after the acute coronary syndrome in diabetic and nondiabetic patients. Cardiology. 128:25–33
Berezovskaya G, Smirnova O, Malev E, Khromov-Borisov N, Klokova E, Karpenko M, Papayan L, Petrishchev N (2018) Thrombin generation test for evaluation of antiplatelet treatment in patients with coronary artery disease after percutaneous coronary intervention. Platelets. 29:185–191
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al (2016) ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). EurHeart J 37:267–315
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V et al (2014) ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35:2541–2619
Undas A, Celinska-Löwenhoff M, Brummel-Ziedins KE, Brozek J, Szczeklik A, Mann KG (2005) Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. ArteriosclerThrombVasc Biol 25:1524–1525
Szczeklik A, Musial J, Undas A, Swadzba J, Gora PF, Piwowarska W et al (1996) Inhibition of thrombin generation by aspirin is blunted in hypercholesterolemia. ArteriosclerThrombVasc Biol 16:948–954
Musial J, Undas A, Undas R, Brozek J, Szczeklik A (2001) Treatment with simvastatin and low-dose aspirin depresses thrombin generation in patients with coronary heart disease and borderline-high cholesterol levels. ThrombHaemost. 85:221–225
Hemker HC, Béguin S (1995) Thrombin generation in plasma: its assessment via the endogenous thrombin potential. ThrombHaemost 74:134–138
Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R et al (2003) Calibrated automated thrombin generation measurement in clotting plasma. PathophysiolHaemostThromb. 33:4–15
Coughlin SR (2002) Thrombin signaling and protease-activated receptors. Nature 407:258–264
Fuster V, Badimon L, Badimon JJ, Chesebro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (first of two parts). N Engl J Med 326(4):242–250
Becker RC, Tracy RP, Bovill EG, Corrao JM, Baker S, Ball SP et al (1994) Surface 12-lead electrocardiographic findings and plasma markers of thrombin activity and generation in patients with myocardial ischemia at rest. J Thromb Thrombolysis 1:101–107
Becker RC, Bovill EG, Corrao JM, Ball SP, Ault K, Mann KG et al (1995) Dynamic nature of thrombin generation, fibrin formation, and platelet activation in unstable angina and non-Q-wave myocardial infarction. J Thromb Thrombolysis 2:57–64
Merlini PA, Ardissino D, Rosenberg RD, Colombi E, Agricola P, Oltrona L et al (2000) In vivo thrombin generation and activity during and after intravenous infusion of heparin or recombinant hirudin in patients with unstable angina pectoris. ArteriosclerThrombVasc Biol 20:2162–2166
Martınez-Sales V, Vila V, Reganon E, Goberna MA, Ferrando F, Palencia MA et al (1998) Elevated thrombotic activity after myocardial infarction: a 2-year follow-up study. Haemostasis 28:301–306
Ardissino D, Merlini PA, Bauer KA, Galvani M, Ottani F, Franchi F et al (2003) Coagulation activation and long-term outcome in acute coronary syndromes. Blood 102:2731–2735
Angiolillo DJ, Ueno M, Goto S (2010) Basic principles of platelet biology and clinical implications. Circ J 74:597–607
Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, Belli C et al (1994) Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 90:61–68
Orbe J, Zudaire M, Serrano R, Coma-Canella I, Martınez de Sizarrondo S, Rodrıguez JA et al (2008) Increased thrombin generation after acute versus chronic coronary disease as assessed by the thrombin generation test. ThrombHaemost 99:382–387
Smid M, Dielis AW, Winkens M, Spronk HM, van Oerle R, Hamulyak K et al (2011) Thrombin generation in patients with a first acute myocardial infarction. J ThrombHaemost 9:450–456
Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P et al (2005) Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 54:2430–2435
Di Minno G, Silver MJ, Murphy S (1983) Monitoring the entry of new platelets into the circulation after ingestion of aspirin. Blood 61:1081–1085
El Haouari M, Rosado JA (2008) Platelet signalling abnormalities in patients with type 2 diabetes mellitus: a review. Blood Cells Mol Dis 41:119–123
DiChiara J, Bliden KP, Tantry US, Hamed MS, Antonino MJ, Suarez TA et al (2007) The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes 56:3014–3019
Brodin E, Seljeflot I, Arnesen H, Hurlen M, Appelbom H, Hansen JB (2009) Endogenous thrombin potential (ETP) in plasma from patients with AMI during antithrombotic treatment. ThrombRes. 123:573–579
Bratseth V, Alfàge P, Trine BO, Harald A, Ingebjørg S (2012) Markers of hypercoagulability in CAD patients. Effects of single aspirin and clopidogrel treatment. Thromb J 10:1–12
Addad F, Chakroun T, Elalamy I, Abderazek F, Chouchene S, Dridi Z et al (2010) Antiplatelet effect of once- or twice-daily aspirin dosage in stable coronary artery disease patients with diabetes. Int J Hematol 92:296–301
Dillinger JG, Drissa A, Sideris G, BalditSollier C, Voicu S, Manzo Silberman S et al (2012) Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am Heart J 164:600–606
Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN et al (1987) Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med 316:1514–1518
Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA (1997) Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 79:1512–1516
Bonten TN, Saris A, van Oostrom MJ, Snoep JD, Rosendaal FR, Zwaginga J et al (2014) Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomized cross-over trial ThrombHaemost 112:1209–1218
Hampton KK, Cerletti C, Loizou LA, Bucchi F, Donati MB, Davies JA et al (1990) Coagulation, fibrinolytic and platelet thromboxane and platelet function in patients on long-term therapy with aspirin 300 mg or 1,200 mg daily compared with placebo. ThrombHaemost 64:17–20
Wallen NH, Ladjevardi M (1998) Influence of low- and high-dose aspirin treatment on thrombin generation in whole blood. Thromb Res 92:189–194
Musial J, Radwan J, Szczeklik A (1997) Aspirin delays thrombin generation in vitro through interaction with platelet phospholipids. Thromb Res 85:367–368
Undas A, Brummel K, Musial J, Mann KG, Szczeklik A (2001) Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood. 98:2423–2431
Osnes LTN, Foss KB, Joo GB et al (1996) Acetylsalicylic acid and sodium salicylate inhibit LPS-induced NF-kB/c-Rel nuclear translocation, and synthesis of tissue factor (TF) and tumor necrosis factor alpha (TNF-alpha) in human monocytes. ThrombHaemost. 76:970–976
Matetzky S, Tani S, Kangavari S et al (2000) Smoking increases tissue factor expression in atherosclerotic plaques: implications for thrombogenicity. Circulation. 102:602–604
Szczeklik A, Krzanowski M, Gora P, Radwan J (1992) Antiplatelet drugs and generation of thrombin in clotting blood. Blood. 80:2006–2011
Villanueva GV, Allen N (1986) Acetylation of antithrombin III by aspirin. SeminThrombHaemost. 12:213–215
Mosesson MW, Siebenlist KR, Meh DA (2001) The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci 936:11–30
Henschen-Edman AH (2001) Fibrinogen non-inherited heterogeneity and its relationship to function in health and disease. Ann N Y Acad Sci 936:580–593
Author information
Authors and Affiliations
Contributions
Addad F, Laaroussi L and Bousoffara MA designed the study.
All authors were involved in patient inclusion and collection of clinical data and specimens.
Sami Kasbaoui and Hbib Triki: recruitment of the patients.
Baccouche H and Bousoffara MA were involved in thrombin generation experiments and analyzed the experimental data.
Bousoffara MA wrote the paper.
Laaroussi L and Baccouche H revised the paper.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent
This study was performed in accordance with the 1964 Helsinki Declaration. All participants gave their informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• There was no variation in thrombin generation in diabetic patients on 100 mg once per day 6 months after an episode of non-ST elevation acute coronary syndrome.
• There was a nonsignificant reduction in thrombin generation on aspirin 160 mg once per day in the study population.
• There was a significant reduction in ETP on aspirin (100 mg twice per day) 6 months after an episode of non-ST elevation acute coronary syndrome.
• After an episode of non-ST elevation acute coronary syndrome, twice-daily aspirin is more effective in reducing thrombin generation than once-daily aspirin in patients with diabetes.
Electronic supplementary material
ESM 1
(DOCX 99 kb)
Rights and permissions
About this article
Cite this article
Boussofara, A., Laroussi, L., Baccouche, H. et al. ImpaCt of aspirin regimen on THrombin generation in diabEtic patients with acute coronary syndrome: CARTHaGE-ACS trial. Eur J Clin Pharmacol 77, 517–526 (2021). https://doi.org/10.1007/s00228-020-02969-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02969-y