Abstract
Purpose
Delirium is reported in over 50% of critically ill ICU patients, and is associated with increased mortality and long-term cognitive consequences. Prevention and early management of delirium are essential components of ICU care. However, pharmacological interventions have not been effective in delirium prevention. This study investigated the effect of aripiprazole in the prevention of delirium in a neurosurgical intensive care unit.
Methods
In this prospective, randomized placebo-controlled small clinical trial, 53 patients, 18 to 80 years old, were randomized to receive enteric aripiprazole (15 mg) or placebo for up to 7 days. Delirium, detected by the Confusion Assessment Method-ICU, ICU events, laboratory studies, aripiprazole safety, time to delirium onset, delirium-free days, delirium prevalence during follow-up and ICU length of stay were recorded.
Results
Forty patients with similar baseline characteristics, including age, sex, neurosurgery types and APACHE II scores, completed the study. Delirium incidence and the mean days to its onset were 20% vs. 55% (p = 0.022) and 2.17 ± 0.41 vs. 2.09 ± 0.30 (p = 0.076) in the aripiprazole and placebo groups, respectively. The mean number of delirium-free days were: 5.6 (95%CI, 4.6-6.5) and 4.3 (95%CI, 3.2-5.4), in aripiprazole and placebo groups, respectively (p = 0.111). The prevalence of delirium during the follow-up was significantly lower in the aripiprazole group (p = 0.018). Serious aripiprazole adverse reactions were not observed.
Conclusions
Aripiprazole can reduce the incidence of delirium in the neurosurgical ICU. Studies with larger sample size in diverse ICU settings and longer follow-up are needed to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delirium is an acute change or fluctuation in mental status manifesting with inattention, disorganized thinking, and an altered level of consciousness, with or without agitation. The DSM-V (Diagnostic and Statistical Manual of Mental Disorders, fifth edition) lists the following criteria for diagnosis of delirium: disturbance of consciousness in a short period (usually hours to days); change in cognition; a direct physiologic consequence of a general medical condition, an intoxicating substance, medication use, or more than one cause induce disturbance of consciousness [1]. By the application of the Confusion Assessment Method for the ICU (CAM-ICU) tool for delirium detection, delirium has been reported to occur in greater than 50% (22–87%) of patients admitted to the ICU, although it remains a poorly recognized ICU complication [2,3,4,5].
Delirium is now considered one of the most important complications in critically ill ICU patients, due to a better understanding of its etiologies, predisposing factors, incidence, and devastating adverse effects on short- and long-term patient outcomes. Also, delirium results in prolonged hospitalization, increased morbidity and mortality, significant family and caregiver burden, and higher health care expenditures [6,7,8]. Therefore, early detection, prevention, and management of delirium are essential components in the delivery of care to ICU patients.
Typical and atypical antipsychotics, including haloperidol, olanzapine, ziprasidone, risperidone, aripiprazole, and quetiapine, are used at different rates worldwide for delirium treatment [9,10,11,12,13,14,15,16,17]. However, investigations exploring the role of these agents in delirium prophylaxis are scarce [18,19,20]. Additional data are required for proposing the routine use of the above agents in preventing delirium in ICU patients [21].
Aripiprazole, a partial agonist at the dopamine (D2) receptor, is classified as a third-generation atypical antipsychotic agent [22]. The incidence of extrapyramidal syndrome (EPS) and QTc prolongation are less frequent for aripiprazole than for other antipsychotic agents [23, 24]. The European Medicine Agency (EMA) and US Food and Drug Administration (FDA) categorize aripiprazole as an agent with no risk for induction of cardiac arrhythmia [25]. Other studies have indicated aripiprazole to be effective in delirium management, with the same efficacy and no clinically significant adverse effects in comparison with haloperidol [26].
We conducted this small clinical study to examine the effect of aripiprazole for delirium prophylaxis in neurosurgical ICU patients. As a validated tool for the evaluation of delirium, we chose the CAM-ICU. Because this tool requires patient cooperation in order to evaluate delirium, and considering our hospital setup, we conducted this study in the neurosurgical ICU.
Materials and method
Patients and setting
This prospective, randomized, double-blind, placebo-controlled study was conducted from January 2017 to August 2017 in a neurosurgical ICU of a 450-bed university hospital, affiliated to Iran University of Medical Sciences, Tehran, Iran. The study protocol was approved by the appropriate ethics committees and written informed consent was obtained from each patient or the patient’s legal guardian (IRCTID: IRCT2017011810178N12). All patients ≥18 years old with stable hemodynamics and breathing spontaneously without mechanical ventilatory support, who were admitted to the ICU post-neurosurgical intervention and without delirium based on the CAM-ICU tool, were consecutively screened for enrollment in the study. The neurosurgical interventions were for the management of traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), brain tumors, hydrocephalus and pituitary adenomas.
Patients with the following conditions were excluded from the study: pregnancy, breastfeeding, preexisting active delirium, current drug overdose or suicide attempt, severe liver failure based on Child-Pugh scores, renal failure (GFR < 15 mL/min), history of prior anti-psychotic use, allergy to aripiprazole, history of neuroleptic malignant syndrome, history of severe dementia (documented history and/or the Informant Questionnaire on Cognitive Decline in the Elderly [IQCODE] score ≥ 4) [27], history of high risk for ventricular dysrhythmia or its presence, patients on drugs known to prolong the QTc interval, baseline QTc ≥ 500 ms in the absence of a bundle branch block, history of torsade de pointes, myocardial infarction in the past 2 weeks, uncompensated stage IV heart failure, refractory electrolyte abnormalities (hypokalemia <3.0 mEq/dL or hypomagnesemia <1.8 mg/dL), and ICU stay of less than 3 days.
Study protocol and assessments
Patients were recruited based on defined inclusion and exclusion criteria and were assigned to the intervention and control groups by a simple randomization method using computer-generated numbers. Usual medical management continued unchanged in all patients at the discretion of the ICU managing teams, who were blinded to the study groups.
The treatment and control groups received 15 mg enteric aripiprazole and placebo daily (Sobhan Pharmaceutical Company), respectively. Placebo tablets were made in an identical fashion to the aripiprazole tablets by the same vendor. Aripiprazole or placebo was continued for up to 7 days, according to the duration of the ICU stay. The non-pharmacological approaches to delirium prevention were employed equally for all patients based on the ICU practice routines.
The Richmond Agitation–Sedation Scale (RASS) was used to evaluate the level of sedation and agitation in our patients [28]. The presence of delirium was determined by a trained investigator, using the CAM-ICU tool, in patients with RASS values of at least −3 at enrollment and during the study period (twice every day) [2, 3, 27, 29]. Patients were followed for 7 days or until discharge or transfer from the ICU or death. The CAM-ICU is a valid and reliable delirium assessment tool suggested by the Society of Critical Care Medicine (SCCM) in its 2013 Pain, Agitation, and Delirium (PAD) guidelines [30].
The following baseline demographics were recorded at the time of enrollment: age, gender, the reason for ICU admission, the severity of illness based on the Acute Physiology and Chronic Health Evaluation II score (APACHE-II) [31], history of drug abuse, and baseline RASS and CAM-ICU scores.
During the study period, QTc intervals in all patients were monitored daily on electrocardiograms. In patients belonging to any group with an episode of QTc-interval prolongation (i.e., ≥ 500 ms or ≥ 60 ms over the baseline value), the study intervention was paused until resolution of the QTc abnormalities. We discontinued the study intervention in the event of concomitant administration of any agent known to cause QTc interval prolongation, such as class Ia or Ic and III antiarrhythmics, cotrimoxazole or azole antifungals.
We monitored for the occurrence of any clinical evidence of EPS. Patients were excluded and a neurological consult was obtained if EPS was detected at any time during the study period.
Patients who were deeply sedated after administration of the study drug, in the absence of other sedative drug use, were excluded.
Other adverse effects of aripiprazole were assessed using laboratory data, such as daily blood sugar, triglyceride, total cholesterol, HDL, and LDL levels at the baseline and the end of the study.
The primary end point was determination of the incidence of delirium during the study period. The secondary end points included safety and tolerability of aripiprazole, time to the onset of delirium, number of delirium-free days, the prevalence of delirium during the study follow-up periods, and the length of stay in the ICU. We also evaluated the effects of dexamethasone and oxazepam doses (for night sedation) during the follow-up between the two groups. The outcome assessments were made by physicians who were blinded to the study interventions.
Sample size
The sample size of the study was calculated with Minitab software using the “test for two proportions” (Fisher exact test) function, considering a type I error of 0.05, power of 0.8, proportion of delirium in the ICU of 70%, and expected relative treatment effect of 50%. The number of participants calculated in each group was 25.
Statistical analysis
Continuous variables were checked for normality, and as they were normal, they were compared by application of the Student t test. The chi-square test or Fisher exact test was used for the categorical variables as appropriate. We employed generalized estimating equation (GEE) models [32, 33] to compare the two groups regarding the status of CAM-ICU during the follow-up periods, using the interaction analysis of time and group. We also used this model to adjust the effect of confounding variables such as medication use on delirium occurrence. A p value of less than 0.05 was considered statistically significant in all cases.
Results
Patient population
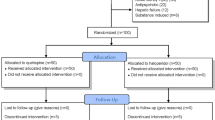
This study was carried out between April 2017 to May 2018. Sixty-five consecutive patients who were admitted to the neurosurgical ICU were evaluated for eligibility, and 53 patients met the study entry criteria. Patients were randomized to the treatment and placebo groups. A total of 13 patients were excluded, as follows: ICU stay less than 3 days in four patients, medication administration errors in eight patients, and suspected drug hypersensitivity reaction in one patient (Fig. 1).
The baseline characteristics, including patient demographics, baseline RASS, history of illicit drug dependence, underlying diseases, types of surgeries, and administration of drugs such as benzodiazepines or systemic steroids, were not statistically different between the two study groups (Table 1).
Effects of aripiprazole on delirium prevention
The CAM-ICU values were measured during the 7 days of the study, and were positive for the presence of delirium in four (20%) and 11 (55%) of the patients in the treatment and placebo groups, respectively [χ2 (1, n = 40) = 5.227, p = 0.022] (Table 2).
The difference in the mean number of days to the onset of delirium was not statistically significant between the two groups (2.17 ± 0.41 days vs. 2.09 ± 0.30 days in the aripiprazole and placebo groups, respectively, t(17) = 0.438, p = 0.076 (Fig. 2). The mean number of delirium-free days in the aripiprazole and the placebo groups were 5.6 (95% CI, 4.6–6.5) and 4.3 (95% CI, 3.2–5.4), respectively [χ2 (1, n = 206) = 2.546, p = 0.111] (Table 2 and Fig. 2). Based on the GEE model, the prevalence of delirium during the study follow-up period was significantly lower in the aripiprazole group than in the placebo group (B = 0.263, p = 0.018) (Fig. 2).
Effects of systemic steroids and benzodiazepines on the occurrence of delirium
The mean doses of dexamethasone were 7.27 ± 7.79 mg (0–24 mg) and 15.3 ± 10.57 mg (0–32 mg) in the aripiprazole and the placebo groups, respectively, [t(204) = −6.188, p < 0.001]. The mean doses of oxazepam were 0.9 ± 2.9 mg (0–10) and 3.79 ± 4.8 mg (0–10 mg) in the aripiprazole and placebo groups, respectively [t(204) = −5.082, p < 0.001]. After the adjustments for the effects of these agents, the incidence of delirium remained significantly lower in the treatment group compared with the placebo group (B = 0.359, p = 0.034).
Effects of aripiprazole on ICU stay
The mean ICU stay was not significantly different when comparing the study groups, 11.55 ± 12.10 days and 13.80 ± 11.91 days in the intervention and placebo groups, respectively [t(38) = −0.593, p = 0.557].
Aripiprazole adverse effects
We did not observe any serious adverse reactions related to aripiprazole, such as the signs or symptoms of neuroleptic malignant syndrome, ventricular arrhythmias or extrapyramidal symptoms. Three patients had prolongation of the QTc interval more than 500 ms, without any ventricular arrhythmias, one in the aripiprazole and two in the in the control group, χ2 (1, n = 40) = 0.360, p = 0.548].
Only one suspected drug-induced skin rash was encountered in the aripiprazole group, which resolved after its discontinuation. After excluding the patient from the study, we evaluated the case using causality assessment methods. We used the Naranjo scale and concluded that the hypersensitivity was due to ceftriaxone, not aripiprazole. No significant differences were found between the two study groups in the serum levels of total cholesterol, HDL, LDL, and triglycerides at baseline and end of study or in daily serum blood sugar levels (p > 0.05).
Discussion
Our pilot study is the first randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of aripiprazole for prevention of delirium in patients in the neurosurgical ICU. The administration of aripiprazole in our study patients resulted in a significant decrease in the rate of ICU-acquired delirium, evaluated using the CAM-ICU tool. The prevalence of delirium was also lower in the aripiprazole group during the follow-up period. Although differences in the number of delirium-free days and the mean ICU length of stay did not reach statistical significance, these values numerically favored patients in the aripiprazole group (5.6 vs. 4.3 days and 11.55 vs. 13.80 days, respectively). We did not observe any significant aripiprazole-related adverse drug reactions in our study. Larger trials with a longer duration of follow-up and diverse ICU populations might be warranted to further elucidate these effects.
Although the pathogenesis of delirium in critically ill patients remains relatively unproven, hypotheses generated by research outside the ICU propose that delirium results from a decrease in acetylcholine and increase in dopamine in the brain, both of which might occur in response to factors promoting cerebral oxidative stress during critical illness [15, 34]. The anticholinergic effects of antipsychotics is a classic issue in delirium. There is evidence that exposure to anticholinergic agents leads to increased severity of symptoms in delirium [35]. However, although both olanzapine and quetiapine are known for their anticholinergic side effects, there is evidence supporting the efficacy of these antipsychotics in delirium, including in ICU populations [14, 13, 36]. Antipsychotics, which exert their effects by altering concentrations of a variety of neurotransmitters in the central nervous system, have therefore been recommended as potentially effective pharmacologic therapies for delirium [9,10,11,12,13,14,15].
The Society of Critical Care Medicine guidelines for pain, agitation, and delirium (PAD) recommends atypical antipsychotics for the treatment of delirium as a grade C recommendation, based mainly on the limited published data linking positive outcomes to quetiapine [14, 30]. However, investigations exploring the role of antipsychotics in delirium prophylaxis are scarce [18,19,20]. The role of haloperidol in preventing delirium has been evaluated in only two randomized controlled trials [18, 19].
Wang et al. found that short-term prophylactic intravenous administration of low-dose haloperidol significantly reduced the incidence of postoperative delirium in elderly patients admitted to the ICU after non-cardiac surgery [19]. However, Al-Qadheeb et al. demonstrated that low-dose haloperidol, initiated early in the ICU stay, did not prevent delirium and had little therapeutic advantage in mechanically ventilated, critically ill adults with delirium [18].
Prakanrattana et al. assessed the efficacy of risperidone for the prevention of postoperative delirium, administered soon after cardiac surgery with cardiopulmonary bypass and found that this reduced the incidence of postoperative delirium [20]. Because of the lack of sufficient evidence, the Society of Critical Care Medicine guidelines do not recommend using a pharmacologic delirium prevention protocol in adult ICU patients [30].
Aripiprazole, an atypical antipsychotic agent, is a dopamine (D2) partial agonist with intrinsic activity at the receptor site through stabilization of D2 receptor-mediated neurotransmission without its prolonged blockage. Aripiprazole has an agonistic effect on serotonin type 1, an antagonistic effect on serotonin type 2, and a blocking effect on the alpha receptors. Because of its higher affinity at the D2 receptors than endogenous dopamine, aripiprazole is less likely to elevate serum prolactin levels and induce EPS than other antipsychotics. Aripiprazole also has high affinity at the dopamine D3 receptors, which explains its partial agonist activity [23, 24]. By blocking the D2 receptor, aripiprazole could be effective in the treatment or prevention of delirium [26].
The CAM-ICU, a validated delirium screening tool, was used to identify delirium and measure the response to treatment in our study patients. The CAM-ICU demonstrates very good psychometric properties (i.e., validity and reliability) and is explicitly designed for use in ICU patients, both on and off mechanical ventilation. Translated into over 20 languages, this tool is currently in use worldwide [30]. We used an available validated Persian-language version of the CAM-ICU (http://www.icudelirium.org/delirium/languages.html).
The definitive role of systemic corticosteroids and benzodiazepines as important risk factors for the development of delirium in the ICU setting remains controversial [21, 37, 38]. Devlin et al. reported that the drug-associated delirium literature consists primarily of case series and uncontrolled cohort studies [37]. Zaal et al. performed a systematic review of risk factors for delirium in the ICU from 2000 to 2013. They evaluated critically ill adults not undergoing cardiac surgery and used either multivariable analysis or randomization to evaluate variables as potential risk factors for delirium [38].
The results for benzodiazepines as a risk factor for the development of delirium were found to be inconclusive. This may be explained by the different definitions used in the studies, but also by the interplay between delirium, the indication for benzodiazepines (anxiety, sleep disorders, or induction of coma), and the potential direct harmful effect of the medication itself [38].
Although systemic steroid use, particularly in high doses, has long been assumed to be a potential risk for delirium, until recently there has been a paucity of rigorous data to confirm this association. Schreiber et al. [34] evaluated a cohort of 520 mechanically ventilated adults with acute lung injury for the development of delirium. Among 20 of the potential delirium risk factors incorporated into the model, only age and the administration of a systemic corticosteroid in the preceding 24 h were independently associated with a transition to delirium. On the other hand, the results of the Dexamethasone for Cardiac Surgery trial suggest that the administration of a corticosteroid prior to cardiac surgery does not influence the prevalence of postoperative delirium [39]. In our study, although the mean doses of benzodiazepines and systemic steroids administered were higher in the placebo group, after adjusting for these confounders, the incidence of delirium remained significantly lower in the treatment group than the placebo group.
Regarding the occurrence of EPS, our results are consistent with the pooled data from the overall clinical program with aripiprazole, which indicated that the incidence of EPS with this agent was similar to that observed with the placebo [40]. QTc interval prolongation on the electrocardiogram has been observed in patients taking certain antipsychotic medications, which can lead to torsade de pointes, a potentially fatal cardiac arrhythmia [41]. In this study, we observed no significant difference in QTc abnormalities between the aripiprazole and placebo groups, in agreement with other studies supporting a low risk of arrhythmic potential for this agent [23].
Hyperglycemia and dyslipidemia have been reported in patients treated with atypical antipsychotics, particularly with olanzapine and clozapine [42]. In our study, there was no significant difference in blood sugar or lipid profile between the two groups, either at the beginning or end of the study.
The limitations of our study included its small sample size, complexities regarding delirium assessment in neurological pathologies, short duration of follow-up, and no more than daily frequency of assessment for delirium. Further studies with larger sample size, more diverse ICU patients, better randomization techniques, more frequent CAM-ICU assessments, longer duration of study, and longer post-ICU follow-up are warranted to study the role of aripiprazole in delirium prophylaxis and its effects on post-ICU cognitive function.
Conclusion
Delirium is associated with poor short- and long-term patient outcome and longer duration of stay in the ICU and hospital. Data regarding pharmacological interventions for delirium prophylaxis in critically ill patients are limited. Recent studies have demonstrated the safety and efficacy of aripiprazole in various psychiatric disorders. Based on our study, aripiprazole can be considered a safe and effective choice of treatment to prevent neurosurgical ICU-acquired delirium. Larger studies are needed to validate our findings.
Abbreviations
- ICU:
-
Intensive care unit
- CAM-ICU:
-
Confusion Assessment Method for the ICU
- EPS:
-
Extrapyramidal syndrome
- TBI:
-
Traumatic brain injury
- SAH:
-
Subarachnoid hemorrhage
- IQCODE:
-
Informant Questionnaire on Cognitive Decline in the Elderly
- RASS:
-
Richmond Agitation–Sedation Scale
- APACHE-II:
-
Acute Physiology and Chronic Health Evaluation II
References
Diagnostic and Statistical Manual of Mental Disorders (2013) Fifth edn. American Psychiatric Association
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286(21):2703–2710
Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK (2001) Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med 29(7):1370–1379
Gilchrist NA, Asoh I, Greenberg B (2012) Atypical antipsychotics for the treatment of ICU delirium. J Intensive Care Med 27(6):354–361. https://doi.org/10.1177/0885066611403110
van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ (2009) Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 37(6):1881–1885. https://doi.org/10.1097/CCM.0b013e3181a00118
Medicare & Medicaid statistical supplement (2013). United State Department of Health and Human Services, Centers for Medicare & Medicaid Services
Inouye SK (1998) Delirium in hospitalized older patients. Clin Geriatr Med 14(4):745–764
Pandharipande PP, Ely EW, Arora RC, Balas MC, Boustani MA, La Calle GH, Cunningham C, Devlin JW, Elefante J, Han JH, MacLullich AM, Maldonado JR, Morandi A, Needham DM, Page VJ, Rose L, Salluh JIF, Sharshar T, Shehabi Y, Skrobik Y, Slooter AJC, Smith HAB (2017) The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med 43(9):1329–1339. https://doi.org/10.1007/s00134-017-4860-7
Han CS, Kim YK (2004) A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 45(4):297–301. https://doi.org/10.1016/S0033-3182(04)70170-X
Lee KU, Won WY, Lee HK, Kweon YS, Lee CT, Pae CU, Bahk WM (2005) Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol 20(6):311–314
Maneeton B, Maneeton N, Srisurapanont M (2007) An open-label study of quetiapine for delirium. J Med Assoc Thail 90(10):2158–2163
Pae CU, Lee SJ, Lee CU, Lee C, Paik IH (2004) A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 19(2):125–127. https://doi.org/10.1002/hup.559
Skrobik YK, Bergeron N, Dumont M, Gottfried SB (2004) Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 30(3):444–449. https://doi.org/10.1007/s00134-003-2117-0
Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E (2010) Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med 38(2):419–427. https://doi.org/10.1097/CCM.0b013e3181b9e302
Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, Bernard GR, Dittus RS, Ely EW, Investigators MT (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med 38(2):428–437
Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM (2016) Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 64(4):705–714. https://doi.org/10.1111/jgs.14076
Boettger S, Breitbart W (2011) An open trial of aripiprazole for the treatment of delirium in hospitalized cancer patients. Palliat Support Care 9(4):351–357. https://doi.org/10.1017/s1478951511000368
Al-Qadheeb NS, Skrobik Y, Schumaker G, Pacheco MN, Roberts RJ, Ruthazer RR, Devlin JW (2016) Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med 44(3):583–591. https://doi.org/10.1097/CCM.0000000000001411
Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, Chen KS, Gu XE, Zhu SN (2012) Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med 40(3):731–739. https://doi.org/10.1097/CCM.0b013e3182376e4f
Prakanrattana U, Prapaitrakool S (2007) Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care 35(5):714–719
Brummel NE, Girard TD (2013) Preventing delirium in the intensive care unit. Crit Care Clin 29(1):51–65. https://doi.org/10.1016/j.ccc.2012.10.007
Stahl SM (2001) Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry 62(12):923–924
Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR (2003) Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 60(7):681–690. https://doi.org/10.1001/archpsyc.60.7.681
Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28(8):1400–1411. https://doi.org/10.1038/sj.npp.1300203
Fanoe S, Pehrson S, Bundgaard H, Fink-Jensen A, Kristensen D, Jensen HK, Toft E, Nielsen J, Videbech P (2014) Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J 35(20):1306–1315. https://doi.org/10.1093/eurheartj/ehu100
Boettger S, Friedlander M, Breitbart W, Passik S (2011) Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry 45(6):477–482. https://doi.org/10.3109/00048674.2011.543411
Jorm AF, Jacomb PA (1989) The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 19(4):1015–1022
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond agitation-sedation scale (RASS). JAMA 289(22):2983–2991. https://doi.org/10.1001/jama.289.22.2983
Monette J, Galbaud du Fort G, Fung SH, Massoud F, Moride Y, Arsenault L, Afilalo M (2001) Evaluation of the confusion assessment method (CAM) as a screening tool for delirium in the emergency room. Gen Hosp Psychiatry 23(1):20–25
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care M (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41(1):263–306. https://doi.org/10.1097/CCM.0b013e3182783b72
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Twisk JW, Smidt N, de Vente W (2005) Applied analysis of recurrent events: a practical overview. J Epidemiol Community Health 59(8):706–710. https://doi.org/10.1136/jech.2004.030759
Stuart R, Lipsitz NML, Harrington DP (1991) Generalized estimating equations for correlated binary data: using the odds ratio as a measure of association. Biometrika 78(1):153–160
Schreiber MP, Colantuoni E, Bienvenu OJ, Neufeld KJ, Chen KF, Shanholtz C, Mendez-Tellez PA, Needham DM (2014) Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med 42(6):1480–1486. https://doi.org/10.1097/CCM.0000000000000247
Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M (2001) Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med 161(8):1099–1105
Riviere J, van der Mast RC, Vandenberghe J, Van Den Eede F (2019) Efficacy and tolerability of atypical antipsychotics in the treatment of delirium: a systematic review of the literature. Psychosomatics 60(1):18–26. https://doi.org/10.1016/j.psym.2018.05.011
Devlin JW, Zaal IJ, Slooter AJ (2014) Clarifying the confusion surrounding drug-associated delirium in the ICU. Crit Care Med 42(6):1565–1566. https://doi.org/10.1097/CCM.0000000000000293
Zaal IJ, Devlin JW, Peelen LM, Slooter AJ (2015) A systematic review of risk factors for delirium in the ICU. Crit Care Med 43(1):40–47. https://doi.org/10.1097/CCM.0000000000000625
Sauer AM, Slooter AJ, Veldhuijzen DS, van Eijk MM, Devlin JW, van Dijk D (2014) Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg 119(5):1046–1052. https://doi.org/10.1213/ANE.0000000000000248
Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, Saha A, Ali M, Iwamoto T (2003) Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 61(2-3):123–136
Gury C, Canceil O, Iaria P (2000) Antipsychotic drugs and cardiovascular safety: current studies of prolonged QT interval and risk of ventricular arrhythmia. Encephale 26(6):62–72
Lacro JP FS, Endow-Eyer RA (2012) Schizophrenia. In: Koda-Kimble (ed) Applied therapeutics, the clinical use of drugs. 10 edn
Author information
Authors and Affiliations
Contributions
MM and MS created the concept. Literature review and drafting of the proposal were done by MF and MJ. MG, SMRH, MN, NG, and GM performed the search and gathered clinical data. MY analyzed the data. All authors reviewed and helped to finalize the article for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was performed at Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
Rights and permissions
About this article
Cite this article
Mokhtari, M., Farasatinasab, M., Jafarpour Machian, M. et al. Aripiprazole for prevention of delirium in the neurosurgical intensive care unit: a double-blind, randomized, placebo-controlled study. Eur J Clin Pharmacol 76, 491–499 (2020). https://doi.org/10.1007/s00228-019-02802-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02802-1