Abstract
Purpose
Ginkgo terpene lactones meglumine injection (GMI) is a novel preparation of traditional Chinese medicine that contains ginkgolides A, B and K (GA, GB, GK, respectively) as its primary components. In this study we evaluated the safety, tolerability and pharmacokinetics of these three ginkgolides after single and multiple intravenous infusions of GMI. We also investigated the effect of GMI on cytochrome P450 3A4 (CYP3A4) in healthy Chinese volunteers.
Methods
In this open-label, placebo-controlled study 15 subjects were randomly assigned to receive GMI or matched placebo (4:1 ratio). All subjects first received midazolam (MDZ) on day 1, followed by a 6-day washout. On Day 8, the subjects were started on once-daily dosing of either GMI or placebo for 14 days. Lastly, on Day 22 the subjects were given second dose of MDZ + GMI or MDZ + placebo. Plasma concentrations of ginkgolides, MDZ and its metabolite 1-hydroxy midazolam were quantified.
Results
The steady-state conditions of GA, GB and GK were achieved after 6 days of daily dosing. Following a single dose of GMI (Day 8) the area under the concentration–timecurve from zero to 24 h after administration (AUC0-24h) of GA, GB and GK (arithmetic ± standard deviation) was 4.10 ± 1.06, 4.61 ± 1.31 and 0.127 ± 0.102 h μg/mL, respectively; the corresponding values following multiple doses of GMI (Day 19) were 3.94 ± 1.16, 5.00 ± 1.55 and 0.118 ± 0.096 h μg/mL, respectively. The mean accumulation ratios were 0.95, 1.08 and 0.89 for GA, GB and GK, respectively. Additionally, the geometric mean [peak concentration (Cmax) and AUC0-24h] ratios of MDZ and 1-hydroxy midazolam were all within the specified acceptance ranges in the MDZ + placebo treatment and MDZ + GMI treatment.
Conclusions
Our results show that GMI was well tolerated during the entire study. There was no systemic accumulation and no significant effects on the pharmacokinetics of MDZ in healthy Chinese male subjects after repeated dosing of GMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginkgo terpene lactones meglumine injection (GMI) is a novel preparation of traditional Chinese medicine containing 25 mg of ginkgo terpene lactones. The major ginkgo terpene lactones of GMI are ginkgolide A (GA; 8.5 mg), ginkgolide B (GB; 14 mg) and ginkgolide K (GK; 1.0 mg) (see Fig 1 for their respective chemical structures). In comparison, the main components of EGb761, a typical Ginkgo biloba extract product, are flavonoids (24%) and terpenoides (6%: 3.1% ginkgolides and 2.9% bilobalide). The main effect of EGb761 is free radical scavenging [1], while GMI has been extracted and purified from G. biloba leaves for the treatment of cerebral apoplexy [2]. It has also been reported that GMI possesses different a variety of pharmacological activities, such as neuroprotective properties and a potential effect on cerebrovascular diseases, but further study is needed to elucidate its mechanism [3].
Ginkgo, a living fossil tree, has a long history of use in traditional Chinese medicine. It’s products have been documented to include a wide range of pharmacological activities, such as neuroprotective, cardioprotective and antitumorigenic effects, antioxidant, stress alleviating, improvement of memory and protection against apoptosis [4–6]. Many G. biloba products are available commercially, and the leaf extract is currently one of the best-selling herbal products worldwide [1, 7]. The most unique components of the leaf extract are the terpene lactones, namely, bilobalide and GA, GB and ginkgolide C [8]. Ginkgo biloba extract is used as a herb drug or dietary supplement and as such is often administered in combination with other therapeutic drugs. Consequently, it is very important to identify potential herb–drug interactions to guide the rational clinical use of drugs.
Most pharmacokinetic (PK) interactions are related to the changes in the functionality or expression of cytochrome P450 (CYP) enzymes [9]. CYP3A4 is considered to be the most important drug-metabolizing enzyme based on its high abundance in the liver and its participation in the metabolism of >60% of all drugs. As such, the inhibition or induction of this enzyme is the source of numerous drug interactions [10], which has led to the effect of G. biloba extract on CYP450 becoming a major subject of research. In in vitro studies, G. biloba extract has been found to have inhibitory effects on a number of drug-metabolizing enzymes, including CYP3A4, CYP2D6 as well as other isoforms [11]. In contrast, the results of some in vivo studies in humans indicate that G. biloba extract has no significant effects on CYP isoforms nor does it significantly inhibit some CYP isoforms. Gurley and co-workers reported that G. biloba extract had no apparent effect on any of the CYP isoforms they studied using the cocktail approach [12]. To the contrary, Smith and associates [13] found that the concentrations of nifedipine (a substrate of CYP3A4) were significantly increased in subjects exposed to ginkgo (120 mg daily) for 18 days, suggesting that the G. biloba extract significantly inhibits CYP3A4.
GMI was found to be a weak inducer of CYP3A4 in an in vitro CYP450 inducer experiment [Report RTC 00400 by XenoBiotic Laboratories, Inc. China; Electronic Supplementary Material (ESM)], indicating a potential capacity to affect the pharmacokinetics of the co-administered drugs. We therefore sought to explore the effect of GMI on the activity of CYP3A4 in normal volunteers. To this end we chose midazolam (MDZ), which is rapidly metabolized by CYP3A4 to its main metabolite 1-hydroxymidazolam (1-OH MDZ) [14, 15], as the marker for CYP3A4 activity. Due to the chemical complexity and multi-components involved in GMI preparation, PK studies of GMI may be helpful for revealing its action mechanism or determining rational dosage regimens for the appropriate application.
Therefore, the aim of this study was to investigate the safety, tolerability and pharmacokinetics of GMI following single or multiple intravenous infusions of GMI at a dose of 25 mg in healthy Chinese subjects. We also evaluated the effects of multiple doses of GMI on CYP3A4 activity and therefore on the pharmacokinetics of MDZ and its metabolite 1-OH MDZ.
Patients and methods
Materials and reagents
Reference standards for GA (purity 95.4%), GB (purity 99.9%), GK (purity 94.6%) and bilobalide (internal standard, purity 100%) were purchased from the National Institute for Control of Pharmaceutical and Biological Products (Beijing, China). MDZ, 1-OH MDZ, D4-midazolam (internal standard) and D4-1-hydroxymidazolam (internal standard) were purchased from Cerilliant Corporation (Round Rock, TX, USA). Midazolam maleate (15 mg/tablet, Lot 20131001) was manufactured by Jiangsu Nhwa Pharmaceutical Corporation (Xuzhou, China). GMI (5 mL/ampoule, containing 25 mg of ginkgo terpene lactones; Lot 140604) was provided by Jiangsu Kanion Pharmaceutical Co. Ltd. (Lianyungang, China). All other reagents were of reagent grade or better and obtained from commercial sources.
Dosing and Administration
Midazolam
On Days 1 and 22, subjects were administered 7.5 mg MDZ (half of a tablet of midazolam maleate) orally with 200 mL of water.
Ginkgo terpene lactones meglumine injection
Prior to the GMI being administered to each subject by intravenous infusion, it was diluted with 250 mL 0.9% sodium chloride injection. The subjects were closely followed by continuous electrocardiographic monitoring during the administration of the GMI via infusion pump at a gradient infusion velocity of 0.5 mL/min for the first 30 min, followed by 0.5 mL/min increases at half-hour intervals up to a the maximum infusion velocity of 1.5 mL/min. The duration of total infusion dosing was approximately 197 min.
Study participants
The enrollment criteria were Chinese ethnicity, male gender, good health, based on medical history, physical examination, vital signs measurement, electrocardiogram (ECG) and clinical laboratory tests, age of 18–40 years, body mass index of 19–25 kg/m2, body weight of ≥50 kg and nonsmoker. Subjects were ineligible for inclusion if they had used any medicines, including herbal products, within the 2 weeks immediately preceding the study. The intake of any food or beverage containing xanthine (e.g. caffeine) must have been discontinued 48 h before dosing. In addition, the consumption of such foods and beverages (i.e. coffee, tea, soda, chocolate) was not permitted at any time while the subjects were domiciled. No grapefruit or grapefruit juice was to be consumed for 14 days prior to dosing until 2 days following the last dose.
Study design
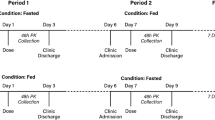
This was an open-label, placebo-controlled, four-period study (NCT02233972) study involving 15 subjects who met the inclusion criteria. These subjects were randomly assigned to receive GMI or matched placebo (4:1 ratio). The study was approved by the Ethics committee of the First Affiliated Hospital–Nanjing Medical University and conducted in compliance with the ethical principles set forth in the Declaration of Helsinki (1989) as well as with local applicable laws and regulations. All subjects provided written informed consent before participating in the study.
The study consisted of (1) a screening (up to 14 days) and a baseline evaluation (Day −1); (2) an initial treatment with a single dose of MDZ 7.5 mg on Day 1; (3) a 6-day washout period/no treatment from Day 2 to Day 7; (4) a multiple-dose treatment regimen in which subjects received a once-daily intravenous infusion of GMI 25 mg or placebo from Day 8 to Day 21; (5) a second single dose of MDZ together with GMI or placebo on Day 22; (6) an end-of-study evaluation on Day 23 (Table 1).
Subjects fasted for at least 10 h prior to the administration of the drug and continued to fast for another 4 h after the administration of the drug. No fluid intake was allowed from 2 h before until 2 h after the dosing; outside of this time period, water was provided ad libitum.
PK assessments
Measurement of drug concentrations
The plasma drug levels of MDZ and ginkgolides (GA, GB and GK) were determined using two different validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay methods (XenoBiotic Laboratories, Inc., Nanjing, China).
Determination of MDZ and 1-OH MDZ
Midazolam and 1-OH MDZ were extracted from plasma samples by protein precipitation. Briefly, 30 μL D4-MDZ/D4-1-OH MDZ (internal standards) and 500 μL acetonitrile were added successively to a tube containing 100 μL plasma. The samples were vortexed for 6 min and then centrifuged at 3200 g, 4 °C for 5 min. A 300-μL sample of supernatant was transferred to another tube and evaporated to dryness. The dry extracts were re-dissolved in 150 μL of 60% methanol, and 10-μL aliquots were injected into the LC-MS/MS system for analysis.
The compounds were analyzed by high-performance liquid chromatography on a Symmetry C8 LC column (50 × 2.0 mm, 5 μm; Waters Corp., Milford, MA). A gradient elution consisting of solvent A (methanol:acetonitrile, 50:50, v:v) and solvent B (10 mM ammonium acetate solution adjusted to pH 4), was delivered at a flow rate of 0.6 mL/min. Detection was performed by MS/MS using an API 5000 mass spectrometer (Applied Biosystems/Sciex, Foster City, CA) in MRM-positive ionization mode. For MDZ and its internal standard (D4-MDZ) the precursor ion mass was m/z 362.3 and 329.9 and the fragmention m/z was 291.2, 295.3, respectively. The precursor ion mass for 1-OH MDZ and its internal standard (D4-1-OH MDZ) was m/z342.1 and 346.0 and the fragment ion was m/z 203.2 and 203.1, respectively. The linear calibration curves were obtained over the concentration range of 0.100–20.0 ng/mL for both MDZ and 1-OH MDZ. The accuracy of MDZ/1-OH MDZ ranged from 85 to 115%, while the relative standard deviations (RSDs) of inter-day precision were all within 15% of the three quality control (QC) levels. The values of inter-day precision were summarized as follows: 2.20, 2.03, 1.56% for MDZ; 4.44, 1.75, 1.78% for 1-OH MDZ (more detailed results were presented in the ESM).
Determination of GA/GB and GK
The ginkgolides A/B and K were extracted from plasma samples by liquid–liquid extraction. Briefly, 50 μL of bilobalide (internal standard) and 40 μL 2 N HCL solution were added successively to a tube containing 200 μL plasma. The mixture was vortexed for 45 min, following which 1 mL ethyl acetate was added and the mixture vortexed for 5 min and then centrifuged (10000 g, 5 min, 4 °C). An 850-μL sample of supernatant was transferred to another tube and evaporated to dryness under the flow of N2 at 35 °C. The dry extracts were re-dissolved in 150 μL methanol: 0.1% formic acid solution (50:50, v:v), and 10-μL aliquots were injected into the LC-MS/MS system for analysis.
The compounds were analyzed using an UFLC 20-AD XR liquid chromatography system on a C18-AR column (50 × 2.1 mm, 3 μm; Shimazu Corp., Kyoto, Japan). A gradient elution consisting of solvent A (acetonitrile) and solvent B (20 mM ammonium acetate solution adjusted to pH 5.4) was delivered at a flow rate of 0.7 mL/min. Detection was performed in MS/MS using an API 5000 mass spectrometer (Applied Biosystems/Sciex) in MRM-positive ionization mode. The precursor ion mass for GA, GB and GK was m/z 453.2, 423.3 and 405.4 and the fragment ion was m/z 351.2, 367.1 and 331.0, respectively. The precursor ion mass for bilobalide was m/z 325.3 and the fragment ion m/z was 163.3. Linear calibration curves for GA, GB and GKwere obtained in the concentration range of 0.200–200, 0.200–200 and 0.189–189 ng/mL, respectively. The accuracy of GA/GB/GK ranged from 85 to 115%, and the RSDs of inter-day and intra-day precision were all within 15% of the three QC levels. The values of intra-day precision were summarized as follows: 7.16, 3.68, 5.33% for GA; 10.63, 2.94, 4.82% for GB; 10.73, 4.52, 4.17% for GK (more detailed results were presented in the ESM).
PK analysis
On Day 1, blood samples (3 mL) were collected into K2EDTA (anticoagulant)-coated tubes at 0, 10 min, 20 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h, 3.28 h (197 min), 4 h, 6 h, 8 h, 12 h, 16 h and 24 h after the administration of MDZ. On Days 8 and 19, blood samples (4 mL) were collected at 0, 30 min, 1 h, 1.5 h, 2 h, 3.28 h (197 min), 3.42 h (205 min), 3.58 h (215 min), 4 h, 5 h, 6 h, 8 h, 12 h and 24 h after the administration of GMI or placebo. On Day 22, blood samples (4 mL) were collected at 0, 10 min, 20 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h, 3.28 h (197 min), 3.42 h (205 min), 3.58 h (215 min), 4 h, 5 h, 6 h, 8 h, 12 h, 16 h and 24 h after the administration of MDZ and GMI or placebo. On Days 14, 17, 18 and 19, blood samples (4 mL) were collected before drug administration to determine the trough concentration (C min) of GA, GB and GK, respectively.
Immediately after the blood samples were collected, they were mixed gently and chilled on ice, following which they were centrifuged at 3000 rpm for 5 min. The plasma samples were then separated and stored at −70 °C until analysis.
Non-compartmental methods using Phoenix WinNonlin software (ver. 6.3; Certara, L.P., Princeton, NJ) were used to evaluate the pharmacokinetic parameters, namely, peak plasma concentration after administration(Cmax), time to reach Cmax (Tmax), area under the concentration–time curve from zero to 24 h after administration (AUC0-24h), AUC from zero to infinity (AUC0-∞), elimination half-life (T½), apparent total body clearance of substance (after oral administration) [CL(/F)] and apparent volume of distribution during terminal phase (after non-intravenous administration) [Vz(/F)]. Cmax and Tmax were obtained directly from the observed plasma concentration–time values. AUC0-24h or AUC0-t was estimated using the linear trapezoidal rule. AUC0-∞ was calculated as AUC0-t + Ct/λz, where Ct was the last detected concentration and λz was the slope of the log-linear regression of the terminal declining phase. T1/2 was calculated as ln2/λz using the best-fit mode. CL(/F) and Vz(/F) were estimated as dose/AUC0-∞ and CL/λz, respectively. The accumulation ratio (Racc) of ginkgolides was calculated as AUC0-24h (Day 19)/AUC0-24h (Day 8).
Safety and tolerability assessments
Safety and tolerability assessments included recording all adverse events (AEs) and serious AEs. Additional safety assessments included monitoring of vital signs, ECG recordings and blood chemistry, urinalysis, and hematology studies.
Statistical analysis
All PK parameters were expressed with the coefficient of variation, with any geometric means indicated, or as the median and range of values. Descriptive statistics were provided for PK concentrations and derived PK parameters. The accumulation ratio of primary PK parameters was assessed using an analysis of variance model that included day as a fixed effect and subject as a random effect. The 90% confidence intervals (CI) for MDZ and 1-OH MDZ when administered with/without GMI were calculated. The 90% confidence intervals for GA, GB and GK were calculated after single or multiple intravenous infusions of GMI.
Results
Demographics
The baseline demographics of the 15 subjects enrolled in this study are summarized in Table 2. All 15 subjects were healthy, Chinese adult men. During the study, all subjects received two once-daily oral doses of MDZ and GMI or placebo (0.9% saline solution) by intravenous infusion once daily for 15 days.
All 15 subjects completed the study and were included in the safety and PK analysis. There were no significant protocol deviations during the duration of the study.
Pharmacokinetics of ginkgolides
Dosages of approximately 8.5, 14 and 1.0 mg of GA, GB and GK, respectively, were administered. Following the 6-day washout, 12 subjects were given GMI by intravenous infusion once daily for 12 days from Day 8 to Day 19. The PK parameters of GA, GB and GK after single and multiple intravenous infusions of GMI are listed in Table 3, and the arithmetic mean plasma concentration–time profiles of GA, GB and GK for each sampling day are shown in Fig 2. The arithmetic mean of the AUC0-24h after a single intravenous infusion of GMI (first dose of GMI, Day 8) was 4.10, 4.61 and 0.127 h μg/mL for GA, GB and GK, respectively (Table 3), and the corresponding AUC0-24h values after multiple intravenous infusions of GMI (12th dose of GMI, Day 19) were 3.94, 5.00 and 0.118 h μg/mL, respectively. The geometric mean of the AUC0-24h accumulation ratios (12th dose to 1st dose) was 0.95, 1.08 and 0.89 for GA, GB and GK, respectively (Table 4). These results indicate that the PK parameters of ginkgolides A, B and K on Days 8 and 19 were similar and that there was no accumulation with the once-daily administration protocol.
The Cmin of GA, GB and GK are shown in Fig 3. This graph demonstrates that the steady state was likely achieved by 6 days of treatment with intravenous infusions of GMI at the dose of 25 mg.
Effect of GMI on the pharmacokinetics of MDZ and its metabolite 1-OH MDZ
The plasma concentration–time profiles of MDZ and 1-OH MDZ at baseline (Day 1) and after GMI or placebo administration (Day 22) are shown in Fig 4a and b, respectively. The corresponding PK parameters and statistics are summarized in Tables 5 and 6, respectively. We found no obvious differences in the PK parameters of MDZ and 1-OH MDZ between the MDZ + placebo treatment and the MDZ + GMI treatment.
The geometric mean ratios of Cmax and AUC0-24h of MDZ (Day 22/Day 1) after the MDZ + GMI treatment were 0.87 (90% CI 0.65–1.16) and 0.88 (90% CI 0.75–1.03), respectively; the corresponding ratios of 1-OH MDZ were 0.94(90% CI 0.59–1.49) and 0.99 (90% CI 0.81–1.20), respectively. Otherwise, the geometric mean ratios (Day 22/Day 1) of Cmax (1-OH MDZ)/Cmax (MDZ) and AUC0-24h (1-OH MDZ)/AUC0-24h (MDZ) were 1.08 (90% CI 0.85–1.37) and 1.12 (90% CI 0.89–1.42), respectively. The results indicated that the ratios were all within the specified acceptance ranges.
Safety
The GMI appeared to be well tolerated throughout the study. There were no serious AEs or clinically significant changes in vital signs or clinical laboratory parameters. Mild sedation occurred and was noted after MDZ administration.
Discussion
To the best of our knowledge, our study is the first to simultaneously evaluate the pharmacokinetics of ginkgolides A, B and K following single and multiple intravenous infusions of GMI to healthy Chinese volunteers. GMI was approved by the Federal Drug Administration of China in 2012 and subsequently became commercially available. The dose regimen of the preparation used in our study was selected based on the label and the results of previous clinical trials [2] which demonstrated that a once-daily intravenous infusion of GMI 25 mg for 14 days had a manageable toxicity profile and encouraging efficacy in the treatment of cerebral apoplexy.
Following the single (Day 1) and multiple (Day 12) intravenous infusions of GMI at the dose of 25 mg, GA, GB and GK directly entered the systemic circulation, with a median Tmax of 197 min, which was the endpoint of the infusion using the pump. The steady states of GA, GB and GK were likely achieved by 6 days of daily dosing. Also, the PK parameters of these three ginkgolides on Day 8 (1st dose of GMI) and Day 19 (12th dose of GMI) were similar. The estimated AUC accumulation ratios were 0.95, 1.08 and 0.89 for GA, GB and GC, respectively, which indicates that no accumulation occurred in vivo after multiple intravenous infusions of GMI. These results are similar to those reported by Wang et al. [19], who showed that there were no obvious differences in PK parameters, such as AUC and T½, after consecutive administration of GMI for 7 days in rats.
Prior to our study, in vitro data were available which suggested that GMI is a weak inducer of CYP3A4 (Report RTC 00400, XenoBiotic Laboratories, Inc., China). Therefore, we further assessed the effect of GMI on the activity of CYP3A4 in vivo. To evaluate this effect, we selected MDZ as the probe drug as this drug has been extensively used for determining CYP3A4 activity in vivo [14, 16]. MDZ is rapidly metabolized by CYP3A4 to its major metabolite 1-OH MDZ.
As shown in Table 6, the geometric mean ratios of MDZ and 1-OH MDZ were all within the specified acceptance ranges in both the MDZ + placebo treatment and the MDZ + GMI treatment. These results indicate that once-daily intravenous infusions of GMI (25 mg) for 14 days had no statistically significant effect on the pharmacokinetics of MDZ or its metabolite 1-OH MDZ. Our results agree with those previously reported by Zadoyan et al., who showed that EGb761 (a typical G. biloba extract product) had no relevant effect on the activity of the major CYP enzymes in humans using the cocktail–phenotyping approach [17]. In another clinical trial, Gurley and co-workers showed that G. biloba had no significant effects on CYP1A2, CYP2D6, CYP2E1, and CYP3A4 using the probe drugs caffeine, debrisoquin, chlorzoxazone and MDZ, respectively [12]. In contrast to these findings, Smith and co-workers reported that an 18-day course of G. biloba (120 mg daily) resulted in a 53% increase in nifedipine plasma concentrations in healthy subjects, leading these authors to conclude that G. biloba significantly inhibits CYP3A4 [13]. Taking these findings together, we suggest that the inconsistent effects of G. biloba on CYP3A4 in humans can likely be attributed to a number of variables, such as different dosages, duration of treatment, or composition of G. biloba extract. Ginkgo biloba extract is the most widely used herbal medicine in the world [18]; therefore, further study is still needed to clarify the effect of different G. biloba extract products on the activity of different CYPs.
In conclusion, the ginkgolides A, B, and K achieved their respective steady state by 6 days with once-daily dosing of GMI at the dose of 25 mg. The systemic exposure to these ginkgolides, as characterized by AUC0-24h, indicated no accumulation following repeated once-daily dosing for 12 days. The administration of MDZ alone, multiple once-daily intravenous infusions of GMI and the co-administration of GMI with MDZ in healthy Chinese male subjects were well tolerated and had an acceptable safety profile. There were no serious or unexpected AEs. In our study, GMI appeared neither to inhibit nor to induce CYP3A4.
References
Zhao B, Wang Z, Ling Y, Zhou S, Wu S, Dong S, Xie Y, Li L, Sun Y, Wang J, Ji H, Yan Y, Li Y, Guo J, Tian T, Xiao W (2013) Phase III clinical trial of Diterpene Ginkgolides Meglumine Injection for syndrome of stagnant phlegm blocking collaterals in convalescence of atherosclerotic thrombotic cerebral infarction. Chin Tradit Herbal Drugs 44(24):3525–30
Zhao B, Wang Z, Ling Y, Zhou S, Wu S, Dong S, Xie Y, Li L, Sun Y, Wang J, Ji H, Yan Y, Li Y, Guo J, Tian T, Xiao W (2013) Phase III clinical trial of Diterpene Ginkgolides Meglumine Injection for syndrome of stagnant phlegm blocking collaterals in convalescence of atherosclerotic thrombotic cerebral infarction. Chin Tradit Herbal Drugs 44(24):3525–30
Chen C, Zhou J, Chen J, Chang X, Zhang L, Wang Z (2014) Protective effects of ginkgo terpene lactones meglumine injection on focal cerebral ischemia reperfusion injury in rats. Chin J Exp Tradit Med Form 20(17):133–6
Mahadevan S, Park Y (2008) Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci 73(1):R14–19
Chan PC, Xia Q, Fu PP (2007) Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25(3):211–44
Ma S, Liu X, Xun Q, Zhang X (2014) Neuroprotective effect of ginkgolide K against H2O2-induced PC12 cell cytotoxicity by ameliorating mitochondrial dysfunction and oxidative stress introduction. Biol Pharm Bull 37(2):217–25
Zheng B, Xing G, Bi Y, Yan G, Wang J, Cheng Y, Liu Y, Ashraf MA, Xie J (2016) Comparative pharmacokinetics of a proliposome formulation of Ginkgo biloba extract and Ginaton in rats by a sensitive ultra performance liquid chromatography-tandem mass spectrometry method. Saudi J Biol Sci 23(1):54–65
Li J, Li D, Hu J, Bi Y, Xiao W, Wang Z (2015) Simultaneous determination of ginkgolides A, B, C and bilobalide by LC-MS/MS and its application to a pharmacokinetic study in rats. Biomed Chromatogr 29(12):1907–12
Mooiman KD, Maas-Bakker RF, Hendrikx JJ, Bank PC, Rosing H, Beijnen JH, Schellens JH, Meijerman I (2014) The effect of complementary and alternative medicines on CYP3A4-mediated metabolism of three different substrates: 7-benzyloxy-4-trifluoromethyl-coumarin, midazolamand docetaxel. J Pharm Pharmacol l66(6):865–74
Zhou W, Ruigrok TJ (1990) Protective effect of Danshen during myocardial ischemia and reperfusion: an isolated rat heart study. Am J Chin Med 18(1–2):19–24
Zou L, Harkey MR, Henderson GL (2002) Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci 71(13):1579–89
Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY (2002) Cytochrome P450 phenotypic ratios for predicting herb-drug interactions inhumans. Clin Pharmacol Ther 72(3):276–87
Smith M, Lin KM, Zheng YP (2001) An open trial of nifedipine-herb interactions with St. John’s wort, ginseng, and ginkgo biloba. Clin Pharmacol Ther 69:86 (abstract)
Garrett M, Smeraglia J, Lin X, Tan L, Tran J (2005) A pilot study to assess simultaneous administration of oral midazolam (MDZ) and fexofenadine (FEX) for the evaluation of cytochrome (CYP) 3A4 and P-glycoprotein (P-gp) activities. Clin Pharmacol Ther 77:45
Seo KA, Bae SK, Choi YK, Choi CS, Liu KH, Shin JG (2010) Metabolism of 1′- and 4-hydroxymidazolam by glucuronide conjugation is largely mediated by UDP-glucuronosyl transferases 1A4, 2B4, and 2B7. Drug Metab Dispos 38(11):2007–13
Lee JI, Chaves-Cnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF (2002) Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther 72(6):718–28
Zadoyan G, Rokitta D, Klement S, Dienel A, Hoerr R, Gramatté T, Fuhr U (2012) Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol 68(5):553–60
Gaudineau C, Beckerman R, Welbourn S, Auclair K (2004) Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem Biophys Res Commun 318(4):1072–8
Wang S, Ouyang B, Aa J, Geng J, Fei F, Wang P, Wang J, Peng Y, Geng T, Li Y, Huang W, Wang Z, Xiao W, Wang G (2016) Pharmacokinetics and tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K after intravenous infusion of ginkgo diterpene lactones in a rat model. J Pharm Biomed Anal 126:109–16
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Shao, F., Zhang, H., Xie, L. et al. Pharmacokinetics of ginkgolides A, B and K after single and multiple intravenous infusions and their interactions with midazolam in healthy Chinese male subjects. Eur J Clin Pharmacol 73, 537–546 (2017). https://doi.org/10.1007/s00228-017-2197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2197-3