Summary
Introduction
Bayesian forecasting has been shown to improve the accuracy of pharmacokinetic/pharmacodynamic (PK/PD) models by adding measured values to a population model. It could be done in real time for neuromuscular blockers (NMB) using measured values of effect. This study was designed to assess feasibility and benefit of Bayesian forecasting during a rocuronium target-controlled infusion (TCI).
Methods
After internal review board (IRB) approval and informed consent, 21 women scheduled for breast plastic surgery were included. Anesthesia was maintained with propofol, alfentanil, and controlled ventilation through a laryngeal mask. Rocuronium was delivered in TCI with Stanpump software and the Plaud population model. The target effect was 50% blockade until insertion of breast prosthesis; thereafter it was set to 0%. Response to train of four (TOF) at adductor pollicis was recorded using a force transducer. In ten patients, drug delivery was based on the population model. In the others, repeated measures values were entered in the software, and the PK model was adjusted to minimize the error in predicted effect. Model precision was compared between groups using mean prediction error and mean absolute prediction error.
Results
At target 50%, model accuracy was not improved with Bayesian adjustments; conversely, post-infusion errors were significantly decreased. The first two measures had the most influence on the model changes.
Discussion
Below clinical utility, such adjustments may be used to explore cofactors influencing interindividual and intraindividual variability in NMB dose-response relationship. Similar tools may also be developed for drugs in which a quantitative effect is available, such as electroencephalography (EEG) for hypnotics.
Implication
Real-time Bayesian forecasting combining measured values of effect with a population model is suitable to guide NMB-agent delivery using Stanpump software.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For most anesthetic drugs, anesthetists may use mathematical functions to describe the dose–concentration–effect relationship, also called pharmacokinetic–pharmacodynamic modeling (PK/PD). In this relationship, the blood concentration is usually linked to the dose by a two- or three-compartment model, and the pharmacological effect is linked to the effect compartment concentration by a linear or sigmoidal E-max model[19]. These models, available in the literature for all anesthetic drugs administered IV allow predicting the effect induced by a known dose but also choosing the dose to achieve a desired effect or driving an infusion in order to maintain a chosen level of concentration and effect, as in target-controlled infusion (TCI) [4] However, for a particular patient, the observed effects of a drug may differ markedly from the predicted, because the patient differs from the population used to establish the model (by age; cardiac, hepatic, or renal function; drug interactions) [9] or because some factors influence the dose–effect relationship over time. Using the population model to adjust drug delivery may then induce detrimental underdosage, overdosage, or delayed recovery[2,13].
Accuracy of a mathematical model for an individual patient may be improved by introducing a measured value of concentration and adjusting the model parameters to minimize the difference between the value predicted by the model and the measure [10]. This process, called Bayesian forecasting, is proposed in several medical contexts in which a narrow therapeutic window makes the precision of the control critical, as with antibiotics [11], immunosuppressive drugs [16], or in special populations such as children [7]. Regarding anesthetic drugs, Maitre combined an alfentanil population model with measured values of concentrations and showed that this Bayesian algorithm markedly improved the performance of the prediction [13]. Few years later, he used a similar approach to select relevant cofactors of midazolam pharmacokinetics [13, 12]. In both papers, this adjustment, based on drug assays, was not done in real time and could not be used to adjust the delivered doses.
Fortunately, although concentration assays still take hs or days, simple and reliable measures of effect are available for some anesthetic drugs and for muscle relaxants, the effects of which can be quantified by the evoked response to a standard train of four (TOF) stimulations. This quantitative measure of effect has been used to describe vecuronium dose response without a single concentration measure [3] or to adjust delivery using a closed-loop system [15].
A few years after Maitre’s paper, a Bayesian adjustment of muscle relaxants based not on measured concentration but on measured percentage of blockade was implemented in the Stanpump software by Steve Shafer and colleagues (http://opentci.org/lib/exe/fetch.php?media=code:stanpump.zip) for pancuronium, vecuronium, rocuronium, and atracurium. Because measured values of effect were available in real time, Bayesian adjustment was expected to improve the precision of neuromuscular blockade administration (NMBA) delivery and to minimize overdosage and residual blockade at recovery, without requiring blood sampling. It may also be used to fit an individual patient model faster than classical post hoc population analysis [23]. Also, real-time adjustment of the model may allow description of the model’s variability over time and the influence of factors such as temperature, cardiac or regional output, and drug interactions.
As far as we know, no clinical study has tested the Stanpump algorithm for both safety and performance. The purpose of this study was to assess prospectively the influence of the Stanpump Bayesian algorithm on the accuracy of rocuronium TCI.
Methods
After institutional review board approval and patient informed consent, 21 women with an Anesthesiologist Society of America (ASA) physical status grades 1–2 who were scheduled for breast plastic surgery under general anesthesia with moderate muscle relaxation were included. Patients who had a history of adverse events during anesthesia, who had hepatic or renal impairment, or had recently ingested drugs known to interact with NMB were excluded. Patients received premedication orally with hydroxyzine 1 mg/kg 1 h before anesthesia. On patient arrival in the operating room, a catheter was inserted IV and general anesthesia was induced propofol 3 mg/kg and alfentanil 30 μg/kg. After controlling ventilation via facial mask, a size 4 laryngeal mask airway (LMA) was inserted. General anesthesia was maintained with propofol infusion (10 mg/kg/h) and boluses of alfentanil 10 μg/kg IV, as required. The lungs were ventilated mechanically to maintain end-tidal carbon dioxide tension between 30 and 40 mmHg with 50% nitrous oxide in oxygen. No inhalation agent was used.
The ulnar nerve was stimulated through surface electrodes at the wrist using supramaximal 2 Hz TOF stimulation every 12 s. The evoked twitch response of the adductor pollicis (AP) was measured with a force transducer, with results displayed on an oscilloscope (Gould V1000, Gould Instrument System Inc., Valley View, OH, USA) and recorded simultaneously on paper with a Gould ES1000 chart recorder. The effect was defined as the percentage of depression of the first twitch (T1) compared with the control value (T0) before NMBA. After achieving a stable twitch response over a 3-min period, a rocuronium bolus of 0.15 mg.kg-1 was injected, which was expected to induce an NMB effect (T1/T0) of around 50%. This was followed by a rocuronium TCI targeting an effect of 50%. Both the initial bolus and the TCI were driven by Stanpump software connected to a Graseby 3400 syringe pump and using the two-compartment PK/PD model described by Plaud et al. [15]. After 15 min of observation, patients were divided into one of two groups:
-
1.
In the control group (n = 10), TCI was continued, with a target effect of 50% based on the predicted concentration and effect, whatever the measured effect was.
-
2.
In the Bayesian group (n = 11), a measured value of effect (T1/T0) was entered into the Stanpump software every 5 min if the difference between measured and predicted effect exceeded 10%. We selected this threshold both to maintain the level of effect adequate for the surgery (50%) and to minimize fluctuations in drug delivery due to the steep slope of the concentration-effect relationship at that target. After entering a measured value, the software calculated the corresponding effect-site concentration according to the Plaud population PD model. It then corrected the PK model (central compartment volume and k10 volume at steady state and clearances) using the Bayesian algorithm described for alfentanil by Maitre[13] in order to minimize the difference between the initial and corrected concentration. Then, a new value of predicted effect was computed using the corrected model. This value was automatically set as the target (this is a safety feature of Stanpump, considering that the previous drug infusion scheme resulted in an adequate level of effect and should be maintained). After each adjustment, the investigator changed the target to set it back to 50% blockade.
The Bayesian algorithm used by Stanpump is the simple maximum a priori probability (MAP) Bayesian algorithm. The model estimated the parameters that minimized the weighted squared error log of the observations while also minimizing the weighted square error of the log of the predictions. The PK model used in Stanpump is based on volume of the central compartment (V1) and microrate constants k10, k12, k21; however, only V1 and k10 are estimated to observed values, then other microrate constants are corrected using the new value of V1. In both groups, TCI was continued with a target of 50% until insertion of breast prostheses. TCI induced a mild level of relaxation usually requested by some plastic surgeons in our institution.. Thereafter, the target was set to 0%, which stopped the infusion while predicting the time course of recovery. Muscle relaxant was reversed following surgery by neostigmine 30 μg/kg and atropine 15 μg/kg when T1/T0 was still <90%. Postreversal data were neither included in graphs nor in performance calculation.
Data recording and analysis
NMB was recorded every of 5 min from initial bolus to recovery in all patients. The error between measured and predicted values was calculated and plotted for each individual considering three separate periods:
-
initial infusion period with a target effect set to 50% based on the Plaud PK model [13]; [15]
-
final infusion when the PK model was adjusted to measured values in half of the patients
-
post-infusion period until recovery
For each period, the median prediction error (MDPE) was computed for each patient as the median value of (measured effect – predicted effect), and the median of these values was calculated in each group. Similarly, the median absolute prediction error (MDAPE) was computed as the median of the absolute values of (measured – predicted effect). At recovery, time from stopping rocuronium infusion to T1/T0 >70% and to T4/T1 >70% were also recorded. Initial volume and clearance values were calculated automatically by Stanpump as the Plaud parameter × weight and were recorded. Final estimates were retrieved from the Stanpump file for patients who had Bayesian adjustment. Finally, changes induced by the Bayesian algorithm on the rocuronium PK model were analyzed.
Statistics
Statistical analysis used Student’s t test for demographic data and Mann–Whitney U test to compare prediction errors in both groups. Successive parameters of the PK model were compared using analysis of variance (ANOVA) then compared with the initial and final values by t test for repeated values, protected by Bonferroni correction. A p value < 0.05 was considered to indicate statistically significant difference. The sample size was based on two previous comparable closed-loop investigations on methohexital and propofol. [18,21]. However, this study should be considered as a pilot study initially designed to assess the feasibility of Bayesian forecasting in clinical practice.
Results
Control and Bayesian groups were similar for demographics and anesthesia time course, except a moderate difference in age (Table 1). The intensity of paralysis was judged as clinically sufficient by the surgeon for all patients.
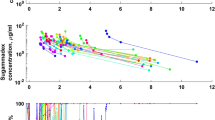
In both groups, measured blockade showed a wide inter- and intraindividual variability around the target (Fig. 1), with both underestimates and overestimates. Consequently, MDPE was low and the MDAPE around 20% (Table 2).
Error between measured and predicted neuromuscular blockade (T1/T0) during infusion (left) and recovery (right) in the control group (upper graph) and in patients who had Bayesian adjustments every 5 min after the 15th min (lower graph). The benefit is more obvious during recovery (right graphs) than during target-controlled infusion (left graphs)
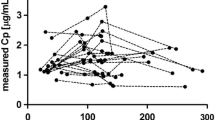
There was no significant difference in MDPE or MDAPE between groups during infusion either during the first 15 min (which was expected, as Bayesian adjustments had not started) or during the adjustment period. Conversely, the predicted postinfusion values were very close to those measured in the Bayesian group (Fig. 1, right graphs), resulting in a significant decrease in both predicted and absolute prediction errors compared with the control group (p < 0.02, Table 2). The delay to recovery from NMB showed a shorter trend in the Bayesian group, but this difference did not reach statistical significance with the number of patients studied. During TCI, 4.8 adjustments per patient were performed (range 1–7), which induced significant changes in all parameters of the rocuronium model (Table 3). In summary, Bayesian adjustments increased V1, V2, and distribution clearance (CL2), and decreased elimination clearance (CL1). For all parameters, the first adjustment was the most significant (Fig. 2), the second and third added a minor change, and after the third the model was not different from the final estimate.
Discussion
This study showed that a Bayesian algorithm as described offline with alfentanil [13] assays could be applied online during a rocuronium TCI, thus improving the recovery control. These results might not be clinically relevant for rocuronium, as it can now easily be reversed by sugammadex[14], but it should be considered as a teaching example of adjustment also suitable for other muscle relaxants insensitive to sugammadex or for other anesthetic drugs in which a measured effect is available in real time, as with the bispectral index of electroencephalography (EEG) for propofol. In these contexts, Bayesian adjustment may improve the control of the pharmacological effect, decrease overdosage due to inter- or intraindividual variability (especially for long-duration delivery), and therefore shorten recovery time. The accuracy of the adjusted model remained low during infusion (median error around 20%) for several reasons. First, the 50% target NMB chosen for the purpose of breast plastic surgery was in the steepest portion of concentration–effect relationship curve, and a minor error in the predicted concentration can induce a major error in the predicted effect. Second, the adjusted period in our study was relatively short (15–30 min), and a better performance may be expected for longer infusion duration. Third, the PK model was adjusted to measured values only every 5 min, and more frequent adjustments may have improved the accuracy of the prediction sooner, as shown in other studies[5] [17] using open- or closed-loop system.
The observed errors (Fig. 1) and the PK model adjustment profile (Fig. 2) suggested that, for our patients, the Plaud PK model [15] underpredicted CL2 and overpredicted CL1, resulting in an initial underpredicted effect observed in both groups and overpredicted late effect and during recovery in the nonadjusted group. This was surprising, as the Plaud study was performed in patients similar to ours, in the same hospital, using IV-administered anesthesia and measuring muscle relaxation using the same force transducer. The dose regimen was higher and shorter in the Plaud study (100 μg/kg-1/min-1 for 5 min), and patients were 15 years younger. Previously published studies found a clearance close to our estimation[6,22] but a lower volume of distribution at steady state, consistent with the Plaud model [6,22,23].
We did not perform rocuronium assays because it was ethically difficult for short-duration plastic surgery and a previous publication demonstrated for vecuronium that including measured plasma concentrations did not change the value of half maximal effective concentration (EC50) or the PK model but only increased the equilibration rate constant (ke0) [8]. It did not change the infusion rate to maintain a chosen level of effect, suggesting that it would not have changed the drug regimen in our TCI.
We could not determine whether these differences were truly related to the PK model or were related to the PD model (EC50 or ke0) and compensated by the Bayesian algorithm by modifying the PK model. Several factors are known to influence rocuronium PK, such as age[23] , acute normovolemic hemodilution [1], cardiac output[20], organ failure requiring intensive care unit admission [20], or drug interactions, such as phenytoin[6], which supports Stanpump's choice of adjusting the PK model. All these studies compared two groups (one control vs. one having the disease) and returned a yes or no conclusion regarding the influence of the parameter. However, they were unable to quantify the influence of the disease on PK changes, whereas the online Bayesian approach can do so. Also, the Bayesian approach takes into account globally all possible factors together (for example, status of an elderly patient with moderately low cardiac output and hemodilution [1]). Nevertheless, the algorithm consistently modified the PK model in all patients. In the stable conditions under which our study was conducted (anesthesia IV without factors known to significantly influence muscle relaxant, PK, or PD), the main purpose of these changes was to reduce the interindividual variability. Figure 2 suggests that it could be done by introducing only one or two measured values into the model, with later measures inducing only minor changes in the PK parameters.
The same Bayesian tool could also be used in different contexts, such as long duration of anesthetic delivery or in the presence of factors known to influence muscle relaxant PK or PD, such as temperature changes, volatile anesthesia, magnesium treatment, or other drug potentiation. In these contexts, a TCI adjusted on measured values at regular intervals would be expected to minimize intraindividual variability, improve control of the effect, and quantify in real time the influence of the covariates on muscle relaxant requirement. The feasibility of this study therefore opens fresh perspectives for these various contexts, both from a clinical and a scientific point of view. The beneficial effect of muscle relaxant Bayesian adjustment will remain limited to a submaximal neuromuscular effect, as at 100% with no twitch response, no adjustment is possible. However, in patients requiring a deep block for surgery, TCI may initially target a submaximal block in order to allow a Bayesian adjustment, then to increase the target and infuse the drug with a minimal interindividual variability.
In conclusion, our study showed that a Bayesian adjustment could be used in real time in clinical conditions to drive muscle relaxant infusion or TCI as far as an evoked response could be measured (submaximal block). For short duration without influencing factors, it reduced interindividual variability at recovery by entering a few measured values at the beginning of infusion. For longer duration or in the presence of factors influencing muscle relaxant pharmacology, it might also help to control and understand intraindividual variability, although this was not the purpose of our study.
References
Dahaba AA, Rehak PH et al (1996) A comparison of mivacurium infusion requirements between young and elderly adult patients. Eur J Anaesthesiol 13(1):43–48
Donati F (2000) Cumulation and flexibility with infusions of neuromuscular blocking drugs. Can J Anaesth 47(10):936–942
Ebeling BJ, Muller W et al (1991) Adaptive feedback-controlled infusion versus repetitive injections of vecuronium in patients during isoflurane anesthesia. J Clin Anesth 3(3):181–185
Egan TD (2003) Target-controlled drug delivery: progress toward an intravenous "vaporizer" and automated anesthetic administration. Anesthesiology 99(5):1214–1219
Eleveld DJ, Kuizenga K et al (2007) A temporary decrease in twitch response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg 104(3):582–584
Fernandez-Candil J, Gambus PL et al (2008) Pharmacokinetic-pharmacodynamic modeling of the influence of chronic phenytoin therapy on the rocuronium bromide response in patients undergoing brain surgery. Eur J Clin Pharmacol 64(8):795–806
Fernandez de Gatta MM, Garcia MJ et al (1996) Bayesian forecasting in paediatric populations. Clin Pharmacokinet 31(5):325–330
Fisher DM, Wright PM (1997) Are plasma concentration values necessary for pharmacodynamic modeling of muscle relaxants? Anesthesiology 86(3):567–575
Hu C, Horstman DJ et al (2005) Variability of target-controlled infusion is less than the variability after bolus injection. Anesthesiology 102(3):639–645
Jelliffe RW, Schumitzky A et al (1993) Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther Drug Monit 15(5):380–393
Lugo G, Castaneda-Hernandez G (1997) Amikacin Bayesian forecasting in critically ill patients with sepsis and cirrhosis. Ther Drug Monit 19(3):271–276
Maitre PO, Buhrer M et al (1991) A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm 19(4):377–384
Maitre PO, Stanski DR (1988) Bayesian forecasting improves the prediction of intraoperative plasma concentrations of alfentanil. Anesthesiology 69(5):652–659
Naguib M (2007) Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg 104(3):575–581
Plaud B, Proost JH et al (1995) Pharmacokinetics and pharmacodynamics of rocuronium at the vocal cords and the adductor pollicis in humans. Clin Pharmacol Ther 58(2):185–191
Rousseau A, Leger F et al (2004) Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit 26(1):23–30
Schumacher GE, Barr JT (1984) Bayesian approaches in pharmacokinetic decision making. Clin Pharm 3(5):525–530
Schwilden H, Schuttler J et al (1987) Closed-loop feedback control of methohexital anesthesia by quantitative EEG analysis in humans. Anesthesiology 67(3):341–347
Sheiner LB, Stanski DR et al (1979) Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther 25(3):358–371
Sparr HJ, Wierda JM et al (1997) Pharmacodynamics and pharmacokinetics of rocuronium in intensive care patients. Br J Anaesth 78(3):267–273
Struys MM, De Smet T et al (2001) Comparison of closed-loop controlled administration of propofol using Bispectral Index as the controlled variable versus "standard practice" controlled administration. Anesthesiology 95(1):6–17
Szenohradszky J, Fisher DM et al (1992) Pharmacokinetics of rocuronium bromide (ORG 9426) in patients with normal renal function or patients undergoing cadaver renal transplantation. Anesthesiology 77(5):899–904
Yang L, Wang HL et al (2012) Population pharmacokinetics of rocuronium delivered by target-controlled infusion in adult patients. Chin Med J (Engl) 123(18):2543–2547
Author information
Authors and Affiliations
Corresponding author
Additional information
This prospective study illustrates the influence of real-time Bayesian pharmacokinetic parameter adjustments on the precision of a rocuronium computer-controlled infusion.
Rights and permissions
About this article
Cite this article
Motamed, C., Devys, JM., Debaene, B. et al. Influence of real-time Bayesian forecasting of pharmacokinetic parameters on the precision of a rocuronium target-controlled infusion. Eur J Clin Pharmacol 68, 1025–1031 (2012). https://doi.org/10.1007/s00228-012-1236-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1236-3