Abstract
Purpose
Blonanserin is a novel potent dopamine D2 and serotonin 5-HT2 antagonist for treating schizophrenia. The aim of this study was to investigate prandial effects on systemic exposure to blonanserin in healthy volunteers, with particular attention paid to the effect of dose timing relative to meal intake.
Methods
Volunteers received a single 2-mg oral dose of blonanserin under the following conditions: fasting, 30 min before eating a standard meal; or 30 min or 2 or 4 h after eating the meal. Plasma concentrations of blonanserin were measured using validated high-performance liquid chromatography coupled with tandem mass spectrometry.
Results
Ratios and 90% confidence intervals of the geometric means compared with the fasting condition indicated that the maximum concentrations of blonanserin (Cmax) significantly increased with dosing 30 min before meal intake, and 30 min and 2 and 4 h after meal intake, yielding by 330%, 239%, 272%, and 138%, respectively. The truncated area under the concentration-time curve (AUClast) also increased by 386%, 201%, 256%, and 155%, respectively. There was no difference in values of the time to reach maximum concentration between the fasting and the four fed states.
Conclusions
Food intake increased the systemic exposure to blonanserin for all time intervals investigated in this study. The marked effect of food on the bioavailability of blonanserin should be taken into account in its dosing schedules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blonanserin [2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta [b]pyridine, AD-5423] is a novel atypical antipsychotic agent structurally unrelated to existing atypical drugs such as risperidone and clozapine [1, 2]. Blonanserin has been shown to have potent dopamine D2 and serotonin 5-HT2 antagonist properties, whereas it is almost devoid of histamine H1 and muscarinic M1 antagonist activities [1]. Clinical trials have demonstrated that blonanserin is effective in treating both positive and negative symptoms of schizophrenia [2, 3]. Blonanserin was well tolerated [3, 4], and its safety profile compared favorably with haloperidol, particularly with respect to prolactin elevation and the frequency of extrapyramidal symptoms [3]. Blonanserin was approved in Japan for treating schizophrenia in January 2008, and the standard maintenance dose is 4–8 mg twice daily after a meal [2]. Previous phase I clinical study data show that blonanserin is rapidly absorbed and reaches maximum plasma concentration at around 1.4–1.7 h [1]. To the best of our knowledge, however, there has been little information regarding the time effects of food intake on the pharmacokinetics of blonanserin.
The aim of this study was to examine the systemic exposure to blonanserin after a single orally administered dose under fasting and fed conditions, with particular attention devoted to the effect of dose timing relative to meal intake on blonanserin absorption.
Methods
Study participants
Ten healthy Japanese volunteers (six men and four women) were studied as per a protocol approved by the Ethics Committee of Hirosaki University School of Medicine after obtaining written informed consent. The mean ± standard deviation (SD) for age was 24.2 ± 2.6 years, and the mean body weight was 54.1 ± 5.0 kg. Individuals with health problems, drug or alcohol abuse, or laboratory abnormalities on screening were excluded.
Study design
This study was conducted as a randomized, open-label, five-sequence, Latin-square, crossover study with a washout interval of 2 weeks. Each participant was randomly assigned to five sequence groups and received a single 2-mg oral dose of blonanserin (Lonasen®, Dainippon Sumitomo Pharma Co., Ltd, Osaka, Japan) with 200 ml tap water at 9 a.m. after an overnight fast (fasting state) or under fed conditions with a high-fat standard Western meal. For fed conditions, participants took the drug 30 min before eating the meal (fed state 1), or 30 min or 2 or 4 h after eating the meal (fed states 2, 3, or 4, respectively). Participants ate the meal at 9:30 a.m., 8:30 a.m., 7:00 a.m. or 5:00 a.m. in fed state 1, 2, 3, or 4, respectively. The high-fat standard Western meal was hamburger (722 kcal). Fat, carbohydrate, and protein contents of the meal were 42.4 g (53.9%), 58.9 g (32.7%), and 20.7 g (14.4%), respectively, based on information provided on the Web sites http://www.mcdonalds.co.jp/. No meal was allowed until 6 h after administration of blonanserin or after breakfast for fed state 1. The consumption of alcohol, tea, cola, or grapefruit juice was forbidden during the test days. A safety and tolerability evaluation was carried out by spontaneous reporting of adverse events and by using the Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale for measuring unwanted effects of psychotropic drugs [5].
Sample collection and blonanserin measurement
Blood samples (10 ml each) to determine plasma levels of blonanserin were taken in heparinized tubes just before and 1, 2, 3, 4, 6, 8, 12, and 24 h after administration of blonanserin. Plasma was separated immediately and stored at −30°C until analysis. Blonanserin (AD-5423) and [2H5]AD-5423 [an internal standard (IS)] were supplied by Dainippon Sumitomo Pharma Co., Ltd (Osaka, Japan). All reagents were purchased from Wako Pure Chemical Industries (Kyoto, Japan) except for 0.1 mol/L phosphate buffer (pH 6.8), which was purchased from Nakalai Tesque, Inc. (Kyoto, Japan). Plasma concentrations of blonanserin were determined by a validated liquid chromatography method with tandem mass spectrometry (LC/MS/MS) developed at JCL Bioassay Co. (Osaka, Japan). Briefly, blonanserin and IS were extracted from human plasma with an OASIS HLB solid-phase extraction cartridge (60 mg/3 cc). After the eluate was evaporated to dryness by centrifugal concentration, the extraction residue was reconstituted with 0.1 vol% formic acid solution/methanol (80:20, v/v). An aliquot of this solution was then injected into the LC/MS/MS system (API4000 system) equipped with a YMC-Pack Pro C18 column. Gradient elution was performed with 0.1 vol% formic acid solution/methanol (80:20, v/v) and 0.1 vol% formic acid solution/methanol (20:80, v/v) as the mobile phases. The measurement was conducted in the multiple reaction monitoring mode with electrospray ionization and positive ion detection. The method was validated for the concentration range 10–1,000 pg/ml. Intra- and interday relative SDs for a concentration of 10 pg/ml were 7.9% and 4.9%, respectively. The limit of quantification was 10 pg/ml.
Statistical analysis
Maximum plasma concentrations (Cmax), time to reach maximum concentration (Tmax), truncated area under the concentration-time curve from time 0 to the time of last quantifiable concentration (AUClast), area under the plasma concentration vs. time curve from time 0 to infinity (total AUC) calculated by linear trapezoidal summation, and elimination half-life (T1/2) were estimated using noncompartmental methods. Statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). The point estimate and 90% confidence interval (CI) [6, 7] for the geometric mean ratio of each fed state relative to the fasting state in log-transformed Cmax and AUClast were calculated by analysis of variance (ANOVA) using a mixed-effects model-fitting sequence, with period and treatment as fixed effects and subject (sequence) as a random effect. A lack of effect of food was concluded if the 90% CI was within the range of 0.80–1.25. Differences in the median of Tmax were compared using the Kruskal–Wallis test. A p value <0.05 was considered statistically significant.
Results
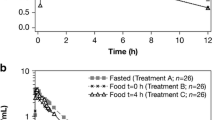
All plasma concentrations of blonanserin were below the limit of quantification before administration. Figure 1 shows the mean concentration-time profile of blonanserin under fasting and fed conditions, and the corresponding pharmacokinetic parameters are summarized in Table 1. Cmax increased by 330%, 239%, 272%, and 138% in fed states 1, 2, 3, and 4, respectively. AUClast increased by 386%, 201%, 256%, and 155% in fed states 1, 2, 3, and 4, respectively. Total AUC also increased by 403%, 208%, 272%, and 162% in fed states 1, 2, 3, and 4, respectively. These results showed remarkable differences in the extent of maximum plasma drug concentrations and drug absorption rates between the fed and fasting conditions (Fig. 1, Table 1). There were no significant differences in the Tmax between the fasting state and fed states (p > 0.05).
Plasma concentration of blonanserin in healthy volunteers after a single 2-mg oral dose under fasting conditions (fasting state), 30 min before meal intake (fed state 1), 30 min after meal intake (fed state 2), 2 h after meal intake (fed state 3), and 4 h after meal intake (fed state 4) (mean ± standard error, n = 10 each)
Mild psychological side effects, e.g., concentration difficulty, latency, and sleepiness were observed from 2 h to 6 h after blonanserin administration in four individuals under both fasting and fed conditions; however, there was no change in the UKU score between fasting and fed conditions (data not shown).
Discussion
To our knowledge, this is the first report of the time effects of food intake on systemic exposure to blonanserin, a novel atypical antipsychotic agent. The findings of this study showed that food has a significant influence on the extent of absorption of blonanserin. Cmax and AUClast were significantly higher under all fed conditions investigated in this study compared with those of the fasting condition, with no significant difference in the Tmax (Fig. 1, Table 1). We thus provide evidence that systemic exposure to blonanserin can be significantly increased if patients receive the drug between 30 min before and 4 h after meal intake. These findings imply that the administration of blonanserin with long periods of fasting might reduce its efficacy, and patients should receive blonanserin between 30 min before and 4 h after eating. Moreover, our findings also suggest that blonanserin can be administered immediately before bedtime, as a 4-h interval between the evening meal and bedtime is normal, thus avoiding psychological side effects, such as latency and sleepiness.
The study is consistent with the literature regarding the significant increases of drug absorption when the drug is administered either concomitantly with or after eating a meal [8–13]. The findings of this study indicated that the increase of systemic exposures to blonanserin continues until at least 4 h after food intake, which is in line with our previous study with quazepam [10]. This is likely due to the amount of time that food, particularly fat, remains in the gastrointestinal tract (3–4 h) [8, 9]. Regarding the mechanisms of the food effects observed in this study, we can speculate that, first, elevation of gastric pH due to food intake may increase solubility of blonanserin because the octanol/water partition coefficient of blonanserin increases with respect to the increase of pH up to pH 8.3 [2]. Second, increased splanchnic blood flow may influence absorption of drugs that are extensively metabolized as a result of changes in the clearance of drugs during the first pass through the hepatoportal system [8, 9, 13]. Third, food consumption enhances biliary activity in response to dietary fat, and the increased activity of bile salts induced by the meal improves stability of the emulsion phase within the gut lumen, which increases drug absorption [8, 9].
The majority of patients with acute schizophrenia reported mild to moderate adverse events, such as insomnia and somnolence, after repeated oral doses (2.5 to 10 mg/day) of blonanserin, and the incidence has a tendency to increase by daily dose amount [3]. For example, the incidences of insomnia were 4.9%, 8.6%, and 9.4% with the repeated daily doses of 2.5, 5, and 10 mg blonanserin, respectively [3]. In this study, mild psychological side effects were observed after a single 2-mg oral dose of blonanserin in four individuals under both the fasting and fed conditions; however, there was no difference in the UKU score between fasting and fed conditions.
There are several limitations of the study. First, food effect on blonanserin with repeated dose was not performed. There has been no information about effect of food intake on repeated-dose pharmacokinetics of blonanserin, although this drug is used for schizophrenia in the repeated-dose setting. Second, we did not use standard Western food but high-fat Western food to examine the effect of food intake on pharmacokinetics, though our previous study showed no difference in food effect on quazepam pharmacokinetics between high- and low-fat meals [14]. Accordingly, further studies with repeated dose and standard Western meal are required.
In conclusion, the study suggests that the effect of food on blonanserin absorption is evident and continues with dosing from 30 min before to 4 h after eating a meal. The marked effect of food on blonanserin bioavailability should be taken into account in its dosing schedules.
References
Heading CE (1998) AD-5423 (Dainippon Pharmaceutical Co Ltd). IDrugs 1(7):813–817
Dainippon Sumitomo Pharma Co., Ltd (2009) Lonasen® (blonanserin). Interview form (in Japanese). http://ds-pharma.jp/medical/product/dbps_data/_material_/product/lonasen/lonasen_tabpow_interv.pdf Accessed 8 March 2010
Garcia E, Robert M et al (2009) The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia: a randomized, double-blind, placebo-controlled, multicentre study. CNS Drugs 23(7):615–625
Kumagai R, Ichimiya Y (2009) Efficacy of blonanserin in combination therapy for treatment-resistant depression. Psychiatry Clin Neurosci 63(4):593–594
Jordan S, Knight J et al (2004) Monitoring adverse drug reactions: scales, profiles, and checklists. Int Nurs Rev 51(4):208–221
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2001) Guidance for industry: Statistical Approaches to Establishing Bioequivalence. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070241.pdf Accessed 8 March 2010
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2002) Guidance for industry: Food-Effect Bioavailability and Fed Bioequivalence Studies. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070241.pdf Accessed 8 March 2010
Singh BN (1999) Effects of food on clinical pharmacokinetics. Clin Pharmacokinet 37(3):213–255
Fleisher D, Li C et al (1999) Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet 36(3):233–254
Yasui-Furukori N, Takahata T et al (2003) Time effects of food intake on the pharmacokinetics and pharmacodynamics of quazepam. Br J Clin Pharmacol 55(4):382–388
Sostek MB, Chen Y et al (2007) Effect of timing of dosing in relation to food intake on the pharmacokinetics of esomeprazole. Br J Clin Pharmacol 64(3):386–390
Karsdal MA, Byrjalsen I et al (2009) Influence of food intake on the bioavailability and efficacy of oral calcitonin. Br J Clin Pharmacol 67(4):413–420
Kang W, Kim K et al (2008) Effect of food on systemic exposure to niflumic acid following postprandial administration of talniflumate. Eur J Clin Pharmacol 64(10):1027–1030
Yasui-Furukori N, Kondo T et al (2002) Effect of dietary fat content in meals on pharmacokinetics of quazepam. J Clin Pharmacol 42(12):1335–1340
Acknowledgments
This study was supported by the Hirosaki Research Institute for Neurosciences. We acknowledge JCL Co., Japan, for technical support for the LC/MS/MS methods.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saruwatari, J., Yasui-Furukori, N., Inoue, Y. et al. Effect of dose timing in relation to food intake on systemic exposure to blonanserin. Eur J Clin Pharmacol 66, 899–902 (2010). https://doi.org/10.1007/s00228-010-0834-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0834-1