Abstract
Objective To explore the influence of hospitalisation on the prescription of drugs in the primary care sector using prescription data of a major statutory health insurance (SHI) organisation, with a special focus on the so-called “Me-Too” drugs – in particular, 3-hydroxy-3-methyl-glutaryl (HMG) CoA reductase inhibitors (statins) and proton pump inhibitors (PPIs).
Methods A comprehensive outpatient drug prescription analysis was conducted on members of a SHI who had been hospitalised during the first 3 months of 2004. The number and costs of all prescriptions of 2426 patients during a 3-month period before admission and after discharge, respectively, were compared using Wilcoxon’s signed rank test. Data are shown in absolute and relative numbers as well as relative risks (RR) and their 95% confidence intervals (CIs).
Results The total number of prescriptions before hospitalisation and after discharge remained nearly the same, while the number of different active substances prescribed per patient decreased by 4%. However, overall costs increased after discharge by 15% due to the higher cost per prescription. Changes in medication affected nearly every patient (98.1%), and 60% had at least five changes. Of the substances prescribed to an individual before admission, 57% were cancelled after discharge, and 55% of all substances prescribed after discharge were novel prescriptions. Significantly more patients received a PPI or statin after hospitalisation (RR for a PPI: 1.27; 95% CI: 1.12 –1.45; RR for a statin: 1.16; 95% CI: 1.02–1.32). The increase in PPI medication was due to a 58% increase in the number of patients receiving pantoprazole, a “Me-Too” drug.
Conclusion Hospitalisation exerts a marked influence on drug therapy in ambulatory care, with a significant increase in the prescription of novel, on-patent drugs instead of less expensive alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The separation of health care in the primary and secondary sector is one factor accounting for a discontinuity in pharmacotherapy [1–3]. One possible consequence of this discontinuity may be confusion and emotional upheaval among both the general practitioners (GPs) and their patients – the GPs because of marked deficiencies in secondary sector care givers in reporting the discharge medication [4] and patients because they are left with a feeling of distrust towards their GP when the drug therapy prescribed by their GP is replaced. Moreover, these changes may have an important economic impact, especially on the primary sector. Drug formularies are usually used in hospitals [5], and the price paid for the listed drugs is negotiated. It appears undisputed that manufacturers, hoping for a ‘knock on-effect’, give hospitals high discounts on newly launched, on-patent drugs designed especially for chronic treatment. In contrast, both the drug prices in the ambulatory sector as well as the prescribing targets of the office-based physicians are strictly regulated. A hospital-initiated therapy with novel drugs therefore represents a burden on the individual prescription limit of the single GP and the purchaser of health care [6].
The latter aspect is of special interest for the so-called “Me-Too” drugs [7]. These drugs offer, in most instances, no considerable advantage over drugs already on the market, but they are usually more expensive [8]. Two examples of “Me-Too” drugs are pantoprazole, a proton pump inhibitor (PPI), prescriptions of which have increased markedly in Germany in recent years, and atorvastatin, a 3-hydroxy-3-methyl-glutaryl (HMG) CoA reductase inhibitor (statin). Until 2004, atorvastatin was the most prescribed drug in many countries, including Germany. At the beginning of 2005 a reference price was introduced in Germany for this drug. The manufacturer of atorvastatin, however, refused to reduce its price to this level, resulting in high out-of-pocket payments by recipients and, consequently, to an exceptional decrease in atorvastatin prescriptions [9] after the end of the study reported here.
Most of the studies carried out to date have been based not on reliable prescription data, but on surveys [e.g. 6] looking at the influence of hospitalisation on ambulatory drug therapy performed on selected populations, such as elderly patients and/or chronic patients [10, 11], or focusing on special therapy [12–14]. Since data collection via surveys is time-consuming, larger studies with sufficient power to detect changes in medication and their costs before and after hospitalisation, especially in single drug classes, are missing. Moreover, research in this field should present a complete and sufficiently clear picture of the medication change that occurs in hospitals so that both hospital doctors and GPs are convinced of an unbiased analysis. Such analyses should take into account that the reason for hospitalisation may have required a drug change for the hospital stay that should also be maintained after discharge.
This study takes advantage of an impressive data set comprising every prescription for all those insured with one major statutory health insurance (SHI) organisation filled before admission to and after discharge from a single hospital. Utilising this information, the first aim of the study was to reliably ascertain the overall influence of hospitalisation on the prescription of all medications in outpatient care as well as the economic impact of the hospital’s influence. The second aim was to evaluate the influence of hospitalisation on the prescription of two drug groups, PPIs and statins, with particular focus on pantoprazole and atorvastatin, which are designated “Me-Too” drugs.
Methods
Design
This follow-up study compared drugs prescribed in the primary care sector before and after hospitalisation. Data were collected both on the global and the patient level, including the prescription costs.
Database
The database for the study comprised prescription data of insured members of one German SHI organisation, the Local Health Care Funds (AOK)-Mecklenburg-Vorpommern. This SHI is by far the largest in Mecklenburg-Vorpommern, insuring about one third of the entire population (1.7 million people) of this state. The study covered all those insured that had at least one hospital stay in a certain hospital during the first 3 months of 2004. The hospital has about 1000 beds and 22 departments and offers standard and specialist care, including gynecology, paediatrics, psychiatry, neurosurgery, among others. This hospital belongs to the second-highest category of in-patient care in Germany [”Krankenhaus der Schwerpunktversorgung“ (hospital of advanced acute care)]. It is the only one in the district. For each patient record, the following data were available:
-
Pseudonymised identification number of the insured person to protect the patient’s personal data but to allow his or her identification for follow-up;
-
Dates of hospital admission and discharge;
-
Central pharmaceutical number, which is an identification number providing every detail of the finished drug product;
-
Number and the date of each prescription.
The age and sex of the patients were not available.
Data analysis
The prescriptions for each patient were analysed for the 3-month period (≤91 days) before and after hospitalisation, respectively. For those patients with more than one hospitalisation within the first 3 months of 2004, drugs prescribed before the first and after the last hospital stay were taken into account. For a detailed analysis of prescriptions of statins and PPIs, we analysed only the last prescription of the respective group before admission and the first after discharge.
Brand name, package size (indicated in Germany as N1 to N3), prices of the drugs and potential savings that could be made by switching to generic drugs and re-imports were taken from the pharmacy price schedule [15]. The indicated costs are gross and include allowances or payments made by the insured patient. Prescription data were linked to the ATC-Code [16] for the active substance.
To compare drug changes on an individual level before admission versus after discharge, we used Wilcoxon’s signed rank test. Relative risks (RR) and corresponding 95% confidence intervals (CIs) of receiving a drug after discharge compared to before admission were calculated. Chi-square statistics were used to test for differences between drug groups.
Results
Patients
A total of 2848 SHI-insured members had at least one stay at the hospital during the first 3 months of 2004. For 254 and 168 patients, respectively, only data before admission or after discharge were available. This may have been due to the cancellation of all medications or receipt of a first-time prescription after discharge; other reasons may have been relocation, change to another SHI or, possibly, death. To refer to the same cohort before and after hospitalisation, we included only those 2426 patients who had prescriptions of drugs before and after hospitalisation within the analysed time frame. Nearly 85% of these people (2044/2426) had one hospital stay and 382 had at least two hospital stays.
Global analysis
The total number of prescriptions before hospitalisation (n = 20,320) and after hospitalisation (19,995) differed only by 1.6%, with the mean number of prescriptions per patient being 8.38 and 8.24, respectively (Table 1). However, the mean cost per patient rose by 15% from 385 € in the 3 months before the hospital stay to 442 € in the following 3-month period (p < 0.001). This corresponds to a 17% increase in the average cost per prescription – from 45.84 € to 53.49 €. (The difference between a 15% increase in the mean cost per patient and the 17% increase in the average cost per prescription results from a slight decrease of about 2% in the total number of prescriptions, as described above.) Consistent with this, there was a sharp 76% decrease in the number of prescriptions after discharge for drugs costing less than 10 €, with drugs in other price brackets being prescribed more frequently. This was most obvious for medications with costing more than 1000 €: these drugs accounted for only 0.2% of all prescriptions, but for 8% of all costs – and for 30% of the increase in costs after hospitalisation.
Table 2 shows the changes in the number of prescriptions and costs according to different ATC-groups. Increases in both prescriptions and costs were most prominent for anti-neoplastic and immunomodulating agents and for drugs affecting blood and blood-forming organs, such as anti-anaemic and anti-thrombotic agents, as well as parenteral nutrition. Anti-anaemic drugs, such as the epoetins erythropoietin and darbepoetin, among others, and the anti-thrombotic substances heparin and clopidogrel showed the highest increase in prescription frequency. Some of the highest increases in anti-neoplastic and immunomodulating agents – temozolmide (+5692 €) or bartezomib (+5500 €), for example – were caused by the treatment of only one additional patient. While the actually number of prescribed medications targeting the musculo-skeletal system decreased, the total cost of such medications increased. A good example of this trend is the use of non-selective non-steroidal anti-inflammatory drugs (NSAIDs): the prescriptions of diclofenac decreased by 43% – from 257 to 157 – while at the same time prescriptions of the expensive coxib rofecoxib, rose by 36% – from 228 to 309 (data not shown).
Analysis on the patient level
The number of different active substances prescribed per patient decreased moderately from 5.62 before hospitalisation to 5.38 after hospitalisation (p < 0.001), possibly attributable to the decrease in the number of patients receiving more than ten different active substances (11.9 vs. 8.5%). However, the overall changes in patient drug treatment regimens were impressive: 60% of patients had at least five changes and 10% more than ten changes, while the medications of only 1.9% remained unchanged (Fig. 1). Of all active substances prescribed to an individual before admission, 57% were cancelled after discharge, whereas 55% of all agents prescribed after a hospital stay were newly started. These changes affected all ATC-groups. Even when our assessment included only the largest package sizes for prescriptions, which are usually an indicator of long-term treatment, more than 50% of all agents were cancelled and newly started after discharge.
Prescription of proton pump inhibitors (PPIs)
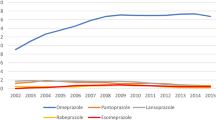
After hospitalisation, 27% more patients were on a PPI therapy regimen (343 vs. 436; RR: 1.27; 95% CI: 1.12 –1.45). Although pantoprazole accounted for 47% of all PPIs before admission (161/343) and was already the predominant PPI, the number of patients receiving this drug after discharge increased significantly by another 58% – from 161 to 255 prescriptions. In fact, pantoprazole currently accounts for 58% (255/436) of all PPIs administered (Table 3). In contrast, no effect was detectable for the only generic PPI available, omeprazole, or the other members of this drug family. The rate of pantoprazole was especially high for those patients on a newly started PPI therapy (64%; 132/207).
Nearly half of all patients with a PPI therapy after hospitalisation had not been on a PPI treatment regimen prior to admission (207/436). This rate was significantly higher in patients with a newly started pantoprazole treatment (52%; 132/255) than in those who commenced a different PPI prescription after discharge (41%; 75/181; χ2 = 4.53, p = 0.033). Figure 2 illustrates the strong increase in pantoprazole prescriptions.
Before admission and after discharge, more than 96% of the prescriptions of omeprazole were generics.
Prescription of HMG CoA reductase inhibitors (statins)
Before admission, simvastatin was the predominant statin prescribed, followed by atorvastatin. After discharge, statins were prescribed to 16% more patients than prior to the admission to hospital. A detailed comparison revealed that the percentage of patients receiving simvastatin increased by 13% (175 to 197), whereas the respective percentage increases for atorvastatin and fluvastatin were 23 and 24% (Table 4). Again, only half of the patients receiving statins after admission had had any prescriptions for a statin prior to admission. A switch from one statin to another occurred only in 3% of the prescriptions. The slightly larger increase in the number of patients prescribed atorvastatin in comparison with simvastatin occurred with the start of a new treatment after discharge rather than with a change from other statins to atorvastatin. In the case of simvastatin, generics with comparable low costs are available, and the prescription rates for such generics were 92% before and 96% after hospitalisation.
Discussion
Despite several efforts [17, 18] to ensure care as a continuum between the primary and secondary sectors, our analyses, based on a large sample of patients and a reliable data set, demonstrate that hospitalisation in Germany is still accompanied by marked changes in pharmacotherapy. More than half of all substances that were prescribed by primary care physicians before admission had been changed after hospitalisation. The costs per prescription significantly increased. Many drug changes in a patient’s medication resulted in a therapy with the so-called “Me-Too” drugs. Based on our results, these changes were justified only in a minority of cases and will ultimately lead to a financial burden for primary care doctors and the ambulatory health sector.
Strengths and limitations of the study
One major advantage of this study is the combination of a broad and reliable data set, covering all prescriptions to every member of one large SHI, with an efficient computer-based analysis. Because the hospital under consideration was the only one in the region and had a broad catchment area, the data should provide a valid and representative overview of the ambulatory prescriptions to patients and changes in their medication due to hospitalisation.
As no diagnoses or other data regarding the hospital stay were available, assumptions about the medical indication and appropriateness of the observed drug changes are possible only to a limited degree. Although not all of the changes can necessarily be attributed to the hospital stay [1, 17, 18], it is known that primary care doctors normally follow hospital recommendations [5], so that the drug change observed in general practice frequently reflects modifications arising from hospitalisation. Indeed, the extent of the changes we observed are roughly in accordance with those reported by others [3, 10].
The follow-up in the present study was limited to a 3-month period after hospitalisation and, consequently, we cannot make any assumptions about the sustainability of the observed changes. However, either these changes continue and confirm our results or they are, again, a matter of modification and even then strengthen our results concerning changes in drug therapy and its consequences for the individual in the context of hospitalisation.
Recent revisions to German social security legislation that came into effect at the beginning of 2004 have, however, complicated the interpretation of the data [19]. Over-the-counter (OTC) drugs were withdrawn from the benefits catalogue of the SHI, and low-priced prescription-only medicines became more expensive, whereas expensive drugs became cheaper. The sharp decrease in the number of drugs costing 10 € or less prescribed after hospitalisation can be ascribed to this legislative change. Therefore, our astonishing results that the number of prescriptions decreased slightly after discharge may actually have reflected the use of OTC-drugs prior to 2004 and masked a real increase in the number of prescriptions initiated by the hospital.
Meaning of the study
Our study provides information on the overall drug change caused by hospitalisation and changes on an individual level. Similar to Beers et al.’s [9] milestone study, the total number of prescriptions before admission and after discharge also remained nearly the same in our study. The sharp increase in medication used for acute or severe illnesses, such as anticancer therapy, anti-anaemic and anti-thrombotic agents, or parenteral nutrition, indicates that the observed changes are largely attributable to the hospitalisation. Therefore, when only the global aspects of medication are assessed, the influence of hospitalisation on the prescribing of drugs in primary care seems to be rather small. Above all, such changes in medication seem more or less justified. Likewise, the total costs of all patients increased only moderately after hospitalisation, with significant increases observed for expensive therapeutics such as anti-neoplastic agents. Within this specialised care, potential savings might be realised only with difficulty without a loss in the quality of care.
Nevertheless, savings are attainable in other therapeutic fields. We were able to demonstrate an effect of hospitalisation on the prescription of economically relevant therapeutic groups, especially in terms of an increase in novel on-patent drugs in comparison to off-patent alternatives. This was most obvious for PPIs. Here, the number of patients receiving pantoprazole after hospitalisation increased sharply. These were mainly patients prescribed a PPI medication for the first time after discharge, whereas the number of those receiving omeprazole, the only generic PPI available, remained unchanged. This discrepancy is a strong indicator for both the preferred use of pantoprazole in the hospital and of a relevant influence of the discharge medication on the prescription of PPIs in primary care. The differences in the effectiveness and/or side effects of the different PPIs are presumed to be marginal [20]. Therefore, the therapeutic advantages of novel drugs in this area over off-patent alternatives are questionable, and the preferred usage of pantoprazole in secondary care seems not to be based simply on scientific evaluation. An increase in the prescription of PPIs after hospitalisation has also been observed by other authors, who believe that these drugs are over-used in hospitalised patients and argue that this results in inappropriate drug consumption in the primary care sector [11, 21, 22].
We found comparable results to those obtained for PPIs for the NSAIDs. The number of patients receiving the on-patent COX-2 specific inhibitor, rofecoxib, increased considerably, while those prescribed the inexpensive non-selective NSAID, diclofenac, decreased. Coxibs had been thought to be safer than the non-selective alternatives in some patients. However, a nationwide drug survey in the USA showed an increasing use of COX-2 inhibitors also among patients who are not at high risk from NSAID-related adverse effects with negative consequences on the overall cost-effectiveness in actual practice [23]. The increase of rofecoxib therapy observed in our study may reflect such a less focused use. Moreover, after the end of our study, a large rofecoxib trial was stopped due an increased risk for confirmed cardiovascular events associated with the use of this medication, with the manufacturer subsequently announcing a worldwide withdrawal of the drug [24].
Cardiovascular diseases are the main reason for admission into hospital, and statins are recommended for the affected patients [25]. Therefore, an overall increase in the number of patients under therapy with statins was expected [12]. Despite better evidence regarding the efficacy of simvastatin [26], for which generics are also available, the increase in such drugs after hospitalisation was slightly higher for the two on-patent statins, atorvastatin and fluvastatin.
We detected a considerable increase in the number of patients treated with atorvastatin, pantoprazole and rofecoxib, as well as increases in prescription costs associated with these drugs. These medications represented three of the four most frequently sold in Germany [14] even though alternative off-patent or generic drugs were also available. Even if two of these no longer belong to the top-selling drugs – atorvastatin because of high co-payments for the insured and rofecoxib because of a worldwide withdrawal due to serious adverse drug events – they are noteworthy examples of the role that hospitals play in establishing drug usage in primary care. Many GPs miss the cooperation of their hospital colleagues towards a more rational prescription policy that also takes costs into consideration – and not just of PPIs and statins, but medications in general. The GPs feel that they are left to convince their patients to change from a brand drug to a less expensive generic version [27].
Apart from economic considerations and the issue of appropriate prescribing, it should be emphasized that changes on an individual level are impressive and affect nearly every single patient. Our study confirmed the results of previous, mostly small surveys. The challenge faced by doctors in both primary and secondary sectors is to harmonise the transition process from hospital to ambulatory care and thereby avoid prescription errors and distrust on the side of the patients [28].
Conclusion
An integrated drug supply at the interface between primary and secondary care is often claimed but, as yet, this has not been realized in many countries including Germany. Increased efforts, especially comprehensive communication structures and shared drug formularies, are deemed necessary to achieve this [29–31]. In the light of the present results, arrangements to a harmonise drug supply should, at least from the view of the health care purchaser, be directed especially to the on-patent drugs frequently used in chronic diseases.
To support such efforts, feasible, timely and efficient evaluations are needed that measure, and inform all participants, about the effects of a programme to harmonise drug therapy at the interface between the primary and secondary care sectors. Such evaluations require both a representative database and a manageable mode of analysis. A large, computerised and detailed analysis as the one presented in this study might be a valuable instrument towards this.
References
Harder S, Fischer P, Krause-Schäfer M, Ostermann K, Helms G, Prinz H, Hahmann M, Baas H (2005) Structure and markers of appropriateness, quality and performance of drug treatment over a 1-year period after hospital discharge in a cohort of elderly patients with cardiovascular diseases from Germany. Eur J Clin Pharmacol 60:797–805
Himmel W, Tabache M, Kochen MM (1996) What happens to long-term medication when general practice patients are referred to hospital? Eur J Clin Pharmacol 50:253–257
Himmel W, Kochen MM, Sorns U, Hummers-Pradier E (2004) Drug changes at the interface between primary and secondary care. Int J Clin Pharmacol Ther 42:103–109
Roth-Isigkeit A, Harder S (2005) Reporting the discharge medication in the discharge letter. An explorative survey of family doctors (in German). Med Klin 100:87–93
Harder S, Thurmann P, Huber T, Rietbrock N (1991) Prescription of drugs not listed in a clinic’s pharmacopoeia: supervision by clinical pharmacologists. Eur J Clin Pharmacol 40:561–564
Feely J, Chan R, McManus J, O’Shea B (1999) The influence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics 16:175–181
Garattini S (1997) Are me-too drugs justified? J Nephrol 10:283–294
Goozner M (2004) The $800 million pill. The truth behind the cost of new drugs (chapter 8: “Me Too”). University of California Press, Berkeley, Calif., London. Available at: http://www.ucpress.edu/books/pages/10083/10083.ch08.html (last accessed 2007/01/15)
Head Associations of Health Insurance Funds (2006) Pharmaceutical Index of the Statutory Health Insurance (GKV-Arzneimittel-Schnellinformation, GAmSi). Essen. Available at: http://www.gamsi.de/dateien/GAmSiBundesbericht_2006_12.pdf (last accessed 2007/05/02)
Beers MH, Dang J, Hasegawa J, Tamai IY (1989) Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc 37:679–683
Cochrane RA, Mandal AR, Ledger-Scott M, Walker R (1992) Changes in drug treatment after discharge from hospital in geriatric patients. BMJ 305:694–696
Parente F, Cucino C, Gallus S, Bargiggia S, Greco S, Pastore L, Bianchi Porro G (2003) Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther 17:1503–1506
Schroder-Bernhardi D, Dietlein G (2002) Lipid-lowering therapy: do hospitals influence the prescribing behavior of general practitioners? Int J Clin Pharmacol Ther 40:317–321
Weltermann B, Martin C, Adl S, Kuching A, Korbonits G, Hopp HW (1997) Prescribing practice for beta blockers at patient discharge to ambulatory care. A health care economic evaluation in a cardiology patient sample with special reference to drug budgeting (in German). Gesundheitswesen 59:258–261
Pharmacy Price Schedule (German), ABDATA Pharma-Daten-Service, Eschborn, Germany
WHO Collaborating Centre for Drug Statistics Methodology (2006). ATC/DDD Index 2006. Available at: http://www.whocc.no/atcddd/indexdatabase (last accessed 2007/01/15)
Adl S, Weltermann BM, Kuching A, Martin C, Korbonits G, Hopp HW (2001) Difficulties in the transfer of drug therapy from inpatient to ambulatory treatment (in German). Gesundheitswesen 63:597–601
Hach I, Maywald U, Meusel D, Konig JU, Kirch W (2005) Continuity of long-term medication use after surgical hospital stay. Eur J Clin Pharmacol 61:433–438
Bundesgesetzblatt (2003) Gesetz zur Modernisierung der gesetzlichen Krankenversicherung. Teil I G 5702, nr. 55. Bonn, Bundesgesetzblatt, pp 2190–2258
McDonagh MS, Carson S (2005) Drug Class Review on Proton Pump Inhibitors. Final Report. Oregon Health & Science University, Portland, Ore.
Jones MI, Greenfield SM, Jowett S, Bradley CP, Seal R (2001) Proton pump inhibitors: a study of GPs’ prescribing. Fam Pract 18:333–338
Mat Saad AZ, Collins N, Lobo MM, O’Connor HJ (2005). Proton pump inhibitors: a survey of prescribing in an Irish general hospital. Int J Clin Pract 59:31–34
Dai C, Stafford RS, Alexander GC (2005) National trends in cyclooxygenase-2 inhibitor use since market release: nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med 165:158–160
EMEA (European Medicines Agency). EMEA statement following withdrawal of Vioxx (rofecoxib) Doc. Ref: EMEA/97949/2004 Available at: http://www.emea.eu.int/htms/hotpress/d9794904.htm (last accessed 2007/ 01/15)
De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S et al. (2003) European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil 10:S1–S10
The Institute of Quality and Economy in Healthcare (2006) Evaluation of the effects of statins (with particular consideration of atorvastatin (in German), ver 1.0. Institute of Quality and Economy in Healthcare, Cologne. Available at: http://www.iqwig.de/download/Nutzenbewertung_Statine.pdf (last accessed 2007/01/15)
Simmenroth-Nayda A, Hummers-Pradier E, Ledig T, Jansen R, Niebling W, Bjerre LM, Kochen MM, Himmel W (2006) Prescription of generic drugs in general practice. Results of a survey of general practitioners (in German). Med Klin 101:705–710
Schnipper J, Kirwin J, Cotugno M, Wahlstrom SA, Brown BA, Tarvin E et al. (2006) Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 166:565–571
Duerden M, Walley T (1999) Prescribing at the interface between primary and secondary care in the UK. Towards joint formularies? Pharmacoeconomics 15:435–443
Hakansson A, Andersson H, Cars H, Melander A (2001) Prescribing, prescription costs and adherence to formulary committee recommendations: long-term differences between physicians in public and private care. Eur J Clin Pharmacol 57:65–70
Wolzt M, Ohrenberger G, Reichardt B (2003) Cost reduction with project based prescription of generic ACE inhibitors. Wien Klin Wochenschr 115:23–28
Acknowledgment
We are indebted to the AOK Mecklenburg-Vorpommern for the permission to perform this study with special thanks to Michael Hewelt for his valuable support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimmsmann, T., Schwabe, U. & Himmel, W. The influence of hospitalisation on drug prescription in primary care – a large-scale follow-up study. Eur J Clin Pharmacol 63, 783–790 (2007). https://doi.org/10.1007/s00228-007-0325-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0325-1