Abstract
Objective
We characterized the kinetics of indomethacin glucuronidation by recombinant UDP-glucuronosyltransferase (UGT) isozymes and human liver microsomes (HLM) and identified the human UGT isozymes involved.
Methods
Indomethacin glucuronidation was investigated using HLM and recombinant human UGT isozymes. Human UGTs involved in indomethacin glucuronidation were assessed in kinetic studies, chemical inhibition studies, and correlation studies.
Results
Among the UGT isozymes investigated, UGT1A1, 1A3, 1A9, and 2B7 showed glucuronidation activity for indomethacin, with UGT1A9 possessing the highest activity, followed by UGT2B7. Glucuronidation of indomethacin by recombinant UGT1A9 and 2B7 showed substrate inhibition kinetics with K m values of 35 and 32 μM, respectively. The glucuronidation of indomethacin was significantly correlated with morphine 3OH-glucuronidation (r = 0.69, p < 0.05) and 3′-azido-3′-deoxythymidine glucuronidation (r = 0.82, p < 0.05), a reaction mainly catalyzed by UGT2B7. Propofol inhibited indomethacin glucuronidation in HLM with an IC50 value of 248 μM, which is between the IC50 value in recombinant UGT1A9 (106 μM) and UGT2B7 (> 400 μM).
Conclusions
These findings suggest that UGT2B7 plays a predominant role in indomethacin glucuronidation in the human liver and that UGT1A9 is partially involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indomethacin, an effective and frequently prescribed non-steroidal anti-inflammatory drug (NSAID), is metabolized by several drug-metabolizing pathways, including those of the cytochrome P450s and UDP-glucuronosyltransferases (UGTs), into one major metabolite, indomethacin glucuronide, and two minor metabolites O-desmethyl indomethacin and N-des-p-chlorobenzoyl-indomethacin [1]. Following an oral dose of 14C-indomethacin, 54–59% and 19–42% of the radioactivity was recovered in the urine and feces, respectively, [2–4]. The urinary recovery of indomethacin glucuronide was 60.7% [5], which means that at least 33% of the dose was excreted as a glucuronide. This implies that the formation of glucuronide constitutes a significant portion of the elimination pathway for indomethacin. Recombinant human UGTs have been used to show that several human UGTs, including UGTs 1A1, 1A3, 1A4, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, and 2B15, are capable of catalyzing the glucuronidation of indomethacin [6]. Earlier findings that indomethacin inhibits UGT1A1 [7] and 1A9 [8] may also support the affinity of indomethacin for the UGT1A isozymes. While the study of Kuehl et al. provided information on indomethacin glucuronidation [6], it focused primarily on the kinetic study in human liver microsomes (HLM) and the respective recombinant UGT isozymes, and not on the determination of the predominant isozymes responsible for glucuronidation. In contrast, the present study provides novel information in that it identifies the primary isozymes involved in the glucuronidation of indomethacin in the human liver. Because the relative abundance of UGTs in the human liver has not been determined, a simple comparison of glucuronidation activity among UGT isozymes is not sufficient to elucidate the main UGTs involved in the liver. Further, because little data are available on specific inhibitors of the respective UGT isozyme [9], the identification of the main UGT isozymes involved in the glucuronidation of drugs is difficult. The glucuronidation of β-estradiol at the 3-OH position, trifluoperazine, and propofol were reported as probe reactions for UGT1A1, 1A4, and 1A9, respectively [10], while morphine and 3′-azido-3′-deoxythymidine (AZT) glucuronidation was reported to be a typical reaction of UGT2B7 [11]. Thus, correlation studies between indomethacin glucuronidation and these probe reactions in HLM, along with inhibition characteristics for indomethacin glucuronidation, should help to identify the UGT isozymes responsible.
The identification of enzymes responsible for drug metabolism is important to our understanding how variations in drug concentrations can lead to differences in drug efficacy and toxicity. It also provides essential information on drug-drug interactions. In the present study, we investigated the main UGT isozymes involved in indomethacin glucuronidation in the human liver using HLM and recombinant human UGT isozymes.
Materials and methods
Chemicals and reagents
Indomethacin (Fig. 1) and AZT were purchased from Sigma (St. Louis, Mo.). Propofol and AZT glucuronide were obtained from Aldrich (Milwaukee, Wis.) and Toronto Research Chemicals (Ontario, Canada), respectively. Pooled HLM and a panel of recombinant human UGT Supersomes (UGT1A1, 1A3, 1A4, 1A6, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17) expressed in baculovirus-infected insect cells were purchased from BD Biosciences (Bedford, Mass.), and individual HLM was purchased from Xenotech LLC (Kansas City, Kan.). Approval of this study using materials from humans was obtained from the Ethics Committee of Astellas Pharma. All other chemicals were of the highest grade.
Identification of indomethacin glucuronide by mass spectrometry analysis
Indomethacin was incubated in reaction mixtures of 0.25 ml of 50 mM Tris-HCl buffer (pH 7.5) containing 8 mM MgCl2, 25 μg/ml alamethicin, 10 mM saccharic acid 1,4-lactone, and HLM for 1 h in the absence or presence of 2 mM UDP-glucuronic acid (UDPGA). The metabolite formed was detected using LCQ Deca (Thermo Electron, San Jose, Calif.), a liquid chromatography with tandem mass spectrometry (LC-MS/MS) system, coupled with a Waters 2690 high-performance liquid chromatography (HPLC) system (Waters, Milford, Mass.). Ionization of the analytes was achieved in positive and negative ion mode with a capillary temperature of 300°C. MS and MS/MS spectra were obtained in the range of m/z 150 to 1000. Collision-induced dissociation was performed using argon as the collision gas, with the collision energy set at 32 eV. The mobile phase consisted of 0.1% formic acid-acetonitrile (6:4, v/v), and the flow rate was 0.5 ml/min. Chromatographic separation was achieved using a reverse phase C18 column, Inertsil Ph (4.6 × 150 mm,;GL Sciences, Tokyo, Japan) at a column temperature of 40°C.
Kinetic study for indomethacin glucuronidation
Indomethacin was incubated in the reaction mixtures described above with recombinant UGT1A9 and 2B7 at a protein concentration of 0.2 mg protein/ml. The indomethacin concentration was set at 4–500 μM for UGT1A9 and 5–500 μM for UGT2B7. After pre-incubation of the reaction mixtures for 5 min at 37°C, the reaction was started by the addition of UDPGA, followed by incubation for the designated times at 37°C. Reaction times were 20 and 40 min for UGT1A9 and UGT2B7, respectively. The kinetic studies were conducted using protein concentrations and incubation times that yielded a linear product formation. The reactions were terminated by the addition of acetonitrile (0.05 ml), 0.01 ml 10% formic acid (v/v), and 0.02 ml of internal standard (niflumic acid, 50 μg/ml), followed by centrifugation at 1870 g for 5 min to obtain the supernatant. Aliquots (0.1 ml) of the supernatant were injected into a HPLC equipped with an ultraviolet (UV) detector (HPLC-UV).
Panel study of UGT isozymes
Indomethacin glucuronidation was measured in reaction mixtures containing recombinant human UGTs 1A1, 1A3, 1A4, 1A6, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17. Assay conditions were as in the kinetic study described above. The indomethacin concentration and protein concentration of the UGT isozymes was 20 μM and 0.2 mg protein/ml, respectively. Reaction time was 30 min for all isozymes.
Indomethacin glucuronidation assay
The peak area of indomethacin glucuronide was analyzed using reverse-phase HPLC (Shimadzu, Kyoto, Japan) as previously described [12]. Briefly, the HPLC system consisted of LC-10AS pumps, a SCL-10A system controller, a SIL-10A autosampler, a SPD-10AV UV detector, and a C-R4AX integrator. Chromatographic separation was achieved using an Inertsil Ph column (4.6 mm × 150 mm, 5 μm) or Capcellpak UG80 column (4.6 mm × 150 mm, 5 μm; Shiseido, Tokyo, Japan). The mobile phase consisted of 0.1% formic acid:acetonitrile (6:4, v/v) and was delivered at a flow rate of 1.0 ml/min. Indomethacin and its glucuronide were detected at a UV wavelength of 300 nm. Because an authentic standard for indomethacin glucuronide was not available, unknown concentrations of indomethacin glucuronide were determined by reference to a standard curve for indomethacin [12]. The peak area of indomethacin glucuronide that was formed was comparable to the reduced peak area of indomethacin during the incubation of indomethacin in the presence of UDPGA. Indomethacin standard curves were linear over the concentration range of 0.2–20 μM with the correlation coefficient values of >0.99. The limit of quantification for the AZT glucuronidation assay was 33 pmol/min per mg protein, and the precision and accuracy were less than 15%.
Correlation study
Indomethacin glucuronidation in microsomes of 16 individual human livers was measured. The indomethacin and HLM concentrations were 40 μM and 0.2 mg protein/ml, respectively. The reaction mixture was incubated for 20 min at 37°C. The correlation between indomethacin glucuronidation and the glucuronidation of β-estradiol at the 3-OH position, propofol, morphine at the 3-OH position, and AZT was determined by applying Pearson’s moment method using Prism ver. 3.02 (Graph Pad Software, San Diego, Calif.). Glucuronidation activities of β-estradiol at the 3-OH position, propofol, and morphine at the 3-OH position in the microsomes from 16 human livers were provided by the manufacturer as typical reference activities for UGT1A1, 1A9, and 2B7, respectively. Along with simple linear regression, stepwise multivariate regression analysis was performed on the activity values for UGT1A9 and 2B7 using Prism ver 3.02. The suitability of the model was judged using Akaike’s information criteria (AIC). A p value of less than 0.05 was considered to be statistically significant.
AZT glucuronidation assay
AZT glucuronidation activity in microsomes from 16 individual human livers was determined in an assay using LC-MS/MS [13]. The reaction mixture of 0.25 ml 50 mM Tris-HCl buffer (pH 7.5) containing 500 μM AZT, HLM (0.2 mg protein/ml), 8 mM MgCl2, 25 μg/ml alamethicin, 10 mM saccharic acid 1,4-lactone, and 2 mM UDPGA was incubated for 20 min at 37°C. The reactions were terminated by the addition of acetonitrile (0.05 ml), internal standard (deuterium isotope of AZT glucuronide; 5 μg/ml, 0.02 ml), and 0.01 ml of 10% formic acid (v/v). The mixtures were then centrifuged at 1870 g for 5 min to obtain the supernatant. Aliquots (0.025 ml) of the supernatant were injected into a LC-MS/MS system. Detection of the glucuronide was carried out using TSQ7000 (Thermo Finnigan, San Jose, California), a LC-MS/MS system coupled with a Surveyor HPLC system (Thermo Finnigan) by monitoring m/z 442→442 in negative ionization mode. Deuterium AZT glucuronide, used as an internal standard, was detected by monitoring m/z 445→445. The capillary voltage was set at 4.5 kV and capillary temperature at 350°C. Collision-induced dissociation was performed using argon as the collision gas, with the collision energy set at 20 eV. The mobile phase consisted of 0.1% formic acid:acetonitrile (7:3, v/v), and the flow rate was 0.5 ml/min. Chromatographic separation was achieved using a reverse-phase C18 column, Capcellpak UG120 (4.6 × 150 mm, 5 μm;Shiseido), at a column temperature of 40°C. Concentrations of AZT glucuronide in reaction mixtures were determined by reference to an authentic standard.
Chemical inhibition
Propofol, a probe substrate for UGT1A9, was tested for its inhibitory effects on indomethacin glucuronidation in HLM, and recombinant UGT1A9 and 2B7. Indomethacin (40 μM) was incubated with HLM, UGT1A9, and UGT2B7 (0.2 mg protein/ml) in the presence and absence of inhibitors. Propofol was dissolved in DMSO, with a final DMSO concentration in the reaction mixtures of 1%. No interference by the inhibitor or its glucuronides was observed in the assay of indomethacin glucuronide.
Data analysis
The kinetics of indomethacin glucuronidation by recombinant UGT1A9 and 2B7 were fitted to the substrate inhibition kinetics (Eq. 1) in order to estimate the Michaelis constant (K m ), substrate inhibition constant (K si ), and maximum velocity (V max). These calculations were carried out using Prism ver. 3.02 [10]
The IC50 value, representing the inhibitor concentration that inhibits 50% of control activity, was estimated using Prism ver. 3.02 [8].
Results
Mass spectrometry analysis of indomethacin glucuronide
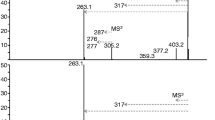
The incubation of indomethacin with HLM resulted in one peak, which was observed in the presence of UDPGA only. The electrospray ionization mass spectra of the peak had ions at m/z 534 and 532 in positive and negative ion mode, respectively (Fig. 2a,b). The product ion spectrum of the peak showed a protonated aglycon ion at m/z 358 in positive ion mode (Fig. 2c), suggesting the loss of a glucuronic acid. These findings indicate that the peak formed by incubating indomethacin in the presence of UDPGA is an indomethacin glucuronide.
Liquid chromatography-mass spectrometry analysis of a reaction mixture following the incubation of indomethacin with UDP-glucuronic acid in pooled human liver microsomes. Positive (a) and negative (b) ion electrospray mass spectrometry (MS) and MS/MS spectra of the peak in the positive ion mode (c) are shown
Indomethacin glucuronidation in UGT isozymes
Of the recombinant UGT isozymes investigated for their ability to glucuronidate indomethacin, UGT1A1, 1A3, 1A9, and 2B7 possessed catalytic activity (Fig. 3). Among these, UGT1A9 showed the highest activity, followed by UGT2B7. The glucuronidation velocity was 70, 90, 701, and 186 pmol equivalents/min per mg protein for UGT1A1, 1A3, 1A9, and 2B7, respectively, while that by recombinant UGT isozymes other than these isozymes was less than 33 pmol equivalents/min per mg protein.
The activity of recombinant human UDP-glucuronosyltransferase (UGT) isozymes in terms of indomethacin glucuronidation. Indomethacin (20 μM) was incubated with recombinant human UGT isozymes (UGT1A1, 1A3, 1A4, 1A6, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17) at the protein concentration of 0.2 mg protein/ml for 30 min. Data represent the mean of duplicate determinations. The limit of quantification for the AZT glucuronidation assay was 33 pmol equivalents/min per mg protein
Kinetic study
The glucuronidation of indomethacin by recombinant UGT1A9 and 2B7 showed substrate inhibition kinetics with K m values of 35 ± 7.1 and 32 ± 17 μM in UGT1A9 and 2B7, respectively (Fig. 4; mean ± computer-calculated standard error (SE)). The respective K si values were 211 ± 55 μM and 40 ± 21 μM, and the respective V max values were 1850 ± 221 and 972 ± 362 pmol equivalents/min per mg protein.
Kinetics for indomethacin glucuronidation by recombinant UDP-glucuronosyltransferase (UGT) 1A9 (a) and UGT2B7 (b). Indomethacin (4 or 5–500 μM) was incubated with protein concentrations of 0.2 mg protein/ml for 20 min in UGT1A9 and 40 min in UGT2B7. Data represent the mean ± standard deviation of triplicate determinations
Correlation studies in HLM
The glucuronidation velocity of indomethacin in microsomes from 16 human livers ranged from 1018 to 2125 pmol equivalents/min per mg protein. As shown in Fig. 5a,b, glucuronidation was significantly correlated with the glucuronidation of morphine at the 3OH-position (r = 0.69, p < 0.05) and AZT (r = 0.82, p < 0.05), which are typical reactions for UGT2B7. In contrast, indomethacin glucuronidation was not significantly correlated with propofol glucuronidation (Fig. 5c) or estradiol 3β-glucuronidation (Fig. 5d), which is a probe reaction for UGT1A9 and 1A1, respectively. Multivariate regression analysis was applied to investigate the possible involvement of UGT1A9 in indomethacin glucuronidation, including the activity probes for 2B7 and 1A9. The coefficient of determination (r 2), corrected by the degree of freedom (r 2*) between indomethacin glucuronidation and the UGT isozymes was improved to 0.59 (from 0.44) by adding UGT1A9 activity as an independent variable. The incorporation of UGT1A9 activity also caused the AIC value to decrease from 183.87 to 180.75.
Correlation analysis between indomethacin glucuronidation and UDP-glucuronosyltransferase isozyme-catalyzed glucuronidation in microsomes from 16 individual human livers. Indomethacin (40 μM) was incubated with microsomes (0.2 mg protein/ml) for 20 min. Data represent the mean ± standard deviation of triplicate determinations. The x-axis represents the activity for morphine 3-OH-glucuronidation (a), 3′-azido-3′-deoxythymidine (AZT) glucuronidation (b), propofol glucuronidation (c), and estradiol 3β-glucuronidation (d); the y-axis represents indomethacin glucuronidation activity
Chemical inhibition
The inhibitory effects of propofol on indomethacin glucuronidation in HLM were evaluated. As shown in Fig. 6a, indomethacin glucuronidation in HLM was inhibited by propofol in a concentration-dependent manner [IC50 value: 248 ± 34 μM (mean ± computer-calculated SE)]. Propofol inhibited indomethacin glucuronidation by UGT1A9 to a similar extent as in HLM (IC50 value: 106 ± 1.7 μM), while 200 μM propofol inhibited UGT2B7 by only 20%, and the IC50 value was >400 μM (Fig. 6b).
Inhibitory effects of propofol on indomethacin glucuronidation. Indomethacin (40 μM) was incubated with human liver microsomes (a), UGT1A9, and UGT2B7 (b) in the absence and presence of propofol. The protein concentration was 0.2 mg protein/ml, and the incubation times were 20 min in HLM and UGT1A9, and 40 min in UGT2B7. Data represent the mean ± standard deviations of triplicate determinations
Discussion
This paper describes the identification of UGT isozymes involved in the glucuronidation of indomethacin in the human liver. The use of recombinant human UGT isozymes to study indomethacin glucuronidation revealed that UGT1A1, 1A3, 1A9, and 2B7 have glucuronidation activity (Fig. 3). This finding is partly consistent with that reported previously [6], in which UGT1A4, 1A7, 1A8, 1A10, 2B4, and 2B15 also showed a slight glucuronidation activity for indomethacin. The reason for the discrepancy between the present and the previous study may be due to differences between the HPLC-UV and LC-MS/MS analysis systems in terms of their sensitivity to the detection of glucuronide.
UGT1A1, 1A3, 1A9, and 2B7, the UGT isozymes exhibiting indomethacin glucuronidation activity in this study, are expressed in the human liver [14], thereby suggesting the possible involvement of these isozymes in indomethacin glucuronidation in the liver. As UGT1A9 and 2B7 demonstrated a relatively high glucuronidation activity for indomethacin, kinetic studies of its glucuronidation were conducted using these isozymes. The kinetic profiles demonstrated substrate inhibition kinetics in both the UGT1A9 and 2B7 systems as being also observed in HLM (Fig. 4). K m values in the UGT1A9 and 2B7 systems were 35 and 32 μM, respectively, and thus similar to those reported previously (68.5 and 17.7 μM, respectively [6]), but relatively lower than those in HLM [12]. This discrepancy may be due to differences in the conditions affecting UGT isozymes in recombinant systems and liver microsomes. It has been reported that K m values of morphine 3OH- and 6OH-glucuronidation and AZT glucuronidation, typical reactions for UGT2B7, in recombinant UGT2B7 are somewhat lower than those in HLM [11]. In order to assess the contribution of UGT1A9 and 2B7 to indomethacin glucuronidation in the liver, we evaluated the correlation between this latter glucuronidation and UGT1A9-specific propofol glucuronidation [15], morphine 3OH-glucuronidation, and AZT glucuronidation, which are mainly catalyzed by UGT2B7 [11], using microsomes prepared from 16 individual human livers. Indomethacin glucuronidation correlated significantly with morphine 3OH-glucuronidation and AZT glucuronidation (Fig. 5a,b), but no significant correlation was observed with estradiol 3β-glucuronidation or propofol glucuronidation (Fig. 5c,d), which are typical reactions for UGT1A1 [16] and 1A9, respectively. These findings suggest that UGT2B7 is the predominant UGT isozyme involved in indomethacin glucuronidation in the human liver. Indomethacin glucuronidation was higher in the recombinant UGT1A9 system than in the UGT2B7 system (Fig. 3). However, the amount of UGT isozymes in the recombinant systems, along with the relative abundance of UGT isozymes in the liver, could not be determined. Thus, comparing glucuronidation activity alone does not provide enough information to determine the isozyme responsible for the glucuronidation of drugs. As stated above, indomethacin glucuronidation correlated significantly with the typical reactions for UGT2B7. In addition, a separate inhibition study has shown that diflunisal’s IC50 value for UGT2B7-catalyzed AZT glucuronidation in the liver (178 μM, [13]) was comparable to that for indomethacin (100–231 μM, [12]). These findings led to the conclusion that UGT2B7 plays a predominant role in the glucuronidation of indomethacin. UGT2B7 has the capacity to conjugate a wide variety of xenobiotics and endogenous steroids. The substrates include androsterone, morphine, codeine, lorazepam, propranolol, and NSAIDs (ibuprofen and ketoprofen) [17]. Further, UGT2B7 is also responsible for the glucuronidation of naproxen [18] and diclofenac [19].
In order to assess the possible contribution of UGT1A9 to indomethacin glucuronidation, an inhibition study was conducted using propofol, a UGT1A9 probe substrate. Figure 6 demonstrates that propofol inhibited indomethacin glucuronidation in HLM with an IC50 of 248 μM, which is similar to that in the UGT1A9 system (106 μM). However, propofol’s inhibition on UGT2B7 was relatively weak, with an IC50 > 400 μM (Fig. 6b). This inhibition study led us to speculate on the contribution of UGT1A9 in indomethacin glucuronidation in the liver. Namely, the inhibition potency of propofol on indomethacin glucuronidation in HLM reflects a previous report that 200–500 μM propofol inhibited 50% of glucuronidation for etodolac, an NSAID that is mainly catalyzed by UGT1A9 [20], suggesting the partial involvement of UGT1A9 in the glucuronidation of indomethacin. In addition, the contribution of UGT1A9 to indomethacin glucuronidation is also supported by multivariate regression analysis in a correlation study. A relatively large intercept in correlation between UGT2B7 activity and indomethacin glucuronidation (Fig. 5a,b) may also support the involvement of isozymes other than UGT2B7. The reason for the low correlation between UGT1A9-catalyzed propofol and indomethacin remains to be clarified, but one possibility might be the relatively low contribution of UGT1A9. UGT1A9 catalyzes the glucuronidation of a wide variety of xenobiotics, including β-adrenoceptor agonists, diuretics, propofol [21], and NSAIDs such as acetaminophen [22], ibuprofen, ketoprofen, diclofenac, naproxen, flurbiprofen, and sulindac [6].
UGT1A1, 1A3, 1A9, and 2B7, which exhibit indomethacin glucuronidation activity, are expressed in the intestine as well as the liver. Given that human intestine microsomes also catalyze the glucuronidation of indomethacin [12], intestinal glucuronidation should also be taken into consideration in assessing the glucuronidation of orally administered drugs such as indomethacin.
In conclusion, these findings indicate that UGT2B7 plays a predominant role in indomethacin glucuronidation in the human liver and that UGT1A9 is also partially involved. The involvement of more than two isozymes in the metabolism of drugs may contribute to the safer and more efficient use of indomethacin due to a reduced drug-drug interaction.
References
Vree TB, van den Biggelaar-Martea M, Verwey-van Wissen CP (1993) Determination of indomethacin, its metabolites and their glucuronides in human plasma and urine by means of direct gradient high-performance liquid chromatographic analysis. Preliminary pharmacokinetics and effect of probenecid. J Chromatogr 616:271–282
Hucker HB, Zacchei AG, Cox SV, Brodie DA, Cantwell NHR (1966) Studies on the absorption, distribution and excretion of indomethacin in various species. J Pharmacol Exp Ther 153:237–249
Duggan DE, Hogans AF, Kwan KC, McMahon FG (1972) The metabolism of indomethacin in man. J Pharmacol Exp Ther 181:563–575
Kwan KC, Breault GO, Umbenhauer ER, McMahon FG, Duggan DE (1976) Kinetics of indomethacin absorption, elimination, and enterohepatic circulation in man. J Pharmacokinet Biop 4:255–280
Van Hecken A, Verbesselt R, Tjandra-Maga TB, De Schepper PJ (1989) Pharmacokinetic interaction between indomethacin and diflunisal. Eur J Clin Pharmacol 36:507–512
Kuehl GE, Lampe JW, Potter JD, Bigler J (2005) Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos 33:1027–1035
Mano Y, Usui T, Kamimura H (2005) In vitro inhibitory effects of non-steroidal antiinflammatory drugs on UDP-glucuronosyltransferase 1A1-catalysed estradiol 3β-glucuronidation in human liver microsomes. Biopharm Drug Dispos 26:35–39
Mano Y, Usui T, Kamimura H (2006) In vitro inhibitory effects of non-steroidal antiinflammatory drugs on UDP-glucuronosyltransferase 1A9-catalysed 4-methylumbelliferone glucuronidation by recombinant human UGT1A9 – potent inhibition by niflumic acid. Biopharm Drug Dispos 27:1–6
Mano Y, Usui T, Kamimura H (2004) Effects of β-estradiol and propofol on the 4-methylumbelliferone glucuronidation by recombinant human UGT isozymes 1A1, 1A8 and 1A9. Biopharm Drug Dispos 25:339–344
Mano Y, Usui T, Kamimura H (2006) Identification of human UDP-glucuronosyltransferase responsible for the glucuronidation of niflumic acid in human liver. Pharm Res 23:1502–1508
Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ (2003) Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos 31:1125–1133
Mano Y, Usui T, Kamimura H (2006) In vitro drug interaction between diflunisal and indomethacin via glucuronidation in humans. Biopharm Drug Dispos 27:267–273
Mano Y, Usui T, Kamimura H (2007) Inhibitory potential of nonsteroidal anti-inflammatory drugs on UDP-glucuronosyltransferase 2B7 in human liver microsomes. Eur J Clin Pharmacol (in press) DOI 10.1007/s00228-006-0241-9
Tukey RH, Strassburg CP (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616
Burchell B, Brierley CH, Rance D (1995) Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci 57:1819–1831
Senafi SB, Clarke DJ, Burchell B (1994) Investigation of the substrate specificity of a cloned expressed human bilirubin UDP-glucuronosyltransferase: UDP-sugar specificity and involvement in steroid and xenobiotic glucuronidation. Biochem J 303:233–240
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Glucuronidation in humans. Clin Pharmacokinet 36:439–452
Bowalgaha K, Elliot DJ, Mackenzie PI, Knights KM, Swedmark S, Miners JO (2005) S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen. Br J Clin Pharmacol 60:423–433
King C, Tang W, Ngui J, Tephly T, Braun M (2001) Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of diclofenac. Toxicol Sci 61:49–53
Tougou K, Gotou H, Ohno Y, Nakamura A (2004) Stereoselective glucuronidation and hydroxylation of etodolac by UGT1A9 and CYP2C9 in man. Xenobiotica 34:449–461
Ebner T, Burchell B (1993) Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab Dispos 21:50–55
Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299:998–1006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mano, Y., Usui, T. & Kamimura, H. Contribution of UDP-glucuronosyltransferases 1A9 and 2B7 to the glucuronidation of indomethacin in the human liver. Eur J Clin Pharmacol 63, 289–296 (2007). https://doi.org/10.1007/s00228-007-0261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0261-0