Abstract
Cephalopod paralarvae were collected in the southeast–south Brazilian outer shelf and continental slope (24°–34°S) from 2009 to 2015 to evaluate their distribution and abundance in relation to water masses during Autumn and Spring seasons. A total of 801 paralarvae were caught with Bongo nets (500 µm mesh) in oblique tows at sample depths of up to 250 m. Fourteen families, 22 genera, and 15 species were identified. The most abundant families were Argonautidae (40.1%), Ommastrephidae (31%), and Enoploteuthidae (23.7%). The highest abundances were recorded on Autumn 2014 (667 ind 1000 m−3) and Argonauta nodosus was the most abundant species in the study area (437 ind 1000 m−3). Ommastrephes sp., Illex argentinus, and Abralia spp. paralarvae were also abundant (124, 131, and 135 ind 1000 m−3, respectively) during Spring 2009, 2010, and 2014, respectively. These species were collected under the influence of Tropical Water (TW), Subtropical Shelf Water (STSW), and South Atlantic Central Water (SACW). Illex argentinus and Ommastrephes sp. paralarvae occupy different niches. The latter was significantly more abundant in the northern area, in TW and TW + SACW water masses, while I. argentinus was more abundant in the outer southern shelf, in the STSW. This is the first study evaluating the composition, distribution, and abundance of cephalopod paralarvae in the study area, while also providing the first record of Bolitaena pygmaea, Egea inermis, Pterygioteuthis sp., and Promachoteuthis sp. paralarvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cephalopods are considered key species in marine ecosystems, acting on the transport of matter and energy between the benthic and pelagic environment, as they perform vertical migrations to eat close to the surface at night (Boyle and Rodhouse 2005). They are key predators of zooplankton in the oceans (Passarella and Hopkins 1991) and important in the diet of elasmobranchs, cetaceans and birds (Clarke 1996; Fonseca and Petry 2007; Petry et al. 2008). As such, they represent an important component in marine trophic webs due to their role as preys and predators (Boyle and Rodhouse 2005; De La Chesnais et al. 2019; Ibáñez et al. 2021).
Cephalopods living at depths greater than 200 m are considered deep water species and includes representatives of almost all the main taxonomic groups of the class, comprising 42 Families of the 50 currently known (Hoving et al. 2014; Urbano and Hendrickx 2018). These families are poorly studied due to the difficulties of sampling this environment and their ability to escape from nets, in addition to the uneven distribution (Vecchione 1987; Collins et al. 2002). Thus, the occurrence of the early phases of the life cycle in the plankton is an indirect, but efficient way of assessing the abundance, occurrence, distribution, life cycle and the population structure of species that inhabit the pelagic and deep-sea environment as adults (Hoving et al. 2014).
Cephalopods are direct developers and lack a true larval phase undergoing metamorphosis. Newly hatched organisms have the same body plan of the adults, but there is varying degree of behavioral, ecological, and morphological differences to the adult conespecifics (Vidal and Shea 2023). In general, newly hatched squids and small-egged octopods are planktonic and called “paralarva” (Young and Harman 1988). Hatchlings of all sepiids, most sepiolids, and large-egged octopods adopt the habitat and behavior of adults immediately after hatching and are called juveniles (Boletzky 1992).
Many studies have been conducted around the world to study the composition and distribution of cephalopod paralarvae on the continental shelf and slope region (Okutani and McGowan 1969; Rocha et al. 1999; Zaragoza et al. 2015a; Lin et al. 2020; Martínez-Soler et al. 2021). Recent studies were conducted in the Gulf of Mexico (Sluis et al. 2021; Guarneros-Narváez et al. 2022) and around oceanic islands in the Southeast Pacific Ocean (Carrasco et al. 2019, 2020), using zooplankton samples to identify cephalopods paralarvae and juveniles and provided new information on their distribution and diversity. In the south western Atlantic Ocean, most studies carried out with cephalopods to date are limited to oceanic islands in the northeast of Brazil and the continental shelf (Haimovici and Perez 1991; Haimovici et al. 2002; Martins and Perez 2006; Vidal et al. 2010; Martins et al. 2014; Araújo and Gasalla 2018, 2019). Currently, the faunal composition and distribution of cephalopod paralarvae on the outer shelf and the continental slope of the southeast–south Brazilian coast is poorly known.
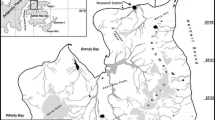
In southern Brazil, the region between Cabo Frio (24°S) and Chuí (34°S) has a dynamic hydrography and is under the influence of the Subtropical Convergent Zone, which occurs due to the confluence between the Brazil Current (BC) and the Malvinas (MC), which is a branch of the Antarctic Circumpolar Current (ACC). The BC transports Tropical Water (TW) toward the south, adjacent to the continental shelf up to 38°S, and the MC flows toward the north and transports cold and relatively fresh subantarctic waters (Piola and Matano 2010). The confluence zone oscillates seasonally between 30 and 46°S (Fig. 1), causing the offshore southward displacement of the BC in the summer, and an alongshore northward penetration of the MC in the winter (Ciotti et al. 1995; Vidal et al. 2013). As a consequence, the region is influenced by a variety of water masses with thermohaline characteristics that vary seasonally (Vidal et al. 2010; Campos et al. 2013). High phytoplankton biomass is associated with the supply of nutrients from the MC, and freshwater discharge from La Plata River and Patos Lagoon Estuary in this region. The biological productivity increases in the vicinity of the Subtropical Convergence, due to the complex hydrography, and the southeastern Brazilian continental shelf is one of the most important fishery grounds of the Brazilian coast (Ciotti et al. 1995; Haimovici et al. 2007).
The upper circulation in the study area is strongly influenced by the BC, which is associated with the South Atlantic Subtropical Gyre (Cirano et al. 2006) (Fig. 1). The BC transports in the upper 200 m, the warm (≥ 18.5 °C), saline (≥ 36) and poor in nutrients TW (Silveira et al. 2000; Cirano et al. 2006). The South Atlantic Central Water (SACW) is found at depths between 200 and 700 m and is characterized by relatively lower temperatures (6–21 °C) and salinities (34.3) (Emery and Meincke 1986; Cirano and Campos 1996). Coastline irregularities around Cabo Frio and prevailing NE winds can cause upwelling of SACW during the Summer, Spring, and early Autumn (Castro et al. 2006).
Over the Southwestern Atlantic continental shelf on the Southern Brazilian coast, there may be a predominance of Subantarctic Shelf Water (SASW) (temperature 5–17 °C and salinity 33.5–34.2) during spring and winter months. This water mass is transported northward by the Patagonian Current together with cold (≥ 10 °C), and low salinity water (≥ 33.5) called the Plata Plume Water (PPW). This water mass is formed by the mixture of fresh waters from the continental discharge of the La Plata River with Subantarctic Shelf Water (Piola et al. 2000, 2008; Möller et al. 2008). Rich in nutrients, the PPW flows north and can be found up to 28°S during the winter and 32°S during summer and influences the primary production in the southern portion of South America (Ciotti et al. 1995). Due to the intense mixing that occurs on the continental shelf of the southern region of South America, the Subtropical Shelf Water (STSW) is a result of the dilution of SACW with water masses of different sources found in the shelf, basically a mix of the PPW with TW and other riverine inputs (Piola et al. 2000; Möller et al. 2008).
The high dynamic between the shelf break and the slope can cause seasonal changes that modify mixing processes, water volumes, and properties between the shelf and the open sea (Matano et al. 2014), which profoundly influences the marine ecosystems in the area off southern Brazil (Acha et al. 2004). Due to seasonal hydrodynamic features of the study area, the distribution and abundance of cephalopod paralarvae in this environment represent valuable information. Thus, the aim of the present study is to determine the faunal composition, distribution, and abundance patterns of cephalopod paralarvae from the outer shelf and continental slope of the southeast–south of Brazil in relation to the main water masses during autumn and spring seasons.
Materials and methods
Sample collection
Zooplankton samples were collected during the Talude Project (Distribution and abundance of cetaceans on south–southeastern Brazilian outer shelf and continental slope ecosystem), in which 10 cruises were carried out on board of the R/V Atlântico Sul of the Federal University of Rio Grande (FURG). Collections were conducted between 2009 and 2015 on Autumn and Spring (Table 1). The sampled area comprised the outer shelf and the continental slope between the 150 and 3000 m isobaths from Chuí (34°S), in the south of Rio Grande do Sul state (RS), to Cabo Frio (24°S), in the Rio de Janeiro state (RJ) over the Brazilian South and Southeast continental shelf, respectively (Fig. 1). Latitudinally, the study area was subdivided into two sectors based on their respective oceanographic characteristics: the northern region (N) (22°S–28°S), which covers the latitudes of the Southern Embayment of Brazil (Palma and Matano 2009) and the southern region (S) (28°S–35°S), which encompasses part of the Southern Subtropical Shelf (Piola et al. 2000; Möller et al. 2008). Temperature and salinity data were obtained through a CTD Seabird (SBE19) in all oceanographic stations from surface to approximately 15 m from the bottom in collections on the shelf, and from surface to 1290 m depth on the slope, always checking bottom depth.
Zooplankton samples were collected using a 60 cm diameter Bongo net fitted with 300 and 500 µm mesh, and equipped with calibrated flow meters (General Oceanics 2030R). A total of 243 tows were carried out, 108 during Spring and 135 during Autumn (Table 1). The hauls were oblique from 250 m deep to the surface in collections on the slope, and up to approximately 5 m from the bottom in collections on the shelf, where the depth was less than 200 m (Muelbert et al. 2018; ICES 2023). After collection, samples were immediately preserved in a 4% formaldehyde solution, neutralized with borax (Steedman 1976). All samples were classified among those that were towed in the outer shelf or in the continental slope for further analysis. Samples were also classified according to the time they were collected, as daytime (0600 h to 1759 h) and nighttime (1800 h to 0559 h).
Identification of cephalopods early-life stages
All cephalopod paralarvae found in the 243 samples from the 500 µm mesh were sorted and identified at the most precise level possible according to Sweeney et al. (1992), Vecchione et al. (2001), Diekmann et al. (2002) and Zaragoza et al. (2015b). Ommastrephidae rhynchoteuthion paralarvae were initially differentiated by the morphology of the proboscis suckers based on Wormuth et al. (1992). Type A rhynchoteuthions have the two lateral suckers enlarged twofold in relation to the remaining six suckers; rhynchoteuthions Type B have the two lateral suckers slightly larger than the other six; and in Type C rhynchoteuthions, all eight suckers have the same size. After paralarvae were differentiated by Type, they were identified to species based on Haimovici et al. (1995) and Wormuth et al. (1992). The only genus with rhynchoteuthions Type A found in the study area is Ommastrephes.
The dorsal mantle length (ML, mm) of each individual was measured with a micrometric scale coupled to the stereomicroscope eyepiece. The mean (± standard deviation—SD) of the ML were calculated for each species.
The relative abundance of paralarvae was estimated as the number of individuals per 1000 m3 (ind 1000 m−3) of filtered seawater. The filtered volume was indirectly estimated by multiplying the area of the net’s mouth by the distance traveled (number of revolutions of the flowmeter). Abundances were categorized in discrete intervals and plotted to show the horizontal distribution of paralarvae.
Water masses
Based on the temperature and salinity data obtained by the CTD (0–1290 m depth), the water masses present in the sampled areas during the Spring (2009, 2010, 2012, 2014), and Autumn (2010, 2011, 2013, 2015) cruises were identified. Due to problems with the CTD, data from Autumn 2014 and Spring 2015 were not available (Table 1).
The water masses were identified and classified seasonally based on thermohaline indices already established for Autumn (April–June) and Spring (October–December) (Emery and Meincke 1986; Piola et al. 2000; Möller et al. 2008; Aseff 2009). Temperature and Salinity (TS) diagrams were made using the Ocean Data View program—ODV (Schlitzer, R., Ocean Data View, http://odv.awi.de, 2023). Horizontal distribution maps of temperature were elaborated for the surface (the average of data obtained between 1 and 10 m) and graphed using the ODV.
Statistical analysis
Paralarvae abundance was log x + 1 transformed prior further analyses. Differences in overall abundance of paralarvae and of the most abundant species in relation to seasonality (Autumn or Spring), latitude (northern or southern area), distance from the coast (outer shelf or continental slope), and time of day (daytime or nighttime) was assessed through the t-test or the Mann–Whitney U test when normality assumptions were not satisfied. The analyses were performed using Past Statistic software (https://palaeo-electronica.org/2001_1/past/issue1_01.htm).
The relationships between paralarval abundances and environmental variables were explored by canonical correspondence analysis (CCA; Ter Braak and Prentice 1988), using the R program (R Development Core Team 2023) and the Vegan package (Oksanen et al. 2012).
The biotic variable was represented by the abundance of paralarvae (ind 1000 m−3) of species with a frequency of occurrence (FO) above 5% (percentage of samples that each species occurred out of the total samples). The environmental variables tested were: bottom depth, mean temperature and mean salinity of the water column (up to the depth reached by the Bongo net), sea surface temperature (SST) and sea surface salinity (SSS) (mean up to 10 m deep, when there was CTD data, or satellite data), and temperature and salinity index (variation in temperature and salinity throughout the water column, calculated by the difference between the values found on the surface and the values found at the maximum depth reached by the Bongo net at each station, Baldoni 2019). To complement the data where it was not possible to collect with CTD, sea surface temperature (SST) data were obtained from MODIS (MODerate Resolution Imaging Spectroradiometers) Aqua satellite from NASA’s Earth observing system, available in https://podaac.jpl.nasa.gov/dataset/MODIS and sea salinity surface (SSS) data were obtained from PO.DAAC Multi-Mission Optimally Interpolated Sea Surface Salinity (OISSS), available in https://podaac.jpl.nasa.gov/dataset/OISSS.
The matrix created with the environmental variables was subjected to a standardization transformation (“standardize” from the Vegan package). This procedure was taken to reduce the effect of different scales between variables. Considering oceanographic features, two models of CCA were created, one for northern region and other for southern region. First, both models were created with all the variables obtained and through an ANOVA test for CCA, it was possible to identify which variables were most significant in the analysis (P < 0.05). Later, the CCA was performed only with significant variables for both regions. Subsequently, an ANOVA test was applied based on 499 permutations to evaluate the significance of the CCA.
Results
Species composition and abundance
A total of 801 cephalopod paralarvae and juveniles were collected in 129 out of 243 samples. Fourteen families were found (Table 2). The most abundant Family was Argonautidae (321 paralarvae, 40.1%) followed by Ommastrephidae (248 paralarvae, 31%) and Enoploteuthidae (187 paralarvae, 23.7%). Species in other families appeared in smaller percentages and were found in lower abundances (Table 2). In the family Cranchiidae, two species and one genus were identified, Egea inermis Joubin 1933, Liguriella podophtalma Issel 1908, and Megalocranchia sp., and one specimen only identified to the family level. In addition, the following species were also collected: Ancistrocheirus lessueurii, Chtenopteryx sicula (Vérany 1851), Onychoteuthis banksii (Leach 1817), Onykia carriboea Lesueur 1821, Bolitaena pygmaea (Verrill 1884), and Scaeurgus unicirrhus (Delle Chiaje [Férussac and d’Orbigny], 1841). Some specimens were identified only to the genus level as Brachioteuthis sp., Chiroteuthis sp., Mastigoteuthis sp., Promachoteuthis sp., and Pterygioteuthis sp. Two specimens of Octopodidae were only identified at the family level. The ML of the specimens ranged from 0.9 to 11.5 mm (Table 2). It was not possible to identify only two paralarvae due to damage during collection. Paralarvae of B. pygmaea, E. inermis, Pterygioteuthis sp., and Promachoteuthis sp. were recorded for the first time in the study area.
Among these species, Promachoteuthis sp. deserves attention as the smallest paralarvae of the genus ever collected, with 3.8 mm ML, 3.0 mm mantle width (Fig. 2). The diagnostic features of paralarva are the large fins that protrudes anteriorly to the anterior part of the mantle (fins width 4 mm; fins length 2 mm). Arms are long (length 3.2 mm) and tentacles very long (7 mm). Arms are more robust at base with two series of large suckers (2 times larger than the tentacles’ suckers) (Fig. 2c), that decrease in size toward the arms tips (Fig. 2a). The brachial formula is II:I:III:IV. Tentacular club is defined, but with small suckers distributed along the tentacle stalk; manus with 8–9 series of suckers and dactyllus with 6 series of suckers. Swimming keels at the tips of the tentacles and in all arms, but more robust and prominent in the arm pair IV (Fig. 2c). Eyes are large, covering most of the ventral area of the head (Fig. 2b).
The horizontal distribution of total paralarval abundances showed the highest values in Spring 2010 and Autumn 2014, and the lowest in Spring 2012 (Fig. 3). In general, abundance was significantly higher on the outer shelf (14 ind 1000 m−3) than on the slope (4 ind 1000 m−3) (Mann–Whitney U test, U = 5795, N1 = 97, N2 = 146, P = 0.01). However, no statistical differences were observed between the abundances in the northern and southern regions (7 and 9 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 7058, N1 = 97, N2 = 146, P = 0.9), between Autumn and Spring collections (8 and 8 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 7102, N1 = 135, N2 = 108, P = 0.6), or during day and night (7 and 11 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 4856.5, N1 = 184, N2 = 59, P = 0.19). The species with the highest abundances were Argonauta nodosus (Lightfoot 1786), Illex argentinus (Castellanos 1960), Ommastrephes sp., and Abralia spp. and their distribution and abundance are described below.
Horizontal distribution of cephalopod paralarvae abundance (ind 1000 m−3) during (a) Spring (2009, 2010, 2012, 2014, and 2015) and (b) Autumn (2010, 2011, 2013, 2014, and 2015) in the southeast–south Brazilian outer shelf and continental slope. The dotted line at 28°S separates the northern region (between Cabo Frio and Santa Marta Grande Cape), and the southern region (until Chuí). Isobaths of 200 and 1000 m are shown
Argonauta nodosus
The horizontal distribution of A. nodosus paralarvae during Spring and Autumn cruises, along with subsurface water temperature (1 up to 10 m depth) are shown in Fig. 4. On the Spring 2012 and 2014, the species did not occur. On Spring 2009 and Autumn 2015 only three individuals were caught, and the abundances recorded were the lowest among all cruises (Table 2). Autumn 2014 showed the greatest abundance of A. nodosus (437 ind 1000 m−3), represented by 238 paralarvae of 1.6 ± 0.5 mm ML (Table 2).
Horizontal distribution of Argonauta nodosus paralarvae abundance (ind 1000 m−3) and subsurface temperature during (a) Spring (2009, 2010, 2012, 2014, and 2015) and (b) Autumn (2010, 2011, 2013, 2014, and 2015) in the southeast–south Brazilian outer shelf and continental slope. The dotted line at 28°S separates the northern region (between Cabo Frio and Santa Marta Grande Cape), and the southern region (until Chuí). Isobaths of 200 and 1000 m are shown
Argonauta nodosus was significantly more abundant in the southern region of the study area than in the northern region (4 and 2 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 5980.5, N1 = 97, N2 = 146, P = 0.016) (Fig. 4). The mean abundance of this species was significantly higher on the outer shelf than on the slope (7 and 0.6 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 5019.5, N1 = 97, N2 = 146, P = 0.000001) and in Autumn than in Spring (5 and 1 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 5801, N1 = 135, N2 = 108, P = 0.002) (Fig. 4). No statistical difference was found between day (3 ind 1000 m−3) and night (4 ind 1000 m−3) (Mann–Whitney U test, U = 4928.5, N1 = 184, N2 = 59, P = 0.42).
Densities peaked in Autumn 2014, in the southern region, at the outer shelf, during the day, and the highest abundance (121 ind 1000 m−3) was registered in a station of 186 m bottom depth. Sixty-four paralarvae were recorded with sizes ranging from 1.0 to 2.7 mm ML. Another station (140 m bottom depth) showed a high abundance of this species (100 ind 1000 m−3) (81 paralarvae, 1.0 to 3.0 mm ML).
Ommastrephidae paralarvae
Illex argentinus was the most common ommastrephid species (138 paralarvae), followed by Ommastrephes sp. (88 paralarvae). The other three species, Hyaloteuthis pelagica (Bosc 1802), Ornithoteuthis antillarum Adam 1957, and Sthenoteuthis pteropus (Steenstrup 1855), appeared in smaller numbers (Table 2).
Illex argentinus showed a significantly higher mean abundance in the southern region than in the northern region (2 and 0.5 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 8090, N1 = 97, N2 = 146, P = 0.0015), and on the outer shelf than on the slope (2 and 0.7 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 5017, N1 = 97, N2 = 146, P = 0.03) (Fig. 5). No statistical difference was found on mean abundance between Spring and Autumn (1.4 and 1.1 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 6634, N1 = 135, N2 = 108, P = 0.6), and day (2 ind 1000 m−3) and night (1 ind 1000 m−3) (Mann–Whitney U test, U = 5029.5, N1 = 184, N2 = 59, P = 0.6). The highest abundance (35 ind 1000 m−3) was registered during Spring 2010, in a station of 150 m bottom depth, in which 19 paralarvae were caught (1.1 to 4.8 mm ML) (Fig. 5). In the same cruise, another station (126 m bottom depth) showed a high density of paralarvae (29 ind 1000 m−3), ranging in size from 1.8 to 3.3 mm ML (n = 6). The two juveniles (8.4 and 10.4 mm ML) were collected in the southern region, during Spring, over the continental slope (1240 m bottom depth).
Horizontal distribution of Illex argentinus paralarvae abundance (ind 1000 m−3) and subsurface temperature during (a) Spring (2009, 2010, 2012, 2014, and 2015) and (b) Autumn (2010, 2011, 2013, 2014, and 2015) in the southeast–south Brazilian outer shelf and continental slope. The dotted line at 28°S separates the northern region (between Cabo Frio and Santa Marta Grande Cape), and the southern region (until Chuí). Isobaths of 200 and 1000 m are shown
Ommastrephes sp. abundance was significantly higher in the northern region (2.0 ind 1000 m−3) than the southern region (0.4 ind 1000 m−3) (Mann–Whitney U test, U = 5586, N1 = 97, N2 = 146, P = 0.0002), and in Spring (2.0 ind 1000 m−3) than in Autumn (0.3 ind 1000 m−3) (Mann–Whitney U test, U = 5850, N1 = 135, N2 = 108, P = 0.001) (Fig. 6). No statistical difference was found on mean abundance on the slope and the outer shelf (1.4 and 0.5 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 6634, N1 = 97, N2 = 146, P = 0.4), and between day and night (0.8 and 2 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 6323, N1 = 184, N2 = 59, P = 0.4). Abundance peaked during Spring 2009 in the northern region at night, on the continental slope (1551 m bottom depth), reaching 61 ind 1000 m−3 (19 paralarvae, 1.2–2.7 mm ML) (Fig. 6). In the same cruise, another station at the northern region (1232 m bottom depth) showed a high density (25 ind 1000 m−3), and eight paralarvae (1.0–2.4 mm ML) were collected. There were no records of the species during Autumn 2011 and Autumn 2013 (Fig. 6, Table 2).
Horizontal distribution of Ommastrephes sp. paralarvae abundance (ind 1000 m−3) and subsurface temperature during (a) Spring (2009, 2010, 2012, 2014, and 2015) and (b) Autumn (2010, 2011, 2013, 2014, and 2015) in the southeast–south Brazilian outer shelf and continental slope. The dotted line at 28°S separates the northern region (between Cabo Frio and Santa Marta Grande Cape), and the southern region (until Chuí). Isobaths of 200 and 1000 m are shown
Enoploteuthidae paralarvae
Among Enoploteuthidae, Abralia reached the largest number of specimens collected (117 paralarvae, 2.4 ± 0.6 mm ML). Forty-one paralarvae were identified as Abralia redfieldi (Voss 1955), while the genus Enoploteuthis and Abraliopsis were found in low abundance (Table 2).
Abralia spp. showed significantly higher mean abundance in the northern region (2 ind 1000 m−3) than in the southern region (1 ind 1000 m3) (Mann–Whitney U test, U = 6119.5, N1 = 97, N2 = 146, P = 0.04), and on the outer shelf than on the slope (3 and 0.3 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 5872, N1 = 97, N2 = 146, P = 0.01) (Fig. 7). A peak of abundance occurred in Spring 2014 (122 ind 1000 m−3), in the southern region, at the outer shelf (138 m bottom depth), during the day and 36 paralarvae were caught (1.2–3.1 mm ML) (Fig. 7). No statistical difference was found on mean abundance between Spring and Autumn (2 and 1 ind 1000 m−3, respectively) (Mann–Whitney U test, U = 6677.5, N1 = 135, N2 = 108, P = 0.6), and day (1.4 ind 1000 m−3) and night (1.5 ind 1000 m−3) (Mann–Whitney U test, U = 4998, N1 = 184, N2 = 59, P = 0.5).
Horizontal distribution of Abralia spp. paralarvae abundance (ind 1000 m−3) and subsurface temperature during (a) Spring (2009, 2010, 2012, 2014, and 2015) and (b) Autumn (2010, 2011, 2013, 2014, and 2015) in the southeast–south Brazilian outer shelf and continental slope. The dotted line at 28°S separates the northern region (between Cabo Frio and Santa Marta Grande Cape), and the southern region (until Chuí). Isobaths of 200 and 1000 m are shown
Canonical correspondence analysis (CCA)
The ANOVA test showed that the CCA was significant for the northern (ANOVA, F = 4.18, P = 0.001) and southern (ANOVA, F = 6.5, P = 0.001) regions. Among the variables tested, the most significant for the CCA analysis were the salinity index, bottom depth and mean temperature for the northern region, and the bottom depth, salinity index, and SST for the southern region (Table 3, Fig. 8). Positive salinity index indicates more saline water on the surface, possibly associated with TW, while negative salinity indices might indicate less saline water on the surface, indicating the presence of shelf waters, possibly PPW in the southern region.
For the northern region, the first three canonical axes generated by the model explained 76% of the accumulated variance in species abundance, while for the southern region it was 78% (Table 3).
The results for the northern region showed that Argonauta nodosus and Illex argentinus were negatively related to temperature and bottom depth, but positively related to salinity index (Fig. 8a). Abralia redfieldi was inversely related to salinity index, depth, and temperature (Fig. 8a), and Abralia spp. was related to temperature, but inversely to salinity index and bottom depth (Fig. 8a). Ommastrephes sp. and Abraliopsis sp. were positively related to the variables salinity index and bottom depth, being found in deeper waters with high surface salinities (Fig. 8a).
For the southern region, Illex argentinus, Abralia redfieldi, Ommastrephes sp. and Abraliopsis sp. were positively related to salinity index and bottom depth (Fig. 8b), with A. redfieldi and Abralia spp. positively related to SST (Fig. 8b), and I. argentinus and A. nodosus inversely related to SST (Fig. 8b). Also, A. nodosus was negatively related to bottom depth and salinity index (Fig. 8b). Therefore, this species was more abundant in southern region, in shelf waters, at shallower bottom depth, with lower salinity index and lower SST.
Water masses and paralarvae occurrence
Six water masses were identified in the study area: Tropical Water (TW), South Atlantic Central Water (SACW), Subtropical Shelf Water (STSW), Plata Plume Water (PPW), Subantarctic Shelf Water (SASW), and Antarctic Intermediate Water (AIW), the temperature and salinity ranges of which are described in the introduction. The TS diagrams from Spring and Autumn cruises show the water masses up to 250 m, which was the maximum depth of collection of the zooplankton net. Thus, only the first five water masses found up to 250 m are mentioned below.
The TS diagrams show that the TW was present between the surface and approximately 200 m depth, both on the slope and on the outer shelf in Autumn and Spring cruises, and in the north and south regions of the study area (Fig. 9a, b). The SACW was found up to 700 m on the slope in both seasons, but was also found in lower depths, as observed in the Spring and Autumn TS diagrams (Fig. 9a, b).
TS Diagram of Spring (a) and Autumn (b) cruises. The water masses found up to 250 m depth are: Plata Plume Water (PPW), Subtropical Shelf Water (STSW), Tropical Water (TW), South Atlantic Central Water (SACW) and Subantarctic Shelf Water (SASW). The gray lines represent the density of seawater (kg m−3)
The SASW, STSW, and PPW water masses were found on the outer shelf of the southern region. The SASW was only found during the Spring cruises, in the southern region, on the outer shelf; additional data are given in Online Resources 1. The STSW was present up to approximately 50 m depth, mainly in the southern region, but also appeared in some stations in the northern region, in both seasons (Fig. 9a, b); (Online Resources 1). The PPW was restricted to the southern region, in both seasons (Online Resources 1). However, on Autumn, this water mass is weaker and appears at few sampling stations (Fig. 9b). The PPW was more frequent in Spring than Autumn (Fig. 9), because during the winter and spring months, this water mass is more intense due to rainfall and winds.
The water masses mixtures in which paralarvae were caught during the cruises and their ML range are shown in Table 4.
Argonauta nodosus paralarvae were more frequently found in stations under the influence of TW. The highest mean abundances occurred in the mixture of TW with other water masses: TW + SACW, TW + STSW, TW + STSW + SACW, PPW, PPW + STSW (Table 4). Illex argentinus paralarvae highest abundances were found associated with STSW and TW + STSW (Table 4). Ommastrephes sp. was caught in greater abundances in TW and in the mixture of TW + SACW. Abralia spp. was found associated with TW and STSW, with specimens being found in several water masses and their mixtures (Table 4).
The other species occurred under the influence of TW, such as Egea inermis and Mastigoteuthis sp., or in mixtures of TW + STSW + SACW as Promachoteuthis sp. and Pterygioteuthis sp., and in the mixture of TW + SACW as Bolitaena pygmaea (Table 4).
Discussion
This is the first study to document cephalopod paralarvae faunal composition and abundance in the area. Argonautidae, Ommastrephidae, and Enoploteuthidae were the most abundant families found in the study area. Argonauta nodosus was the most abundant species followed by Illex argentines, Abralia spp., and Ommastrephes sp.. The faunal composition and distribution of cephalopod paralarvae on the outer shelf off the southeast–south Brazilian coast is poorly known, while on the continental slope there is information only for I. argentinus paralarvae (Haimovici et al. 1995). More than 30 cephalopods species have been recorded in the study region (Haimovici and Perez 1991), some of them identified as paralarvae here, such as A. nodosus, A. redfieldi, Ornithoteuthis antillarum, Hyaloteuthis pelagica and Scaeurgus unicirrhus. New records of paralarvae were found, such as Ancistrocheirus lessueurii, Sthenoteuthis pteropus, Chtenopteryx sicula, Onykia carriboea, Egea inermis, Liguriella podophtalma and Bolitaena pygmaea, as well as paralarvae of the genera Enoploteuthis, Abraliopsis, Mastigoteuthis, Promachoteuthis, Pterygioteuthis, and Megalocranchia.
The study area is extremely dynamic with a mixture of water masses with different properties and from different sources (Piola et al. 2008). During the cruises, however, TW influenced the entire study area, and higher paralarval abundances were recorded in this water mass, as well as in its mixture with SACW and STSW. The SACW is more prominent near the continental slope, but can upwell into subsurface on the outer shelf and even shallower close to the shore. This water mass was found between 100 and 200 m depths close to the shelf break during Autumn and Spring in the present study, being more prevalent on Spring cruises. The SACW coastal upwelling phenomena is well documented in Cabo Frio (24°S) and Santa Marta Grande Cape (28°S) during spring and summer (Gonzalez-Rodriguez et al. 1992; Vidal et al. 2010, 2013; Coelho-Souza et al. 2012; Campos et al. 2013), when prevailing northeast winds cause the transport of surface waters offshore, favoring the intrusion of SACW on the shelf and consequently increasing primary productivity. High paralarvae abundances were observed in sampling stations with mixtures of SACW with TW and STSW. During autumn, upwelling of SACW was reduced due to minimum wind action, leading to increased volume of TW over the shelf and slope, especially in the northern region (Cerda and Castro 2013).
Lower paralarvae abundances were found on the shelf under PPW and STSW influence. These water masses showed few points in the TS diagram in the northern region.
Argonauta nodosus was the most abundant species in the study area, and most paralarvae collected were hatchlings. This suggests the occurrence of spawning areas and nursery grounds in the vicinity. Other two studies report the distribution and abundance of Argonautidae paralarvae, one on the continental shelf of the northern region of our study area (Southeastern Brazilian Bight; 23°–28°40′S) (Araújo and Gasalla 2019, 2022) and the other on the Santa Marta Grande Cape (28°S; southern part of our study area) (Vidal et al. 2010). Both regions are known for upwelling events of SACW during summer and spring months, respectively. Vidal et al. (2010) found small Argonauta nodosus paralarvae (< 3 mm ML) on the outer shelf associated with TW and high abundances of small juvenile males and females in midshelf at stations with high concentrations of Chl a under the influence of SACW. Previous analysis of Argonauta paralarvae, found a weak association with SACW and low temperatures and suggested a cross-shelf transport of paralarvae from open waters to the shelf by cyclonic meanders and eddies (Araújo and Gasalla 2022), due to the intrusion of the TW from the BC over the shelf, above SACW (Campos et al. 2000).
In the present study, A. nodosus paralarvae were found mainly under influence of TW and SACW, and the peak of abundance registered in the mixture of TW + STSW + SACW during Autumn, when an increased volume of TW is found over the area. High abundances were also found associated with PPW, TW + STSW and PPW + STSW in the southernmost area outer shelf. Paralarvae mean abundances were lower only in the core of TW. The high abundances of A. nodosus paralarvae found associated with PPW and STSW in the southernmost area suggest that paralarvae prefers cold and less saline waters than the core of TW. The CCA analysis also indicated a negative relationship between A. nodosus paralarvae with salinity index, greater depths and high surface temperatures in the southern region. Adult females with brooding chamber carrying eggs have been collected in the outer shelf and slope area (Haimovici and Andriguetto 1986). These studies suggest that paralarvae may hatch on the slope under influence of TW and are transported to the shelf where juveniles have been collected (Vidal et al. 2010) due to onshore intrusion of TW promoted by meanders and eddies of the BC (Araújo and Gasalla 2022). Our results, along with previous studies show that A. nodosus is abundant in the southeast–south Brazilian shelf during Autumn, Spring, and Summer months.
The Argentine shortfin squid, Illex argentinus, is the most abundant ommastrephid species in the southwestern–south Atlantic Ocean and occurs from Rio de Janeiro, Brazil to southern Argentina (22°S–55°S), and offshore around the Falkland Islands, the tip of Tierra del Fuego, Staten Island and Burwood Bank (Jereb and Roper 2010). Accordingly, I. argentinus was the most abundant ommastrephid paralarvae in the study area, agreeing with other studies that also found paralarvae and small juveniles in high abundances in the region during winter and spring (Haimovici et al. 1995; Vidal et al. 2010). The abundances recorded with Bongo nets over the shelf (28°–34°S) by Haimovici et al. (1995) ranged from 0.31 to 11.7 ind 1000 m−3, while in the present study (24°–34°S) were higher, from 3–131 ind 1000 m−3. Illex argentinus paralarvae develop in warmer waters and in meanders of the Brazil Current (Vidal 1994; Jereb and Roper 2010). Small paralarvae and juveniles have been found associated with the Subtropical Shelf Front, slope waters, and subtropical waters, while small juveniles were recorded over the shelf in colder waters (Vidal 1994; Haimovici et al. 1995). Indeed, this pattern is in accordance with the results of the present study, where the highest abundances of I. argentinus paralarvae were associated with STSW water mass and in its mixture with TW, while the lowest were found in the TW and PPW. The STSW was more evident in the southern region of the study area and on the outer shelf, where paralarvae were more abundant, while TW and SACW were prevalent in the slope and in the northern region, where paralarvae abundances were low. The influence of PPW on the abundance of I. argentinus paralarvae was restricted to the southern region of the study area, mainly during Spring cruises in 2010 and 2014, when paralarvae were found in mixtures of this water mass with STSW and SACW.
Four subpopulations of I. argentinus are recognized, each one with its own maturation and spawning season. In one of these subpopulations, which is probably associated with the paralarvae found in our study region, adults mature over the outer shelf in autumn and concentrate for spawning over the slope in winter, between 35°S and 39°S. Spawning extends until early spring to the north, between Santa Marta Grande Cape (28°S) and Chuí (34°S) (Haimovici and Perez 1990). In addition, the northward transport of egg masses in subtropical waters from Argentina and Uruguay subpopulations could also explain the high abundances of paralarvae recorded during winter and spring in our study area. Hence, paralarvae found in the study area may originate from adults spawning off the Southern Brazilian shelf, and from winter and spring spawners from Uruguay and Argentina (Haimovici et al. 1995). The presence of small paralarvae (2.4 ± 1.3 mm ML) during the surveys provides further evidence that spawning grounds and nursery areas occurs in our study area, with high abundances recorded in Spring 2010, and in Autumn 2014, mainly in the southern region.
The third most abundant paralarvae was the ommastrephid, Ommastrephes sp. Recent molecular analysis indicated that Ommastrephes bartramii, considered a cosmopolitan species with a discontinuous distribution (Jereb and Roper 2010), includes other cryptic species (i.e., O. brevimanus, O. caroli, and O cylindraceus) with distinct geographical distributions (Fernández-Álvarez et al. 2020). The species distributed in the South Atlantic Ocean would be Ommatrephes cylindraceus; however, morphological characters of both adults and early-life phases and stages are still needed to separate O. bartramii from O. cylindraceus. Thus, the rhynchoteuthion Type A collected in the present study were attributed to Ommastrephes sp.. Ommastrephes species inhabits oceanic waters with a true oceanic distribution, different from ‘offshore’ squids more associated with the continental shelf break, as Illex argentinus (Jereb and Roper 2010; Fernández-Álvarez et al. 2020). Similarly, paralarvae of Ommastrephes sp. and I. argentinus, the two most abundant ommastrephid paralarvae in the present study, occupy different niches. Illex argentinus paralarvae occurred in high abundances in the outer shelf, in the southern area, in the STSW water mass. Instead, Ommastrephes sp. paralarvae was significantly more abundant at the northernmost area, associated with TW and TW + SACW water masses. As already mentioned, TW and SACW are water masses commonly found in the slope region, but were also present on the outer shelf in the present study. Here, no statistical difference was found in Ommastrephes sp. abundance between the outer shelf and slope, but the results suggest that paralarvae are associated with slope waters, and may be transported to the outer shelf through water masses exchange, as they were found in low abundances in shelf waters and their mixtures. Our study area is within the known geographic distribution area of reproduction and spawning of Ommastrephes sp. in the western part of the South Atlantic that occurs in subtropical waters, mainly between 20°S and 35°S, while foraging areas occur in temperate waters (35°S–50°S) during the summer (Jereb and Roper 2010).
Enoploteuthid paralarvae were represented by Abralia spp., A. redfieldi, Abraliopsis sp., and Enoploteuthis sp.. Abralia spp. paralarvae are difficult to identify to the species level, because the three photophores on the eyes that are diagnostic characters of species develops in larger specimens than those collected in the present study (Young et al. 1992). In the Brazilian coast, two species are known to occur, mainly on the slope, A. redfieldi and A. veranyi (Haimovici 1997; Haimovici et al. 2007). Here, only A. redfieldi were identified, and found frequently under TW influence, and in lesser abundance on shelf waters (STSW, PPW) in the southern region of the study area. The same was observed for Abralia spp. of smaller sizes, which were significantly more abundant in the northern area. Both A. redfieldi and Abralia spp. paralarvae were more abundant on the outer shelf, but there were no differences by season and time of day. Due to the small size of the paralarvae collected, we can infer that spawning occurs on the outer shelf of our study area. Our results also suggest that Abralia spp. hatch in the northern region of the study area, under influence of TW, and are transported southward in the BC, developing on the continental shelf before migrating to slope waters.
The amphitretid holopelagic octopod, Bolitaena pygmaea has a wide distribution, occurring in tropical and subtropical waters worldwide, from 100 to 1400 m. To our knowledge, there are no records of the occurrence of B. pygmaea paralarvae in the Southwest Atlantic. Although two paralarvae (2.5 and 2.8 mm ML) were collected at the tropical seamounts and oceanic islands off the northeastern coast of Brazil (1–4°N) (Haimovici et al. 2002). In a study in the Mexican Pacific, with a benthic sampling gear that operated as mid-water trawls during the ascent of the nets, only two specimens of B. pygmaea (5.92 and 10.5 mm ML) were collected at a sampling depth between 1050 and 1600 m (Urbano and Hendrickx 2018). In the northern Gulf of Mexico, B. pygmaea (6.0–64.0 mm ML) were collected from 0 to 1500 m deep, with the highest abundances between 600 to 1000 m and lower from 0 to 200 m deep. The species probably displays an ontogenetic migration to deeper waters, since all individuals larger than 20 mm ML were caught below 600 m deep (Judkins and Vecchione 2020). Our results support these observations, as the two paralarvae recorded in the present study were smaller (3.0 and 5.5 mm ML) and collected at 0–200 m, at bottom depths of 183 and 566 m, although it was suggested that the species is deep meso and bathypelagic non-migrators (Judkins and Vecchione 2020).
Little is known about the abundance and distribution of cranchiid paralarvae off Brazil. Megalocranchia sp. and Liguriella podophtalma paralarvae were recorded in St. Peter and St. Paul Archipelago (0°55′N, 29°20′W) off the northeastern coast of Brazil (Haimovici et al. 2002), while Egea inermis juveniles have been found in the northeastern Brazil (Haimovici et al. 2009). However, these are the first records of these paralarvae in the study region, and the first record of an E. inermis paralarva on the Brazilian coast. Egea inermis and L. podophtalma were collected on the slope, while Megalocranchia spp. was found on the outer shelf. All paralarvae collected were found in warmer and saline waters of TW. Cranchiid paralarvae are epipelagic, occurring from the surface to 200 m day and night, but undergoes an ontogenetic migration to mesopelagic and bathypelagic zones (Voss et al. 1992).
This is the first record of a Pterygioteuthis paralarva in the region. This specimen was collected during Spring 2010 in the southern slope region (800 m bottom depth, 32°18′S, 050°06′W), at night, and the water masses present were TW, STSW, and SACW. Haimovici (1997) report the occurrence of Pterygioteuthis giardi adult off southern Brazil. This species is cosmopolitan, occurring in tropical and subtropical meso and bathypelagic oceanic waters.
The family Promachoteuthidae is poorly known due to extreme rarity and for including truly bathypelagic to abyssopelagic species (Jereb and Roper 2010). Individuals ranging in size from 10.5 to 104 mm ML were captured from 1550 to 3431 m deep. Specimens are known from Japan (Promachoteuthis megaptera), North Atlantic Ocean (Promachoteuthis sloani), South Atlantic Ocean (Promachoteuthis sulcus), eastern and western South Pacific Ocean, western North Atlantic Ocean (Promachoteuthis sp. B), and eastern South Atlantic Ocean (Promachoteuthis sp. D) (Jereb and Roper 2010). Also, a recent study reported the possible occurrence of P. sloani (1000 mm ML) by unobtrusive cameras at a depth of 647 m in the northern Gulf of Mexico (Robinson et al. 2021). The Promachoteuthis sp. paralarva collected during the present study was the smallest specimen of the family ever found (3.8 mm ML) (Voss 1992; Young et al. 2007; Jereb and Roper 2010). This specimen was found at the same collection station as Pterygioteuthis sp. paralarva mentioned above. The morphological characteristics of the paralarva included: large fins (length about 100% ML, width 50% ML) protruding anteriorly to the anterior part of the mantle; arms long (85% ML) with two series of large suckers; tentacles very long (185% ML) with 8–9 rows of small suckers. But, some features of this paralarva differ from the five species currently described: the mantle is not fused to the head, the tentacles are wider than arm pair III, eyes are large, covering most of the ventral area of the head (Jereb and Roper 2010). However, our specimen is smaller and may undergo changes in morphology during growth. The closest described species to our study area is P. sulcus (25 mm ML), collected off Tristan da Cunha (36°49′S, 12°17′W) 3504 km away (Young et al. 2007).
In this first study, we evaluated the paralarval composition and abundance from the shelf and slope region of the southeast–south Brazil over six successive years, filling a gap in the distribution of cephalopods in this region and allowing to deepen knowledge on unreported species from Brazilian waters. Future studies on well-defined strata and at greater depths, and the use of other fixatives that preserve the chromatophore patterns and allow molecular analysis should be encouraged.
Conclusion
Argonauta nodosus was the most abundant species, followed by Illex argentinus, Ommastrephes sp., and Abralia spp. The small size of paralarvae indicates the occurrence of spawning and nursery grounds in the southern area for A. nodosus and I. argentinus, and in northern area for Abralia spp. The TW influenced the entire study area, and high paralarvae abundances were recorded in this water mass, as well as in its mixture with SACW and STSW. Bolitaena pygmaea, Egea inermis, Pterygioteuthis sp., and Promachoteuthis sp. were recorded for the first time in the study area. Promachoteuthis sp. paralarva was the smallest specimen collected so far providing new morphological information for the genera.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J (2004) Marine fronts at the continental shelfs of austral South America physical and ecological processes. J Mar Syst 44:83–105. https://doi.org/10.1016/j.jmarsys.2003.09.005
Adam W (1957) Notes sur les Céphalopodes. XXIII–Quelques espèces des Antilles. Bull Inst R Sci Nat Belg 33(7):1–10
Araújo CC, Gasalla MA (2018) Distribution patterns of loliginid squid paralarvae in relation to the oceanographic features off the South Brazil Bight (22°–25°S). Fishs Oceanogr 27:63–75. https://doi.org/10.1111/fog.12238
Araújo CC, Gasalla MA (2019) Biodiversity of cephalopod early-life stages across the Southeastern Brazilian Bight: spatio-temporal patterns in taxonomic richness. Mar Biodivers 49(5):2429–2443. https://doi.org/10.1007/s12526-019-00980-w
Araújo CC, Gasalla MA (2022) Influence of ocean dynamics on the route of argonauts in the Southeastern Brazil Bight. Prog Oceanogr 209:102906. https://doi.org/10.1016/j.pocean.2022.102906
Aseff CRC (2009) Estudo da variação sazonal na composição físico-química das massas de água da Plataforma continental do Atlântico Sul (PCASO) entre Mar del Plata (Argentina, 38°S) e Itajaí (SC, 26°S). Dissertation, Universidade Federal do Rio Grande
Baldoni LC (2019) Pterotracheoidea (Mollusca, Gastropoda) na plataforma externa e talude sudeste e sul do Brasil. M.Sc. Dissertation, Universidade Federal do Rio Grande
Boletzky SV (1992) Evolutionary aspects of development, life style, and reproductive mode in incirrate octopods (Mollusca, Cephalopoda). Rev Suisse Zool 99(4):755–770
Bosc LAG (1802) Histoire Naturelle des Vers: contenant leur description et leurs moeurs, avec figures dessinées d’après nature. Guilleminet. chez Deterville, Paris, pl 1, 46
Boyle P, Rodhouse P (2005) Cephalopods: ecology and fisheries. Blackwell Science, Oxford
Campos EJD, Velhote D, Silveira ICA (2000) Shelf break upwelling driven by Brazil current cyclonic meanders. Geophys Res Lett 27(6):751–754. https://doi.org/10.1029/1999gl010502
Campos PC, Moller OO, Piola AR, Palma ED (2013) Seasonal variability and coastal upwelling near Cape Santa Marta (Brazil). J Geophys Res Oceans 118:1420–1433. https://doi.org/10.1002/jgrc.20131
Carrasco SA, Meerhoff E, Yannicelli B, Ibáñez CM (2019) First records and descriptions of early life stages of cephalopods from Rapa Nui (Easter Island) and the nearby Apolo seamount. Pac Sci 73(1):161–174. https://doi.org/10.2984/73.1.8
Carrasco SA, Varela AI, Ibáñez CM, Sellanes J, Thiel M (2020) Paralarval and juvenile stages as a proxy of cephalopod diversity in the Juan Fernández and Desventuradas Ecoregion. Southeast Pacific Ocean Bull Mar Sci 96(2):263–280. https://doi.org/10.5343/bms.2019.0055
Castellanos ZJA (1960) Una nueva especie de calamar Argentino, Ommastrephes argentinus sp. nov. (Mollusca, Cephalopoda). Neotropica 6(20):55–58
Castro BM, Miranda LB, Silveira ICAA, Lorenzzetti J (2006) Diagnóstico do conhecimento atual sobre a estrutura e circulação entre o Cabo de São Tomé (RJ) e o Chuí (RS). Programa REVIZEE–Relatório Técnico
Cerda C, Castro BM (2013) Hydrographic climatology of South Brazil Bight shelf waters between São Sebastião (24°S) and Cabo São Tomé (22°S). Cont Shelf Res 89(15):5–14. https://doi.org/10.1016/j.csr.2013.11.003
Ciotti AM, Odebrecht C, Fillmann G, Moller OO Jr (1995) Freshwater outflow and subtropical convergence influence on phytoplankton biomass on the southern Brazilian continental shelf. Cont Shelf Res 15(14):1737–1756. https://doi.org/10.1016/0278-4343(94)00091-z
Cirano M, Campos EJD (1996) Numerical diagnostic of the circulation in the Santos Bightwith COROAS hydrographic data. Rev Bras Oceanogr 44(2):105–121
Cirano M, Mata MM, Campos EJ, Deiró NF (2006) A circulação oceânica de larga-escala na região oeste do Atlântico Sul com base no modelo de circulação global OCCAM. Rev Bras De Geofís 24:209–230. https://doi.org/10.1590/S0102-261X2006000200005
Clarke MR (1996) Cephalopods as prey: cetaceans. Phil Trans R Soc Lond B 351:1053–1065
Coelho-Souza SA, López MS, Guimarães JRD, Coutinho R, Candella RN (2012) Biophysical interactions in the Cabo Frio upwelling system, southeastern Brazil. Braz J Oceanogr 60(3):353–365
Collins MA, Yau C, Boyle PR, Friese D, Piatkowski U (2002) Distribution of cephalopods from plankton surveys around the British Isles. Bull Mar Sci 71(1):239–254
De La Chesnais T, Fulton EA, Tracey SR, Pecl GT (2019) The ecological role of cephalopods and their representation in ecosystem models. Rev Fish Biol Fisheries 29:313–334. https://doi.org/10.1007/s11160-019-09554-2
Diekmann R, Piatkowski U, Schneider M (2002) Early life and juvenile cephalopods around seamounts of the subtropical eastern North Atlantic: illustrations and a key for their identification. Berichteausdem Institut fur Meereskunde an der Christian-Aabrechts-Universitat Kiel No 326
Emery WJ, Meincke J (1986) Global water masses-summary and review. Oceanol Acta 9(4):383–391
Fernández-Álvarez FÁ, Braid HE, Nigmatullin CM, Bolstad KS, Haimovici M, Sánchez P, Sajikumar KK, Ragesh N, Villanueva R (2020) Global biodiversity of the genus Ommastrephes (Ommastrephidae: Cephalopoda): an allopatric cryptic species complex. Zool J Linn Soc 190(2):460–482. https://doi.org/10.1093/zoolinnean/zlaa014
Fonseca VSS, Petry MV (2007) Evidence of food items used by Fulmarus glacialoides (Smith 1840) (Procellariiformes: Procellariidae) in Southern Brazil. Polar Biol 30:317–320. https://doi.org/10.1007/s00300-006-0185-7
Gonzalez-Rodriguez E, Valentin JL, Andre DL, Jacob SA (1992) Upwelling and downwelling at Cabo Frio (Brazil): comparison of biomass and primary production responses. J Plankton Res 14:289–306. https://doi.org/10.1093/plankt/14.2.289
Guarneros-Narváez PV, Rodríguez-Canul R, De Silva-Dávila R et al. (2022) Loliginid paralarvae from the Southeastern Gulf of Mexico: abundance, distribution, and genetic structure. Front Mar Sci 9:941908. https://doi.org/10.3389/fmars.2022.941908
Haimovici M (1997) Cephalopods. In: Seeliger U, Odebrecht C, Castello JP (eds) Subtropical convergence environments: the coast and sea in Southwestern Atlantic. Springer, New York
Haimovici M, Andriguetto JM (1986) Cefalópodes costeiros capturados na pesca de arrasto do litoral sul do Brasil. Arq Biol Tecnol 29(3):473–495
Haimovici M, Perez JAA (1990) Distribución y maturación del calamar argentino, Illex argentinus (Castellanos, 1960) (Cephalopoda: Ommastrephidae), en el sur de Brasil. Sci Mar 54(2):179–185
Haimovici M, Perez JAA (1991) Coastal cephalopod fauna of Southern Brazil. Bull Mar Sci 49(1–2):221–230
Haimovici M, Vidal EAG, Perez JAA (1995) Larvae of Illex argentinus from five surveys on the continental shelf of southern Brazil. ICES Mar Sci Symp 199:414–424
Haimovici M, Piatkowski U, Santos RA (2002) Cephalopod paralarvae around tropical seamounts and oceanic islands off the north-eastern coast of Brazil. Bull Mar Sci 71(1):313–330
Haimovici M, Costa PAS, Santos RA, Martins AS, Olavo G (2007) Composição de espécies, distribuição e abundância de cefalópodes do talude da região central do Brasil. In: Costa PAS, Olavo G, Martins AS (eds) Biodiversidade da fauna marinha profunda na costa central brasileira. Museu Nacional, Rio de Janeiro
Haimovici M, Santos RA, Fischer LG (2009) Class Cephalopoda. In: Rios EC (ed) Compendium of Brazilian sea shells. Evangraf. Rio Grande, RS, p 610–649
Hoving HT, Perez JAA, Bolstad KSR, Braid HE, Evans AB, Fuchs D, Judkins H, Kelly JT, Marian JEAR, Nakajima R, Piatkowski U, Reid A, Vecchione M, Xavier JCC (2014) The study of deep-sea cephalopods. In: Vidal EAG (ed) Advances in marine biology, vol. 67. Academic Press, Oxford
Ibáñez CM, Riera R, Leite T et al (2021) Stomach content analysis in cephalopods: past research, current challenges, and future directions. Rev Fish Biol Fisheries 31:505–522. https://doi.org/10.1007/s11160-021-09653-z
ICES (2023) Working Group on Surveys on Ichthyoplankton in the North Sea and adjacent Seas (WGSINS; outputs from 2022 meeting). ICES Scientific Reports. Report. https://doi.org/10.17895/ices.pub.22146905.v1
Issel R (1908) Diagnosi preliminari di un nuovo genre e di due nuove specie di cefalopodi appartenenti alla fam. Cranchiidae raccolti dalla r. nave “Liguria.” Monit Zool Ital 19:102–104
Jereb P, Roper CFE (2010) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO species catalogue for fishery purposes No 4. Vol 2.
Joubin L (1933) Notes préliminaires sur les Céphalopodes des croisières du DANA (1921–1922), 4e Partie. Ann Inst Océanogr 13(1):1–49
Judkins H, Vecchione M (2020) Vertical Distribution Patterns of Cephalopods in the Northern Gulf of Mexico. Front Mar Sci 7:47. https://doi.org/10.3389/fmars.2020.00047
Leach WE (1817) Synopsis of the orders, families and genera of the class Cephalopoda. The Zoological Miscellany; being descriptions of new or interesting animals 3(30):137–141.
Lesueur CA (1821) Descriptions of several new species of cuttlefish. J Acad Nat Sci Phila 2:86–101
Lightfoot J (1786) A Catalogue of the Portland Museum, lately the property of the Dutchess Dowager of Portland, deceased; which will be sold by auction by Mr. Skinner & C, London
Lin D, Walters A, Bestley S, Zhu G, Chen X, Trebilco R (2020) Distribution of larval and juvenile pelagic squids in the Kerguelen Axis region: Oceanographic influence on size structure and evidence of spawning locations. Deep Sea Res Part II Top Stud Oceanogr 174:104615. https://doi.org/10.1016/j.dsr2.2019.07.003
Martínez-Soler E, Gómez-Gutiérrez J, De Silva-Dávila R, González-Rodríguez E, Aburto-Oropeza O (2021) Cephalopod paralarval species richness, abundance and size structure during the 2014–2017 anomalous warm period in the southern Gulf of California. J Plankton Res 43(2):224–243. https://doi.org/10.1093/plankt/fbab010
Martins RS, Perez JAA (2006) Occurrence of loliginid paralarvae around Santa Catarina Island. Southern Brazil Pan-Am J Aquat Sci 1(1):24–27
Martins RS, Camargo R, Gasalla MA (2014) The São Paulo shelf (SE Brazil) as a nursery ground for Doryteuthis plei (Blainville, 1823) (Cephalopoda, Loliginidae) paralarvae: a Lagrangian particle-tracking individual-based model approach. Hydrobiologia 725:57–68. https://doi.org/10.1007/s10750-013-1519-4
Matano RP, Combes V, Piola AR, Guerrero R, Palma ED, Ted Strub P, James C, Fenco H, Chao Y, Saraceno M (2014) The salinity signature of the cross-shelf exchanges in the Southwestern Atlantic Ocean: numerical simulations. J Geoph Res Oceans 119(11):7949–7968. https://doi.org/10.1002/2014JC010116
Möller OO, Piola AR, Freitas AC, Campos EJD (2008) The effects of river discharge and seasonal winds on the shelf off southeastern South America. Cont Shelf Res 28:1607–1624. https://doi.org/10.1016/j.csr.2008.03.012
Muelbert JH, de Macedo-Soares LCP, Favareto LR, Costa MDP (2018) Ichthyoplankton surveys in the southern Brazilian region from 1970 to 2010. PANGAEA. https://doi.org/10.1594/PANGAEA.884342
NASA Ocean Biology Processing Group (2015) MODIS Aqua Global Level 3 Mapped SST, version 2014.0, NASA Ocean Biology Distributed Active Archive Center. Accessed 10 Mar 2023
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2012) Vegan: Community Ecology Package. R package Version 2.0–4. http://CRAN.Rproject.org/package=vegan
Okutani T, McGowan JA (1969) Systematics, distribution, and abundance of the epiplanktonic squid (Cephalopoda, Decapoda) larvae of the California current, April, 1954-March, 1957. Bulletin of the Scripps Institution of Oceanography, University of California, San Diego
Palma ED, Matano RP (2009) Disentangling the upwelling mechanisms of the South Brazil Bight. Cont Shelf Res 29(11–12):1525–1534
Passarella KC, Hopkins TL (1991) Species composition and food habits of the micronektonic cephalopod assemblage in the eastern Gulf of Mexico. Bull Mar Sci 49(1–2):638–659
Petry MV, Fonseca VSS, Krüger-Garcia L, Piuco RC, Brummelhaus J (2008) Shearwater diet during migration along the coast of Rio Grande do Sul, Brazil. Mar Biol 154:613–621
Piola AR, Matano RP (2010) Brazil and Falklands (Malvinas) Currents. In: Steele JH, Thorpe SA, Turekian KK (eds) Ocean currents. Academic Press, New York
Piola AR, Campos EJD, Möller OO Jr, Charo M, Martinez C (2000) Subtropical Shelf Front off eastern South America. J Geophys Res 105(C3):6565–6578
Piola AR, Romero SI, Zajaczkovski U (2008) Space–time variability of the Plata plume inferred from ocean color. Cont Shelf Res 28(13):1556–1567
Robinson NJ, Johnsen S, Brooks A, Frey L, Judkins H, Vecchione M, Widder E (2021) Studying the swift, smart, and shy: unobtrusive camera-platforms for observing large deep-sea squid. Deep-Sea Res Part I 172:103538. https://doi.org/10.1016/j.dsr.2021.103538
Rocha F, Guerra A, Prego R, Piatkowski U (1999) Cephalopod paralarvae and upwelling conditions off Galician waters (NW Spain). J Plankton Res 21(1):21–33
Silveira ICA, Schmidt ACK, Campos EJD, Godoi SS, Ikeda Y (2000) A corrente do Brasil ao largo da costa leste brasileira. Rev Bras Oceanogr 48:171–183
Sluis MZ, Judkins H, Dance MA, Vecchione M, Cornic M, Sutton T, Rooker JR (2021) Taxonomic composition, abundance and habitat associations of squid paralarvae in the northern Gulf of Mexico. Deep Sea Res Part I Oceanogr Res Pap 174:103572. https://doi.org/10.1016/j.dsr.2021.103572
Steedman HF (1976) Monographs on oceanography methodology no. 4—Zooplankton fixation and preservation. UNESCO Press, Paris
Steenstrup J (1855) Kjaeber af en kolossal Blaeksprutte. Oversigt over Det Kongelige Danske Videnskabernes Selskabs Forhandlinger 1855(5/6):199–200
Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky SV (1992) “Larval” and Juvenile Cephalopods: a manual for their identification. Smithsonian Contrib Zool 513:1–282
Ter Braak CJF, Prentice IC (1988) A theory of gradient analysis. In: Begon M, Fitter AH, Ford ED, Macfadyen A (eds) Advances in ecological research. Academic Press, New York, p 271–317. https://doi.org/10.1016/S0065-2504(08)60183-X
Urbano B, Hendrickx ME (2018) Offshore cephalopods (Mollusca: Cephalopoda) collected off the west coast of Mexico during the TALUD cruises. Molluscan Res 1–16. https://doi.org/10.1080/13235818.2018.1495799.
Vecchione M (1987) Juvenile ecology. In: Boyle PR (ed) Cephalopod life cycles, vol 2. Academic Press, London, p 61–84
Vecchione M, Roper CFE, Sweeney MJ, Lu CC (2001) Distribution, relative abundance and developmental morphology of paralarval cephalopods in the Western North Atlantic Ocean. NOAA Technical Report NMFS 152
Vérany GB (1851) Mollusques méditeranéens [sic] observés, décrits, figurés et chromolitographiés d’après le vivant. 1. Céphalopodes de la Méditerranée. Gênes [Genova], imprimerie des Sourds-Muets. pp i-xvi, 1–132
Verrill AE (1884) Second catalogue of mollusca recently added to the fauna of the New England Coast and the adjacent parts of the Atlantic, consisting mostly of deep sea species, with notes on others previously recorded. Trans Conn Acad Arts Sci 6(1):139–294
Vidal EAG (1994) Relative growth of paralarvae and juveniles of Illex argentinus (Castellanos 1960) in southern Brazil. Antarct Sci 6:275–282
Vidal EAG, Haimovici M, Hackbart VCS (2010) Distribution of paralarvae and small juvenile cephalopods in relation to primary production in an upwelling area off Southern Brazil. ICES J Mar Sci 67:1346–1352. https://doi.org/10.1093/icesjms/fsq080
Vidal EAG, Marian JEAR, Martins RS (2013) Doryteuthis sanpaulensis, São Paulo squid. In: Rosa R, O’Dor RK, Pierce GJ (ed) Advances in squid biology, ecology and fisheries. Part I—Myopsida squids. Nova Biomedical, p 257–30
Vidal EAG, Shea EK (2023) Cephalopod ontogeny and life cycles patterns. Front Mar 10:1162735. https://doi.org/10.3389/fmars.2023.1162735
Voss GL (1955) The Cephalopoda obtained by the Harvard-Havana Expedition off the coast of Cuba in 1938–39. Bull Mar Sci Gulf Caribb 5(2):81–115
Voss NA (1992) Family Promachoteuthidae Naef, 1912. In: Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky Sv (eds) “Larval” and juvenile cephalopods: a manual for their identification. Smithsonian Contributions to Zoology, vol 513, pp 183–185
Voss NA, Stephen SJ, Dong Zh (1992) Family Cranchiidae Prosch, 1849. In: Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky SV (eds) “Larval” and juvenile cephalopods: a manual for their identification. Smithsonian contributions to zoology, vol 513. p 187–210
Wormuth JH, O’Dor RK, Balch N, Dunning MC, Forch EC, Harman RF, Rowell TW (1992) Family Ommastrephidae Steenstrup, 1857. In: Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky Sv (eds) “Larval” and juvenile cephalopods: a manual for their identification. Smithsonian contributions to zoology, vol 513. p 105–119
Young RE, Harman RF (1988) “Larva”, “Paralarva” and “Subadult” in Cephalopod terminology. Malacologia 29(1):201–207
Young RE, Mangold KM, Vecchione M (1992) The Enoploteuthid group of families. In: Sweeney MJ, Roper CFE, Mangold KM, Clarke MR, Boletzky SV (eds) “Larval” and juvenile cephalopods: a manual for their identification. Smithsonian contributions to zoology, vol 513. p 55–66
Young RE, Vecchione M, Roper CFE (2007) A new genus and three species of decapodiform cephalopods. Rev Fish Biol Fish 17:353–365. https://doi.org/10.1007/s11160-007-9044-z
Zaragoza N, Quentglas A, Hidalgo M, Álvarez-Berastegui D, Balbín R, Alemany F (2015a) Effects of contrasting oceanographic conditions on the spatiotemporal distribution of Mediterranean cephalopod paralarvae. Hydrobiologia 749:1–14. https://doi.org/10.1007/s10750-014-2132-x
Zaragoza N, Quentglas A, Moreno A (2015b) Identification guide for cephalopod paralarvae from the Mediterranean Sea. ICES Coop Res Rep 324
Acknowledgements
We would like to acknowledge the researchers and crew on board of the Research Vessel “Atlântico Sul” of the Federal University of Rio Grande-FURG for carrying out the sampling and Prof. Eduardo R. Secchi and Dra. Juliana Di Tullio, general and executive coordinators of TALUDE Project. Surveys were funded by Chevron Brasil Upstream Frade Ltda (Grant number CW702315), PETROBRAS, through the Brazilian Inter-Ministerial Commission for the Resources of the Sea (CIRM), that supplied maritime diesel for the ships. The authors also thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) for providing Ph. D. scholarships to DOO and LB, financing the access to the “Portal de Periódicos” and giving financial support through the “Programa de Excelência Acadêmica – PROEX”. The project is a contribution to the “Instituto Nacional de Ciência e Tecnologia (INCT)—Biodiversidade da Amazônia Azul” of the Brazilian National Research Council (CNPq—Grant #405999/2022-4). We would like to extend our acknowledgments to Octavio E. Garrote and Rubens Torquato for their assistance in providing satellite-derived data and physical data, which significantly contributed to the success of this scientific research endeavor. EAG Vidal would like to thank CNPq (Grants # 426797/2018-3 and #316391/2021-2). E Muxagata would like to thank CNPq (Grants # 307633/2023-3).
Funding
EAGV was supported by the Brazilian National Research Council (CNPq—Grants # 426797/2018–3 and #316391/2021–2). The “Instituto Nacional de Ciência e Tecnologia (INCT)—Biodiversidade da Amazônia Azul” (CNPq—Grant #405999/2022–4) and the “Programa de Excelência Acadêmica—PROEX”. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. DOO data collection and analysis, preparation of figures and tables and drafting the manuscript, reviewing and editing. LB data collection and analysis preparation of figures, reviewing. EM data collection and analysis, supervision, reviewing. EAGV data analysis, supervision, reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. EAG Vidal served as guest editor of the TC Advances in Cephalopod Research.
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: R. Villanueva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortiz de Ortiz, D., Baldoni, L.C., Muxagata, E. et al. Cephalopod paralarvae from the southeast–south Brazilian outer shelf and slope. Mar Biol 171, 84 (2024). https://doi.org/10.1007/s00227-024-04401-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04401-w