Abstract
The development of in situ observational tools has significantly contributed to the study of deep-sea cephalopods and exploration of their habitat in the last decades. In this paper, we report in situ observations of rarely observed deep-sea Mediterranean cephalopods (Chiroteuthis veranyi, Chtenopteryx sicula, and Octopoteuthis sicula). These cephalopods were encountered during a scientific expedition, aimed at characterizing the biodiversity of a deep-sea area in the northern Ionian Sea. Images and video were collected by a remotely operated vehicle (ROV) between 537 and 1248 m. Chromatic, postural, locomotor, and bioluminescent behavioral components were reported for each species. This was the first time that O. sicula was filmed in its habitat and all individuals showed hovering and an arm spread posture with the arm tips exposed, producing an intermittent bioluminescence. Furthermore, our observations on six living specimens of C. sicula represent exceptional events, since this species was only observed once in the eastern Mediterranean in 2012. Overall, five females and a mature male of C. sicula were observed; the male had a large dorsal light organ. Finally, an individual of C. veranyi was observed consuming a large lanternfish (Myctophidae). In the near future, in situ explorations in the Mediterranean should be implemented to shed light on deep-sea cephalopods inhabiting this basin and fill information gaps on the biology, ecology, and behavior of elusive species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of deep-sea cephalopods is often problematic due to the difficulty of collecting samples and observing alive animals in their environment as well as the high costs of dedicated research expeditions (Hoving et al. 2014). Moreover, most species are not of economic importance for human consumption and are generally discarded by fishermen, and consequently several aspects of their biology, distribution, abundance, and ecology are poorly known (Pedà et al. 2022). Numerous pelagic cephalopods are rarely caught by professional fishing gear, and in most cases also avoid conventional sampling equipment (Clarke 1977, 1996a; Roper 1977; Wormuth and Roper 1983) thanks to their fast swimming and a developed sensorial system (Budelmann 1996). These difficulties can lead to underestimation of cephalopod abundance and biodiversity in certain marine habitats. In this context, the recent development and improvement of high-tech scientific tools (Kubodera and Mori 2005; 2007; Widder 2013; Hoving et al. 2019; Robinson et al. 2017; 2021), such as the underwater vehicles (e.g., submersibles and remotely operated vehicles), baited remote underwater video stations and systems (BRUVs), and stationary, towed, or passively drifting camera platforms, has recently stimulated new studies of deep-sea organisms and exploration of deep marine environment. Furthermore, sighting and filming deep-sea cephalopods in their habitat is not easy because of their sensitivity to noise and vibrations, as well as to excessive lighting used in underwater monitoring devices. Indeed, some species can perceive low-frequency sounds and are disturbed by bright light (Mooney et al. 2010; Hanlon et al. 2018; Robinson et al. 2021). For this reason, scientists have often adapted different observation systems for approaching these cephalopods, using particular video devices, selected wavelengths, baited cameras or optical lures (Kubodera et al. 2007; Widder 2013; Hoving et al. 2019; Robinson et al. 2021). One of the most successful and intriguing experiments was carried out by Widder and her collaborators using a bioluminescence-mimicking optical lure (E-Jelly) mounted on the “Medusa” camera system, which allowed them to record a video of a live giant squid in Japanese waters (Widder 2013).
During the last decades, studies based on in situ observations have improved knowledge on various aspects of the biology and ecology of deep-sea cephalopods, with regards mimicry (Burford et al. 2015), coupling, and reproduction (Roper and Vecchione 1996; Hoving et al. 2012; Vecchione 2019), posture and locomotion (Vecchione et al. 2002; Bush et al. 2009; Burford et al. 2015), feeding (Hoving and Robison 2012; Choy et al. 2017; Osterhage et al. 2020; Golikov et al. 2023), hunting strategies (Kubodera et al. 2007; Hoving et al. 2013), bioluminescence (Bush et al. 2009; Burford et al. 2015; Burford and Robison 2020), social structure (Burford and Robison 2020), and occurrence of parasitic infections (Stenvers et al. 2022). Most of these studies were conducted in the Atlantic and Pacific Oceans, partly due to the efforts of institutions that have significant investment in deep exploration (e.g., MBARI, NOAA, Smithsonian Institution, National Science Museum of Tokyo). There is a significant lack of data regarding the behavior and in situ observations of Mediterranean deep-sea cephalopods. Most Mediterranean studies are based on ROV surveys focused on biodiversity patterns and abundance of fauna in deep-sea habitats, occasionally reporting information on some cephalopods (e.g., Mastrototaro et al. 2010). In total, 70 cephalopod species have been reported in the Mediterranean basin, belonging to the orders Bathyteuthida (2), Myopsida (5), Octopoda (16), Oegopsida (26), Sepiida (4), and Sepiolida (17) (Pedà et al. 2022), including non-indigenous species (Bello et al. 2020). Most of them inhabit deep-sea waters and have key roles in the Mediterranean ecosystem, representing important food sources for several large marine predators (Bello 1991, 1996, 1999; Karakulak et al. 2009; Romeo et al. 2012; Battaglia et al. 2013, 2022; Garibaldi and Potestà 2014; Pedà et al. 2015; Foskolos et al. 2020). They are also able to perform movements in the water column to prey on micronekton, following the diel vertical migration and contributing to the energy transfer from upper waters to the deep sea (Roper and Young 1975; Boyle and Rodhouse 2005).
For these reasons, the aim of this paper is to improve the current knowledge on some Mediterranean deep-sea cephalopods using the data, images, and videos collected by ROV surveys in the central Mediterranean.
Materials and methods
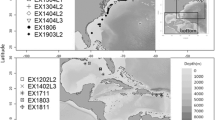
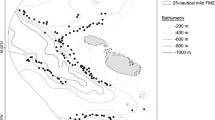
Underwater observations were carried out aboard the Offshore Support Vessel NG Worker (LOA = 89 m; GT = 3923 t) using the remotely operated vehicle (ROV) Schilling HD—unit no. 29. The research cruise planned to explore and assess marine biodiversity in areas devoted to the establishment of an offshore wind power plant. As part of this cruise, we investigated a deep-sea area (size: 115 km2) in the northern Ionian Sea, off the Calabrian coast, Italy (Fig. 1), between 25 July and 2 August, 2022. The deepest part of this area was characterized by muddy bottoms interrupted rarely by slightly outcropped rocks at depths ranging from 537 to 1248 m.
In total, 19 ROV transects were performed during both night and daylight for a total length of 107.92 linear km and 182:55 h of HD ROV footage, at an average speed of 0.26 km/h. The ROV was equipped with an IMENCO Spinner II Shark—High End HD Zoom Camera (https://imenco.no/product/spinner-ii-shark-high-end-hd-zoom-camera) and an ultra-high-definition camera Z-CAM E2-F6 full frame 6 K Cinema Camera, with an objective CANON EF 16–35 mm f/2.8L III USM. The camera settings were controlled live from the ship via a remote controller. All video and control signals were transmitted using a fiber optic multiplexer. The ROV was also equipped with two hydraulic manipulator arms (Titan 4 and Atlas) devoted to sample collection.
The ROV transects were preliminarily designed based on the morphobathymetric layer created through a multibeam echosounder (MBES) data acquisition survey (Fig. 1).
Overall, the ROV explorations allowed us to approach one Chiroteuthis veranyi (Férussac 1835), six Chtenopteryx sicula (Vérany 1851) and three Octopoteuthis sicula Rüppell 1844 (Table 1). An individual code was assigned to each cephalopod. The observations were associated with the following data: transect name, date, depth, geographical coordinates, duration of observation. Moreover, chromatic, postural, locomotor, and bioluminescent behavioral components of body patterns of cephalopods were assessed and described according to previous studies on Octopoteuthis deletron (Bush et al. 2009) and Chiroteuthis calyx (Burford et al. 2015).
Results
The investigation allowed us to film a predation event in C. veranyi for the first time. A single individual of this species was observed (Fig. 2) during the entire scientific cruise, at a depth of about 843 m. This squid had caught a lanternfish (Myctophidae), probably a Lampanyctus crocodilus, which had a relatively large size (the prey total length was larger than the mantle length of the C. veranyi) (Fig. 2a,b). At the time of the sighting, the C. veranyi was holding the lanternfish in its arms (Fig. 2a), with the fish head facing the cephalopod buccal opening, and swimming horizontal to the seafloor with spread arms and tentacles fairly extended, spread, and tilted 45°. During the observation, the squid began to show signs of stress, probably due to the light of the headlights and the presence of the ROV, to the point of abandoning the prey on the bottom and trying to escape (Fig. 2b). In this phase, the squid occasionally lost its orientation, sometimes spinning and swimming into the seabed (Fig. 2c). This behavior was characterized by a series of rapid convulsions, followed by a new behavioral sequence (jolt behavior). This change of behavior was observed for several minutes; after that, the squid moved away definitively. The chromatic pattern of C. veranyi was particularly interesting (Fig. 2d,e,f). All arms were dark (brown-orange) with the exception of the tips of the IV arms, which displayed a white color as well as the tentacles; however, the tips of the tentacles and their suckers appeared dark blue in color. The dorsal midline of the head was characterized by a dark eyebrow patch, with dark brown–red pigmentation concentrated between the eyes, followed by a pale band between the eye dorsal margins and the mantle. The mantle had the same color of the arms, but it became lighter toward the tail, which was characterized by dark fin centers and pale margins. The video analysis allowed us to observe in some frames (when the individual spun) the presence of the typical photophores of the species, on the eyeballs, IV arms as well as on the viscera.
Chiroteuthis veranyi (Chi-ver-1) observed by ROV during the research cruise. a predation event on a lanterfish; b C. veranyi abandons the prey on the bottom and try to escape; c the squid lose its orientation, probably disturbed by ROV lights; d, e, and f particulars of the chromatic pattern and swimming behavior of C. veranyi
Videos of the comb-finned squid C. sicula (Fig. 3) were captured between 1093 and 1158 m depths. The first encounter was preceded by an ink jet that was noticed during the ROV transect; after about 20 s, a C. sicula (Cht-sic-1; Fig. 3a) appeared and it was smimming above the bottom by about 50 cm. The following shots of this species showed individuals (Cht-sic-2; Cht-sic-3; Cht-sic-4; Cht-sic-5) in a stationary swimming phase, i.e., hovering, raised above the bottom about 50–100 cm (3b, c). In contrast, Cht-sic-6 (Fig. 3d, e, f) was lying on the bottom, and as soon as the ROV approached, it lifted off the bottom with a snap, staying for more than a minute near the camera, and then running away. Curiously, Cht-sic-5 appeared in the video while the ROV was busy filming a longnosed skate Dipturus oxyrinchus (Fig. 3c). The Cht-sic-2, Cht-sic-3, and Cht-sic-4 specimens were observed in the same area, about 3 min apart from each other. Two of these specimens were females (Cht-sic-2 and Cht-sic-3), while Cht-sic-4 was a mature male, as demonstrated by the presence of a large sex-specific dorsal light organ (Fig. 3b). The individuals observed in the other dives were all females. Moreover, in some frames it was also possible to observe a large photophore on the ventral surface of the eyeball.
Individuals of Chtenopteryx sicula observed during the ROV survey. a Frame grab from the HD video of the first individual of C. sicula (Cht-sic-1) detected during the research cruise; b image of a male (Cht-sic-4) having a large dorsal light organ (white arrow); c Cht-sic-5 swimming near a longnosed skate, Dipturus oxyrinchus d, e, f particulars of the chromatic pattern and stationary behavior (Cht-sic-6)
The chromatic pattern of C. sicula was almost hyaline, so that it was possible to observe the internal organs (Fig. 3). The fin ribs as well as their membranes were brownish and this color pattern was limited to the external margins of fins; the same pattern was observed in the margins of the III arms (Fig. 3d, e, f). The pigmentation in the dorsal midline of the head between the eyes formed a dark blue eyebrow patch, most concentrated around the eyes (Fig. 3d, e).
Octopoteuthis sicula was observed in deep waters between 687 and 1139 m (Fig. 4). The first individual (Oct-sic-1) was encountered as soon as the ROV reached the seafloor, at the beginning of a transect. Oct-sic-1 was in a “hovering” position (i.e., the individual was maintaining its position in the water column, remaining motionless and sometimes using the fins for stabilization), with the dorsal mantle up, arms spread and fins mainly curved ventrally (Fig. 4a, b, c). The arms remained up and curled. The observations lasted about 17 min, and during this time, a Chauliodus sloani was also detected swimming behind the squid. The color pattern of this individual was characterized by a pale mantle and dark fins, with pale fin edges. The arms were dark on their external side and pale internally (Fig. 4a, b, c). In some frames of the video, it was possible to observe the intermittent bioluminescence produced in the light organs located on the arms’ tips. However, the light production in these eight photophores was not synchronized. During flashes, arm-tip chromatophores were strongly contracted and this portion was distinctly pale. Furthermore, the analysis of some video-frames showed that Oct-sic-1 had pale structures on the dorsal mantle, probably spermatangia implanted on the skin (Fig. 4c).
Specimens of Octopoteuthis sicula observed during the ROV survey. a, b Oct-sic-1 in a “hovering” position; c particulars of the color pattern of Oct-sic-1; spermatangia on the dorsal mantle can be observed (white arrow); d Oct-sic-2 in a “hovering” position; e dark color pattern of Oct-sic-2; f particular of the color pattern of Oct-sic-3 having a dark body with a pale edge of fins
The other two specimens of O. sicula (Oct-sic-2 and Oct-sic-3) at the arrival of the ROV were displaying the same behavior (hovering) of Oct-sic-1. However, after an initial attempt to escape, Oct-sic-2 (Fig. 4d, e) approached the seabed (a few cm from the bottom) and was filmed there for few minutes. This individual had a darker color pattern, with pale fin edges. In some frames, a pale arm crown band can barely be observed in this squid. The last individual (Oct-sic-3) remained for the entire time of observation near the camera with arms spread and curled tips, maintaining the fins being out. The color of this specimens was the darkest observed, although as in the other individuals the edges of the fins were pale (Fig. 4f).
The postural, chromatic, bioluminescent, and locomotor components observed in the three species during this study and described above are summarized in Table 2.
Discussion
This paper presents one of the first detailed in situ observations of Mediterranean deep-sea cephalopods through ROV investigations. This is the first time that O. sicula has been filmed in its habitat and an in situ predation event involving C. veranyi was reported. Furthermore, to date, there is only one rare ROV video of C. sicula captured in the eastern Mediterranean during a research expedition aimed at exploring Thessaloniki Mud Volcan; this comb-finned squid was observed at about 1300 m of depth off the coast of southern Turkey (Young and Vecchione 2016). Thus, our observations of six specimens of C. sicula represent an exceptional event.
Several deep-sea cephalopods are cryptic or elusive, or more simply live in poorly investigated waters. In some cases, these cephalopods display mesopelagic behaviors and often escape to conventional sampling procedures, leading to an underestimation of the consistence of their populations (Clarke 1977). However, indirect information on the occurrence of these species has been retrieved by studies on trophic ecology of large predators (Romeo et al. 2012) such as billfishes and tunas (e.g., Battaglia et al. 2013, 2022; Rosas-Luis et al. 2016), sharks (Bello 1996; Smale and Cliff 1998; Rosas-Luis et al. 2016) or cetaceans (Clarke 1996b; Pedà et al. 2015; Foskolos et al. 2020), which can be defined as efficient cephalopod collectors (Clarke 1986; Bello 1996; Pedà et al. 2022). In recent times, the use of highly technological devices such as ROVs or submersibles has made an important contribution to deep-sea exploration and knowledge of uncommon cephalopods (Vecchione and Roper 1991; Kubodera et al. 2007; Widder 2013; Hoving et al. 2019; Robinson et al. 2021; Jamieson and Vecchione 2022). The captured videos and images during these studies have shown peculiar biological and ecological aspects of cephalopods, that can only be detected and understood after meticulous analysis. Frame-by-frame, survey-by-survey, researchers from different areas of the globe are putting together the various pieces of a complicated puzzle that clarify still unknown aspects of the lives of deep-sea cephalopods.
Our investigations revealed that C. veranyi is an active predator, able to feed on relatively large prey. The observed individual was trying to consume a lanternfish (Myctophidae), probably a L. crocodilus. Data on the feeding ecology of C. veranyi are still totally missing, and the few specimens examined so far in Mediterranean waters all had empty stomachs (Cuccu et al. 2009). Our observations support the hypothesis that C. veranyi, like other deep-sea organisms, generally feeds on large prey to maximize energy intake and reduce metabolism costs in a food-poor environment. However, the specimen did not have enough time to consume the lanternfish as it was disturbed by the ROV and abandoned its prey on the seafloor. As also observed by other authors, ROVs are large, noisy and bright, thus inducing defensive responses and sometimes behavioral changes in deep-sea animals (Lorance and Trenkel 2006; Bush et al. 2009; Budford et al. 2015). In this case, in some frames, ROV disturbance provoked a behavioral change in C. veranyi, consisting of rapid convulsions, orientation loss, rotating movements and directional changes, as also reported for other species, such as Taningia danae (Kubodera et al. 2007), O. deletron (Bush et al. 2009) and C. calyx (Budford et al. 2015). On the contrary, C. sicula and O. sicula, seemed to be less affected by the presence of the ROV, displaying jetting or cloud ink release in few cases.
Although we observed only one individual of C. veranyi, it was possible to note some resemblances to the congeneric species C. calyx. Indeed, some behavioral and chromatic components (Tables 2 and 3 of the present paper) were similar to those described by Budford et al. (2015), which used in situ ROV footage to describe the first behavioral ethogram for C. calyx.
The six records of the cryptic squid C. sicula provided remarkable information on this species. To date, the biology and ecology of C. sicula are almost unknown because collection of fresh individuals of this species is a rare event. In the Mediterranean basin, it was occasionally collected by bottom trawl (e.g., D’Onghia et al.1998; Pedà et al. 2022), pelagic trawl (Orsi Relini and Garibaldi 2005; Krstulović Šifner et al. 2014), zooplankton collection (Naef 1923), stranded biological material in the Strait of Messina (Naef 1923; Spartà 1933; Torchio 1966) or found in the stomach content of large predators (Joubin 1900; Foskolos et al. 2020). Interestingly, in the present study, images of three specimens were captured in a very restricted area, indicating an unusual concentration of individuals of this species, which may also be related to reproductive reasons, as both females and a mature male were observed. Several authors indicated that mature males of C. sicula develop a large, dorsally directed photophore in the mantle cavity (Nesis 1987; Guerra 1992; Jereb and Roper 2010; Escánez et al. 2018). In our male individual, the dorsal luminous organ was evident and highly reflected the light of ROV headlights and include the first images of a C. sicula male in its natural environment. Unfortunately, our videos did not provide any indication of the presence/absence of a visceral photophore, as these squids have never shown the ventral zone during ROV observations. The question of whether or not C. sicula possesses this visceral photophore has long been debated by several authors (Naef 1923; Nesis 1987; Guerra 1992; Jereb and Roper 2010; Escànez et al. 2018; Young et al. 2019) and still remain unresolved, although Escànez et al. (2018) suggested that “C. sicula should have a large photophore on the ventral surface of the eyeball and a visceral photophore; however, sometimes these are not discernible as they are in other descriptions”. This uncertainty stems from the fact that the first descriptions of a Mediterranean C. sicula were made on previously preserved (Veranyi 1851) or very small individuals (Pfeffer 1912) as well as specimens in poor conditions found in the stomach of a dolphin (Joubin 1900). Recently, Krstulović Šifner et al. (2014), examining two males and four females from the southern Adriatic Sea, only observed a ventral photophore in females. Thus, the ventral and the dorsal luminous organs in C. sicula may be related to a sexual dimorphism (i.e., a ventral photophore in females, a dorsal one in males). The dominant transparent color pattern, the dark eye circle and eyebrow patches, and the presence of a downward-directed photophore under each eyeball of C. sicula suggests that this squid is well adapted to the mesopelagic realm, where chromatic components and counterillumination are crucial to hiding the body silhouette in an environment with few hiding places (Herring 2002; Zylinski and Johnsen 2011). Based on current knowledge (Young 1978), squids of the genus Chtenopteryx have mesopelagic habits and make diel vertical migrations, stationing near the bottom during daylight hours and ascending to upper waters at night. Our data seems to confirm this trend because the sightings of C. sicula occurred near the seafloor only during daylight (five individuals between 12:56 and 13:50 and one individual at 06:50), whereas no individuals were observed on the bottom during the night. The same color pattern of C. sicula spotted in the eastern Mediterranean near the Thessaloniki Mud Volcan (Young and Vecchione 2016) can be found on Cth-sic-6, having brownish/dark fin ribs, external margins of fins and margins of the III arms. This behavior could be a reaction to the approach of the ROV, as it was observed in both cases when the vehicle was very close to the squid.
Regarding O. sicula, there is very little information in the literature since sample collection has always been problematic due to the elusive behavior of this species and the high depth at which it lives. In addition, the gelatinous consistency of its body means that the few known intact specimens were mostly found stranded as well as collected by zooplankton or hand nets in the Strait of Messina and Gulf of Naples (Rüppell 1844; Naef 1923; Spartà 1933; Torchio 1966; Villari and Ammendolia 2009), while those caught by trawl nets (D’Onghia et al. 1995; Cuccu et al. 2013) were often damaged (in particular on the arm tips). A recent comprehensive revision of the systematic status of the genus Octopoteuthis in the Mediterranean Sea confirmed the presence of a single species in these waters, i.e., O. sicula (Jereb et al. 2016). To date, O. sicula had not yet been filmed in its habitat, while some videos and in vivo observations are available on the congeneric species Octopoteuthis megaptera (Vecchione et al. 2002) and O. deletron (e.g., Bush et al. 2009; Bush 2012; Hoving et al. 2012). Interestingly, Hoving et al. (2012), during a ROV survey in the Pacific Ocean, observed implanted spermatangia on several parts of the body in O. deletron males and females, concluding that the reproductive strategy of this species aims to “maximize success by inducing males to indiscriminately and swiftly inseminate every conspecific that they encounter”. Previously, Hoving et al. (2008) had found spermatangia implanted in several parts of the body in both females and males of O. sicula by examining 39 individuals from South Africa and collected by trawls or removed from the stomachs of sperm whales and tunas. This is also confirmed by a capture of a female O. sicula in Sardinianwaters (Mediterranean) showing the presence of spermatangia deeply implanted in the mantle tissue (Cuccu et al. 2013). In the present study, similar structures were observed in O. sicula, distributed on the dorsal mantle of Oct-sic-1. It is plausible to think that the two species may adopt similar reproductive strategies.
An extensive description of the postural, locomotor, chromatic and bioluminescent components in O. deletron was provided by Bush et al. (2009), who examined videos of 76 individuals, discovering that this squid can exhibit a broad range of behavioral patterns. Although our observations are limited to three individuals of O. sicula, it was possible to record 17 different components. All specimens showed hovering and arm spread posture withhe tarm tips exposed, producing an intermittent bioluminescence. A similar posture was also observed by Vecchione et al. (2002) in O. megaptera. According to Bush et al. (2009), this behavior is related to a hunting strategy aimed at attracting prey by bioluminescent lures, as also happens in other deep-sea cephalopods (Robison 2004; Hoving et al. 2013). Light emission may also be used for blinding or illuminating prey to assess the target distance in a dark deep environment (Kubodera et al. 2007). Another possible hypothesis concerns a defensive tactic, consisting of the use of luminous signals to alert, blind or confuse predators (Bush et al. 2009); in this case, this tactic was used against the ROV, probably seen as a potential predator. However, during our observations, the individuals were neutrally buoyant in the water column in a hovering posture and essentially motionless, causing us to lean more toward the first hypothesis.
In the near future, we aim to continue exploring the Mediterranean basin to shed light on deep-sea cephalopods and fill wide information gaps on the biology and ecology of cryptic and elusive species.
Data availability
The datasets analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Battaglia P, Pedà C, Sinopoli M, Romeo T, Andaloro F (2013) Cephalopods in the diet of young-of-the- year bluefin tuna (Thunnus thynnus L. 1758, Pisces: Scombridae) from the southern Tyrrhenian Sea (central Mediterranean Sea). It J Zool 80:560–565. https://doi.org/10.1080/11250003.2013.837105
Battaglia P, Pedà C, Malara D, Milisenda G, MacKenzie BR, Esposito V, Consoli P, Vicchio TM, Stipa MG, Pagano L, Longo F, Romeo T (2022) Importance of the lunar cycle on mesopelagic foraging by Atlantic bluefin tuna in the upwelling area of the Strait of Messina (Central Mediterranean Sea). Animals 12(17):2261. https://doi.org/10.3390/ani12172261
Bello G (1991) Role of cephalopods in the diet of the swordfish, Xiphias gladius, from the eastern Mediterranen sea. Bull Mar Sci 49(1–2):312–324
Bello G (1996) Teuthophagous predators as collectors of oceanic cephalopods: the case of the Adriatic Sea. Boll Malacol 32:71–78
Bello G (1999) Cephalopods in the diet of albacore, Thunnus alalunga, from the Adriatic Sea. J Molluscan Stud 65:233–240. https://doi.org/10.1093/mollus/65.2.233
Bello G, Andaloro F, Battaglia P (2020) Non-indigenous cephalopods in the Mediterranean Sea: a review. Acta Adriat 61(2):113–134. https://doi.org/10.32582/aa.61.2.1
Boyle PR, Rodhouse P (2005) Cephalopods: ecology and fisheries. Blackwell Science, Oxford
Budelmann BU (1996) Active marine predators: the sensory world of cephalopods. Mar Freshw Behav Physiol 27(2–3):59–75. https://doi.org/10.1080/10236249609378955
Burford BP, Robison BH (2020) Bioluminescent backlighting illuminates the complex visual signals of a social squid in the deep sea. Proc Natl Acad Sci 117(15):8524–8531. https://doi.org/10.1073/pnas.1920875117
Burford BP, Robison BH, Sherlock RE (2015) Behaviour and mimicry in the juvenile and subadult life stages of the mesopelagic squid Chiroteuthis calyx. J Mar Biol Assoc UK 95(6):1221–1235. https://doi.org/10.1017/S0025315414001763
Bush SL (2012) Economy of arm autotomy in the mesopelagic squid Octopoteuthis deletron. Mar Ecol Progr Ser 458:133–140. https://doi.org/10.3354/meps09714
Bush SL, Robison BH, Caldwell RL (2009) Behaving in the dark: locomotor, chromatic, postural, and bioluminescent behaviors of the deep-sea squid Octopoteuthis deletron Young 1972. Biol Bull 216(1):7–22. https://doi.org/10.1086/BBLv216n1p7
Choy CA, Haddock SH, Robison BH (2017) Deep pelagic food web structure as revealed by in situ feeding observations. Proc R Soc B 284(1868):20172116. https://doi.org/10.1098/rspb.2017.2116
Clarke MR (1977) Beaks, nets and numbers. Proc Zool Soc Lond 38:89–126
Clarke MR (1986) A handbook for the identification of cephalopods beaks. Clarendon Press, Oxford
Clarke MR (1996a) The role of cephalopods in the world’s oceans: an introduction. Philos Trans R Soc B 351:979–983
Clarke MR (1996) Cephalopods as prey. III. Cetaceans. Philos Trans R Soc B 351(1343):1053–1065
Cuccu D, Mereu M, Masala P, Cau A, Jereb P (2009) Chiroteuthis veranii and Ommastrephes bartramii (Cephalopoda: Teuthidae) in the Sardinian waters. Biol Mar Medit 16(1):334–335
Cuccu D, Cannas R, Mereu M, Follesa MC, Jereb P, Cau A (2013) On the first record of the genus Octopoteuthis (Cephalopoda, Octopoteuthidae) in the Sardinian Channel (central western Mediterranean Sea). Molluscan Res 33(2):135–142. https://doi.org/10.1080/13235818.2013.782795
D’Onghia G, Maiorano P, Panetta P (1995) Octopoteuthis sicula (Rüppell, 1844) and Brachioteuthis. Boll Malacol 31(5–8):137–142
D’Onghia G, Maiorano P, Panza M, Panetta P (1998) Rinvenimento di Chtenopteryx sicula (Verany, 1851)(Mollusca, Cephalopoda) nel Mar Ionio settentrionale. Biol Mar Medit 5:690–693
Escánez A, Roura Á, Riera R, González ÁF, Guerra Á (2018) New data on the systematics of comb-fin squids Chtenopteryx spp. (Cephalopoda: Chtenopterygidae) from the Canary Islands. Zool Stud 57:40. https://doi.org/10.6620/ZS.2018.57-40
Férussac AEJPJF d’Audebard de (1835) Note sur deux genres de Céphalopodes encore peu connus, les genres Calmaret et Cranchie, et sur une nouvelle espèce fort remarquable de chacun de ces deux genres. Magasin De Zoologie, Classe V, pp 65–66
Foskolos I, Koutouzi N, Polychronidis L, Alexiadou P, Frantzis A (2020) A taste for squid: the diet of sperm whales stranded in Greece, Eastern Mediterranean. Deep Sea Res Part I 155:103164. https://doi.org/10.1016/j.dsr.2019.103164
Garibaldi F, Podestà M (2014) Stomach contents of a sperm whale (Physeter macrocephalus) stranded in Italy (Ligurian Sea, north-western Mediterranean). J Mar Biolog Assoc UK 94(6):1087–1091. https://doi.org/10.1017/S0025315413000428
Golikov AV, Stauffer JB, Schindler SV, Taylor J, Boehringer L, Purser A, Sabirov RM, Hoving H-J (2023) Miles down for lunch: deep-sea in situ observations of Arctic finned octopods Cirroteuthis muelleri suggest pelagic–benthic feeding migration. Proc R Soc B 290:20230640. https://doi.org/10.1098/rspb.2023.0640
Guerra Á (1992) Mollusca, Cephalopoda. In: Ramos MA et al (eds) Fauna Ibérica, vol 1. Museo Nacional de Ciencias Naturales. CSIC, Madrid
Hanlon R, Vecchione M, Allcock AL (2018) Octopus, squid and cuttlefish. A visual scientific guide to the oceans’ most advanced invertebrates. University of Chicago Press, Chicago, p 224
Herring P (2002) The biology of the deep ocean. Oxford University Press, New York
Hoving HJ, Robison BH (2012) Vampire squid: detritivores in the oxygen minimum zone. Proc Royal Soc B 279(1747):4559–4567. https://doi.org/10.1098/rspb.2012.1357
Hoving HJT, Lipiński MR, Videler JJ (2008) Reproductive system and the spermatophoric reaction of the mesopelagic squid Octopoteuthis sicula (Rüppell 1844) (Cephalopoda: Octopoteuthidae) from southern African waters. Afr J Mar Sci 30(3):603–612. https://doi.org/10.2989/AJMS.2008.30.3.13.647
Hoving HJ, Bush SL, Robison BH (2012) A shot in the dark: same-sex sexual behaviour in a deep-sea squid. Biol Lett 8(2):287–290. https://doi.org/10.1098/rsbl.2011.0680
Hoving HJ, Zeidberg LD, Benfield MC, Bush SL, Robison BH, Vecchione M (2013) First in situ observations of the deep-sea squid Grimalditeuthis bonplandi reveal unique use of tentacles. Proc Royal Soc B 280(1769):20131463. https://doi.org/10.1098/rspb.2013.1463
Hoving HJT, Perez JAA, Bolstad KS, Braid HE, Evans AB, Fuchs D, Judkins H, Kelly JT, Marian JEAR, Nakajima R, Piatkowski U, Reid A, Vecchione M, Xavier JCC (2014) The study of deep-sea cephalopods. Adv Mar Biol 67:235–359
Hoving HJT, Christiansen S, Fabrizius E, Hauss H, Kiko R, Linke P, Neitzel P, Piatkowski U, Körtzinger A (2019) The pelagic in situ observation system (PELAGIOS) to reveal biodiversity, behavior, and ecology of elusive oceanic fauna. Ocean Sci 15:1327–1340. https://doi.org/10.5194/os-15-1327-2019
Jamieson AJ, Vecchione M (2022) Hadal cephalopods: first squid observation (Oegopsida, Magnapinnidae, Magnapinna sp.) and new records of finned octopods (Cirrata) at depths> 6000 m in the Philippine trench. Mar Biol 169(1):1–5. https://doi.org/10.1007/s00227-021-03993-x
Jereb P, Roper CFE (2010) Cephalopods of the world. an annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes 4., FAO. 630 pp
Jereb P, Cannas R, Maiorano P, Bello G, Garibaldi F, Mereu M, Ancona FG, Ammendolia G, Battaglia P, Duysak Ö, Hoving HJT, Lefkaditou E, Lipinski MR, Melis R, Peristeraki PN, Ragonese S, Romeo T, Salman A, Santos MB, Villari A, Cuccu D (2016) The deep-water squid Octopoteuthis sicula Rüppell, 1844 (Cephalopoda: Octopoteuthidae) as the single species of the genus occurring in the Mediterranean Sea. Mar Biol 163(9):192. https://doi.org/10.1007/s00227-016-2965-0
Joubin L (1900) Cephalopodes provenant des campagnes de la Princesse-Alice (1891–1897). Resultats Des Campagnes Scientifiques Accomplies Sur Son Yacht Par Albert Ier Prince Souverain De Monaco 17:1–135
Karakulak FS, Salman A, Oray IK (2009) Diet composition of bluefin tuna (Thunnus thynnus L. 1758) in the Eastern Mediterranean Sea, Turkey. J Appl Ichthyol 25:757–761
Krstulović Šifner S, Petrić M, Isajlović I, Vrgoč N, Ikica Z, Piccinetti C (2014) Insight in some aspects of the reproductive biology, morphometry and age of Chtenopteryx sicula (Cephalopoda: Chtenopterygidae) in the Adriatic Sea. Acta Adriat 55(1):31–42
Kubodera T, Mori K (2005) First-ever observations of a live giant squid in the wild. Proc R Soc B 272(1581):2583–2586. https://doi.org/10.1098/rspb.2005.3158
Kubodera T, Koyama Y, Mori K (2007) Observations of wild hunting behaviour and bioluminescence of a large deep-sea, eight-armed squid, Taningia danae. Proc R Soc B 274(1613):1029–1034. https://doi.org/10.1098/rspb.2005.3158
Lorance P, Trenkel VM (2006) Variability in natural behaviour, and observed reactions to an ROV, by mid-slope fish species. J Exp Mar Biol Ecol 332(1):106–119. https://doi.org/10.1016/j.jembe.2005.11.007
Mastrototaro F, D’Onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A (2010) Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): an update. Deep-Sea Res II 57(5–6):412–430. https://doi.org/10.1016/j.dsr2.2009.08.021
Mooney TA, Hanlon RT, Christensen-Dalsgaard J, Madsen PT, Ketten DR, Nachtigall PE (2010) Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J Exp Biol 213(21):3748–3759. https://doi.org/10.1242/jeb.048348
Naef A (1923) Die Cephalopoden. Fauna e Flora de Golfo di Napoli. Bardi G. (Ed.), monograph 35, 863 pp
Nesis KN (1987) Cephalopods of the world (English edition). Neptune City, NJ and London. Nesis KN. 1982. Kratky opredelitel´ golovonogikh molluskov Mirovogo okeana (Trans: Levitov BS.), Moscow
Orsi Relini L, Garibaldi F (2005) Diversità dei cefalopodi mesopelagici del Santuario dei cetacei in base a campionamenti diretti e osservazioni sull’alimentazione dello Zifio. Ziphius Cavirostris Biol Mar Medit 12(1):106–115
Osterhage D, MacIntosh H, Althaus F, Ross A (2020) Multiple observations of bigfin squid (Magnapinna sp) in the Great Australian Bight reveal distribution patterns, morphological characteristics, and rarely seen behaviour. PLoS One 15(11):e0241066. https://doi.org/10.1371/journal.pone.0241066
Pedà C, Battaglia P, Scuderi A, Voliani A, Mancusi C, Andaloro F, Romeo T (2015) Cephalopod prey in the stomach contents of odontocete cetaceans stranded in the western Mediterranean Sea. Mar Biol Res 11(6):593–602. https://doi.org/10.1080/17451000.2014.966724
Pedà C, Battaglia P, Romeo T, Stipa MG, Longo F, Malara D, Consoli P, Andaloro F (2022) Photographic atlas of cephalopod beaks from the Mediterranean Sea. Eta Beta ed., pp. 109, ISBN: 9791259686381
Pfeffer G (1912) Die Cephalopoden der Plankton-expedition. Ergebnisse Der Plankton-Expedition Der Humboldt-Stiftung 2:1–815
Robinson NJ, Johnsen S, Brooks A, Frey L, Judkins H, Vecchione M, Widder E (2021) Studying the swift, smart, and shy: unobtrusive camera-platforms for observing large deep-sea squid. Deep Sea Res Part I 172:103538. https://doi.org/10.1016/j.dsr.2021.103538
Robison BH (2004) Deep pelagic biology. J Exp Mar Biol Ecol 300:253–272. https://doi.org/10.1016/j.jembe.2004.01.012
Robison BH, Reisenbichler KR, Sherlock RE (2017) The coevolution of midwater research and ROV technology at MBARI. Oceanography 30(4):26–37. https://doi.org/10.5670/oceanog.2017.421
Romeo T, Battaglia P, Pedà C, Perzia P, Consoli P, Esposito V, Andaloro F (2012) Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgol Mar Res 66:295–306. https://doi.org/10.1007/s10152-011-0270-3
Roper CFE (1977) Comparative captures of pelagic cephalopods by midwater trawls. Proc Zool Soc Lond 38:61–87
Roper CF, Vecchione M (1996) In situ observations on Brachioteuthis beanii Verrill: paired behavior, probably mating (Cephalopoda, Oegopsida). Am Malacol Bull 13(1/2):55–60
Roper CF, Young RE (1975) Vertical distribution of pelagic cephalopods. Smith Contrib Zool 209:1–51
Rosas-Luis R, Loor-Andrade P, Carrera-Fernández M, Pincay-Espinoza JE, Vinces-Ortega C, Chompoy-Salazar L (2016) Cephalopod species in the diet of large pelagic fish (sharks and billfishes) in Ecuadorian waters. Fish Res 173:159–168. https://doi.org/10.1016/j.fishres.2015.07.002
Rüppell E (1844) Intorno ad alcuni cefalopodi del mare di Messina. Lettera del Dr. Eduardo Rüppell di Frankfurt sul Meno al Prof. Anastasio Cocco. Giornale Del Gabinetto Letterario Di Messina 5:129–135
Smale MJ, Cliff G (1998) Cephalopods in the diets of four shark species (Galeocerdo cuvier, Sphyrna lewini, S. zygaena and S mokarran) from Kwazulu-Natal South Africa. Afr J Mar Sci 20(1):241–253. https://doi.org/10.2989/025776198784126610
Spartà A (1933) Osservazioni compiute nello Stretto di Messina sul comportamento dei pesci e cefalopodi all’azione di sorgenti luminose. C Ferrari Mem R Com Talassogr 206:206–220
Stenvers VI, Sherlock RE, Reisenbichler KR, Robison BH (2022) ROV observations reveal infection dynamics of gill parasites in midwater cephalopods. Sci Rep 12(1):1–12. https://doi.org/10.1038/s41598-022-11844-y
Torchio M (1966) Euribatia di Teutacei spiaggamenti ed apporto di acque di origine continentale. Atti Soc Ital Sc Nat Mus Civ Storia Nat 105:317–342
Vecchione M (2019) ROV observations on reproduction by deep-sea cephalopods in the central Pacific Ocean. Front Mar Sci 6:403. https://doi.org/10.3389/fmars.2019.00403
Vecchione M, Roper CF (1991) Cephalopods observed from submersibles in the western North Atlantic. Bull Mar Sci 49(1–2):433–445
Vecchione M, Roper CFE, Widder EA, Frank TM (2002) In-situ observations on three species of large-finned deep-sea squids. Bull Mar Sci 71(2):893–901
Vérany GB (1851) Mollusques méditeranéens observés, décrits, figurés et chromolitographiés d’après le vivant. Part 1. Céphalopodes de la Méditerranée. Imprimerie des Sourds-Muets, Gênes
Villari A, Ammendolia G (2009) On a beached specimen of Octopoteuthis sicula (Cephalopoda: Octopoteuthidae) in the Strait of Messina. Boll Malacol 45:9–11
Widder E (2013) The Kraken revealed: the technology behind the first video recordings of live giant squid. Sea Technol 54:47–54
Wormuth JH, Roper CFE (1983) Quantitative sampling of oceanic cephalopods by nets: problems and recommendations. Biol Oceanogr 2(2–4):357–377. https://doi.org/10.1080/01965581.1983.10749466
Young RE (1978) Cephalopods from Hawaiian waters. Fish Bull 76(3):583–615
Young RE, Vecchione M (2016). Chtenopteryx sicula (Verany 1851). Version 16 November 2016 (under construction). http://tolweb.org/Chtenopteryx_sicula/19441/2016.11.16 in The Tree of Life Web Project, http://tolweb.org/. Accessed 1 Mar 2023
Young RE, Vecchione M, Mangold KM (1922–2003) (2019) Cephalopoda Cuvier 1797. Octopods, squids, nautiluses, etc.. Version 26 March 2019 (under construction). http://tolweb.org/Cephalopoda/19386/2019.03.26 in The Tree of Life Web Project, http://tolweb.org/. Accessed 1 Mar 2023
Zylinski S, Johnsen S (2011) Mesopelagic cephalopods switch between transparency and pigmentation to optimize camouflage in the deep. Curr Biol 21(22):1937–1941. https://doi.org/10.1016/j.cub.2011.10.014
Acknowledgements
The authors are grateful to Dr Teresa Romeo, Dr Daniela Pica, Dr Frine Cardone, Dr Valentina Costa, Dr Antonio Giova, Dr Valeria Palummo, Dr Gennaro Ucciero, Dr Martina Genovese, Dr Francesco Stenico.
Funding
This study was funded by Renantis—Falck Renewables.
Author information
Authors and Affiliations
Contributions
PB and SC contributed to the study conception. SC, ES and SG contributed to the sampling design. SC and SE supervised the ROV survey at sea. PB performed the video and data analysis, writing also the first draft. All authors reviewed and revised the first draft and gave final approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Surveys were conducted by ROV and no cephalopod samples were collected during the study. Activities were cpnducted with all applicable permits in place.
Additional information
Responsible Editor: Henk-Jan Hoving.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Video_1 Octopoteuthis sicula in its habitat showing an arm spread posture with the arm tips exposed, producing an intermittent bioluminescence (MOV 118414 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battaglia, P., Canese, S., Salvati, E. et al. In situ observations of three deep-sea cephalopods in the central Mediterranean Sea. Mar Biol 170, 151 (2023). https://doi.org/10.1007/s00227-023-04264-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04264-7