Abstract
Unlike most cephalopods, the firefly squid Watasenia scintillans is highly monoandrous. We investigated this unusual phenomenon by considering the rare occurrence of polyandrous mating and the extremely male-biased operational sex ratio (OSR) at the beginning of the mating season. Theoretically, male-biased OSR can intensify competition for mates, leading to increased polyandry. We estimated OSR (male/female) as 32.7 and 9.1 at the beginning and end of the mating period, respectively. Next, we estimated the rate of polyandry in a population on a weekly basis, based on our new finding that the rate of polyandry can be estimated stochastically from the total number of spermatangia attached to one of the two seminal receptacles within a female. We found that the female squid sustains their highly monoandrous mating regime despite the fact that the OSR is extremely male-biased. Furthermore, there were no significant changes in the polyandry rate during the reproductive season. These results suggest that the squid mating system was not influenced by seasonal changes in OSR. Here, we discuss the evolutionary mechanism of how monoandry persists in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broadly, the points at which females choose males as mating partners, referred to as female choice, call special attention to evolutionary biologists (Emlen and Oring 1977; Eberhard 1996; Rosenthal and Ryan 2022). However, how females decide the number of males to mate, especially single or multiple males, remains largely unknown (Jennions and Petrie 2000; Kvarnemo 2018). In theory, mate number is disproportionally correlated with offspring number in females (Bateman 1948); however, polyandry (female mating with multiple males) is prevalent in diverse taxa (Taylor et al. 2014) owning to direct and indirect benefits gained from multiple mates (Reynolds 1996; Arnqvist and Nilsson 2000; Firman 2011; Matsumura et al. 2021). It is generally considered that the mode of reproduction, either monoandry (female mating with a male) or polyandry, is attributed to each species as a fixed trait (Whiteman and Cote 2004; Hughes et al. 2008; Davies and Gardner 2018; Young et al. 2019). However, monoandry (or monogamy) is considered to have been adopted to maximize reproductive success for one or both sexes as the consequences of biparental care (Tumulty et al. 2014) and severe constraints on the accessibility and availability of reproductive or environmental resources (Komers and Brotherton 1997; Stockley 1997). Therefore, it is often observed that the choice of reproductive mode is determined conditionally by environmental, intrasexual, or intersexual contexts, and hence, differently in populations (Uller and Olsson 2008; Brown et al. 2010).
In this case, the operational sex ratio (OSR), the ratio of males to females who are ready to mate at any one time (Emlen and Oring 1977), may influence the reproductive mode decision in a given population (Arnqvist and Nilsson 2000; Kokko and Jennions 2008; Janicke and Morrow 2018), but see Head et al. (2008) and Plesnar-Bielak et al. (2020). If the OSR skews toward one sex, the intensity of competition for mates increases (Trivers 1972), but see Klug et al. (2010), resulting in altered mating behaviors, such as either an increase or decrease in competitive aggression, courtship, pre- or postcopulatory mate guard, and frequency or duration of copulation (Kvarnemo and Ahnesjo 1996; Weir et al. 2011). We assumed that in species without aggression, courtship, mate guarding and parental care, a possible behavioral change that may occur in response to increased male-biased OSR is a higher frequency of copulation with higher levels of polyandry (Lode et al. 2004; Naud et al. 2004). In this regard, the firefly squid Watasenia scintillans offers a model system suitable for testing this hypothesis because (1) they are semelparous and unlikely to engage courtship and mate guarding due to a considerable length of time gap between copulation and spawning (Hayashi 1995); (2) females are approximately 5% polyandrous (Sato et al. 2020), allowing us to detect any small increase in the rate of polyandry; (3) adult sex ratio changes greatly from male-biased to female-biased (Sato et al. 2020), hence OSR may also change accordingly, during the mating season in the wild; and (4) the level of polyandry can be estimated at a large population scale with a newly developed anatomical method (described in the present study). In addition to this question concerning the possible impact of OSR on monoandry-polyandry decisions, we are intrigued by the highly monoandrous reproductive mode in W. scintillans, because most cephalopods are thought to adopt a polyandrous mating strategy, as suggested by field observations and paternity tests with DNA fingerprinting (Hanlon et al. 1997; Naud et al. 2004, 2016; Iwata et al. 2005; Sato et al. 2014). In the wild population, males mature in advance and await females’ receptivity to mate. Most females copulate once at sexually premature stage during the short mating period of 〜3 weeks and store male-delivered sperm sacs (spermatangia) for the rest of their life for no longer than 3 months (Sato et al. 2020). Microsatellite DNA-based paternity analysis revealed behavioral monogamy (all sperm sacs stored in the female were transferred by a single male), and as the consequence, genetic monoandry (all eggs in the same clutch, though investigated with limited sample size, were fathered by a single male) (Sato et al. 2020). From these data, we speculated two possibilities: (1) polyandry is an alternative common strategy that occurs if certain conditions are met, or (2) polyandry occurs as a consequence of unusual coincidences or pathological actions. However, microsatellite DNA analysis requires considerable effort and, therefore, limits large-quantity analyses, such as population dynamics, which hinders the feasibility to address these queries. Nevertheless, it is of great interest to investigate whether the rate of polyandry in a population can change in response to seasonal dynamics of the OSR.
In firefly squid, the adult sex ratio changes drastically from male-biased to female-biased as the mating season progresses (Sato et al. 2020). Accordingly, at the beginning of the mating season, the OSR is strongly male-biased (males are fully mature while females just begin sexual maturation, (Sato et al. 2020)), and thereafter becomes strongly female-biased because of the massive disappearance of males (presumably by early death). Thus, we assume that male-male competition for mate would be much stronger at the beginning of the mating season than in the later season. If copulation is largely under male control, that is, if mate choice by female is inefficiently operated, the rate of polyandry should be much higher in the early season. Conversely, if the rate of polyandry remains constant at lower levels throughout the season, observing polyandry may be accounted for as accidental and physiologically irrelevant, and the squid sustains a highly monoandrous mating regime with an unknown mechanism by which remating is severely prohibited.
Materials and methods
Specimen collection
The squid specimens (W. scintillans) were purchased from the local fisheries during the fishery season (Jan-May) between 2015 and 2022. Generally, the fisheries catch the squids by using bottom trawls towed near the Oki islands (Shimane prefecture, Japan) and Sakaiminato-port off (Tottori prefecture, Japan), and transported them as dead specimens in ice-cold containers to the marketplaces on the day of fishing. The fresh specimens were kept frozen ( – 20℃) until use. The data collected in 2019 and 2020 were used for the analysis of OSR with a total of 51 sampling days and at least 50 individuals were investigated for basic measurements (sex, mantle length, body weight, testis mass, gonadosomatic index and ovary weight) at each day. Relative ovary weight (OSI) was calculated as 100 x (ovary weight) x (body weight)−1.
Genotyping of female-storing spermatangia using microsatellite DNA markers
Genotyping was carried out as described previously (Sato et al. 2020). We first quantified the number of spermatangia on each seminal receptacle, and the females were grouped according to these numbers. In particular, because females with more than 10 spermatangia are rare, we always analyzed their genotypes. And genotyping was always carried out with the spermatangia collected from only one site of female nuchal pockets (by choosing the pocket with the higher number of spermatangia). In females with 7–9 spermatangia per site, we picked at least 20 females randomly.
Spermatangia were retrieved from the seminal receptacle of mated females. They were placed into a 70% ethanol-filled Petri dish in which they were separated into each spermatangium with fine forceps. Number of spermatangium stored at each location was counted. Each spermatangium was lysed in 50 µl of 50 µg/ml Proteinase K-containing CTAB (100 mM Tris–Cl pH 8.0, 1.4 M NaCl, 20 mM ethylenediaminetetraacetic acid, 2% cetyltrimethylammonium bromide) for 4 h at 52 °C in a 1.5-ml test tube with continuous agitation followed by centrifugation at 14,000 rpm for 10 min at 4 °C. The supernatant was transferred to a fresh tube, followed by genomic DNA extraction with standard phenol/chloroform protocol. Genomic DNA (gDNA) was precipitated using 0.3 M sodium acetate (pH 5.2) and 70% ethanol, washed with 70% ethanol following air-drying, and dissolved in 40 µl milli-Q water. Agarose-gel electrophoresis was done with 0.8% agarose gel for the quantification of gDNA. Genotyping was carried out with microsatellite markers as previously reported (Sato et al. 2020) with some modification. Briefly, polymerase chain reaction (PCR) was carried out using KAPA2G Robust PCR Kit (NIPPON Genetics) with 0.2 μM primers with FAM, Hex, Cy3, and PET-tagged sense oligonucleotides and 100–300 ng gDNA. The PCR condition was 95 °C for 3 min, 30 cycles of 95 °C for 15 s, 60 °C for 15 s and 72 °C for15 sec, followed by 72 oC for 5 min. The fragment length analysis was done using ABI PRISM 3130xl genetic Analyzer with GeneScan™600 LIZ dye size standards. OSIRIS-2.15.1 (National Institute of Health, USA) was used to analyze the peaks obtained.
Calculation of operational sex ratio
Initially, OSR was calculated as the ratio of mature males to females that are ready to mate in a given population. Because all male individuals possess spermatophores in their storage organs at least one week before the onset of the mating period (Supplemental Fig. 1) until their complete disappearance, we regarded them as fully mature and always ready to mate throughout the mating period. In contrast, females had poorly developed ovaries (Supplemental Fig. 1), and the proportion of virgin females gradually declined to zero over the next three weeks. Because mature males and females with or without stored spermatangia cohabitate during this period (as being caught in the same net), we regarded the virgin females as “not being ready to mate”. Therefore, a daily increase in the percentage of non-virgin females represents a substantial increase in the number of females that became ready to mate in the last 24 h.
OSR was measured empirically as the number of males and females prepared to mate (Kvarnemo and Ahnesjo 1996), considering two separated scenarios for female mating regime: polyandry and monoandry. In the polyandry regime, the reproductive status of female individuals was considered to shift from “not being ready to mate” to “being ready to mate” at one time in the reproductive season. In the monoandry regime, the reproductive status of “being ready to mate” was considered to terminate by the first mating, resulting in the status of “not being ready to mate" until the end of the reproductive season. The virgin females collected during the estimated mating period (February 4–March 4) were regarded as “not being ready to mate” because they did not mate despite being together with mature males. Given these conditions, temporal (daily) OSRs during the mating period were calculated as follows:
The daily increase in the proportion of non-virgin females represents the transition rate from “not being ready to mate” to “being ready to mate”. This transition was fitted to a linear regression equation, allowing us to estimate the average transition rate as 3.73%/day in the female population. In addition, the linear decline in the male population during the mating period allowed us to simplify the OSR calculations. Using linear regression equations, we calculated the OSRs.
Results
A correlation between rate of polyandry and the number of spermatangia attached to female
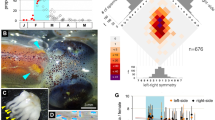
Previously, we found that one exceptional female with extra-large numbers of spermatangia (12 on the left and 13 on the right) was polyandrous, whereas most monoandrous females had an average of 6 spermatangia at each seminal receptacle (SR) (Sato et al. 2020). Hence, we wondered whether the number of attached spermatangia on the female SR may have implications for female multiple mating. Thus, we genotyped every spermatangium in half of a pair of SRs from 141 females. We found that females with ≥ 12 spermatangia on either one of the two SRs within a female were all polyandrous, whereas those with ≤ 7 spermatangia were exclusively monandrous (Fig. 1A). The rate of polyandry increased with an increasing number of spermatangia in females storing between 8 and 11 spermatangia (Fig. 1A). In females with ≥ 8 spermatangia in the SR, the larger the spermatangium number, the more the sire number (Fig. 1B). These results suggest that the probability of polyandrous mating can be estimated based on the number of attached spermatangia.
Spermatangium number correlates with the level of polyandry. A Correlation between the percentage of polyandry and number of spermatangia attached to the seminal receptacle (# spermatangia/site). For each female, we genotyped all spermatangia attached to one of the seminal receptacles, specifically the one containing the higher number of spermatangia. The number of females analyzed was labelled in each plot. B Frequencies of females with different numbers of sires are shown as a function of the number of spermatangia per site
Estimation of operational sex ratio during the mating period
We estimated the rate of increase in mated females in a population and found that when the mating period was narrowed by trimming the ambiguous starting and ending points of the mating period (Fig. 2A; dashed red box), the rate of increase can be approximated using a linear regression model (Linear regression, r2 = 0.857, F1,19 = 102.13, P < 0.0001), resulting in a constant increase of 3.73% per day. In addition, we were able to estimate the sex ratio at any given date based on the data collected in the last three years at the same fishing ground, which revealed a linear decline (Linear regression, r2 = 0.831, F1,35 = 162.62, P < 0.0001) in the male population (1.18%/day) during the mating period (Fig. 2B; dashed red box). Taking these estimations and polyandry/monoandry mating regimes into account (Fig. 2C), we calculated the OSR and found an exponential decline in male-biased OSR over time (Fig. 2D; purple plots). Notably, the OSR shifts from male-biased to female-biased in the middle of the mating period because of a rapid decline in the number of male individuals.
Estimation of operational sex ratio in polyandrous and monoandrous mating regimes. A B Seasonal changes in the percentage of non-virgin females (A) and males (B) are shown with extracted data points (dashed red boxes) for approximate linearization (insets). The data collected in 2019–2022 were combined in the same plots. C Schematic diagram showing transitions of female status under polyandrous and monoandrous mating regimes. In polyandrous mating regime, females gain their receptivity to mate at a certain growth point where female status changes irreversibly from “not being ready to mate” (open box) to “being ready to mate” (grey box). In monoandrous mating regime, females reverse their status from “being ready to mate” (grey box) to “not being ready to mate” (open box) when they mate once (broken orange line with arrow). D, Daily changes in OSR calculated using the method in accordance with either polyandrous (purple plots) or monoandrous (green plots) mating regimes. The inset represents the semilogarithmic scale
Next, we considered their strict monoandrous mating system and tested on an alternative model where females do not engage remating activities after making a copulation with first male, therefore they are regarded to have lost their receptivity to second male’s copulation and thus becoming “not ready to mate” (Fig, 1A; green box). In this scenario, although the male-biased OSR significantly decreased (from 32.7 to 9.1 during the mating period), it remained substantially at high levels (Fig. 1D; green plots).
Estimation of the rate of polyandry during the reproductive season
From these results, we formulated the equation using the spermatangium number-related probability of polyandry. Based on this equation, we estimated the percentage of polyandry of individuals that were caught in the same week of the year using a total of 5,303 females obtained in 2015–2022 (Fig. 3). On a weekly average, the rate of polyandry ranged between 4.06 and 11.72% without any consistent trend of changing patterns throughout the reproductive season (from February to May). The overall rate of polyandry of this species was estimated to be 8.03 ± 2.63%.
Seasonal dynamics of polyandry in W. scintillans. According to the results shown in Fig. 1A, we estimated the percentage polyandry of mated female populations on a weekly basis throughout the entire fishery season. The sample size is shown in each bar. Overall % polyandry (mean ± SEM) is shown on the right (Sum)
Discussion
In sexually reproducing organisms, it has been well documented that females often mate with multiple males (polyandry). Polyandry is also prevalent in cephalopods, with very few exceptions (Nigmatullin et al. 1995; Sato et al. 2020; Murai et al. 2021). One exception is the deep-sea squid W. scintillans (Sato et al. 2020). Our previous analyses with microsatellite DNAs and large-scale, season-wide anatomical investigations estimated that polyandry occurs in only 〜5% of this species (Sato et al. 2020). Because the mating period is assumed to be as short as 3–4 weeks (in February) and males disappear from the fishery grounds after this period, the time constraint for available mate search may be a possible factor affecting remating frequency and motivation. In addition, the relative testis weight of males, the proxy for male promiscuity, is extremely small (Sato et al. 2020), and each male stores only 〜30 spermatophores on average, suggesting that males’ low fecundity may also deter from multiple copulations. Furthermore, females store male-delivered spermatangia with approximately six on average at each pair of seminal receptacles (〜12 spermatophores/female) and are capable of long-term sperm storage until the end of the reproductive season. All these situations are likely to favor a monogamous mating strategy; however, the evolutionary mechanism by which monogamy has been adopted in this species remains unknown.
It has been thought that monogamy evolves if intrinsic mechanisms as well as environmental conditions constrain the pursuit of a second mate (Manning 1962; Wedell 2005; Guevara-Fiore et al. 2009; Ruther et al. 2010; Xochipiltecatl et al. 2021). In the case of firefly squid, mature males that are ready to mate cohabit with females, suggesting that mating decisions depend primarily on females’ receptivity to mate. If so, we assumed that the operational sex ratio (OSR) may be skewed toward males at the beginning of the mating period. It has been reported that one sex-biased OSR may affect many aspects of male mating behaviors, including remating frequency (Pitnick 1993; Markow 2002). Thus, we attempted to measure the exact values of OSR in two separated scenarios; the “polyandrous mating regime” in which females, once mated, continue their receptive status and are continuously ready to mate afterwards, and the “monoandrous mating regime” in which females lose their receptivity to mate once they copulate with a first male. We calculated the OSR according to each scenario and found that the OSR is extremely male-biased in the beginning (in the polyandrous mating regime) or throughout (in the monoandrous mating regime) the mating period (Fig. 1D). We regarded the second scenario of “monoandrous mating regime” to be suitable for this species based on our previous finding that ~ 95% females were behaviorally monoandrous at mating (Sato et al. 2020). In this scenario, the OSR decreased 〜fourfold in the 4-weeks mating period, raising the question of whether this robust change in OSR may affect the propensity to monoandry.
To address this question, we took a new approach to make a precise estimation of population-scale polyandry and its seasonal changes. Previously, we genotyped 272 spermatangia from 19 females using four microsatellite loci (Sato et al. 2020). However, using this approach, it was difficult to determine the population dynamics of polyandry. Therefore, we combined genotyping and morphological analysis based on our new finding that there is a correlation between the number of attached spermatangia and the probability of multiple mating (Fig. 2A). This new approach allowed us to incorporate a large sample size, collected over the last eight years, into the mathematical analysis for polyandry estimation. Consistent with our previous report, the rate of polyandry was extremely low throughout the reproductive season. On weekly based statistics, the rate of polyandry fluctuates from 4.06 to 11.72% (Fig. 2A), which lacks the consistent trends in changing patterns over time. We assume that the rate of polyandry may depend on the precise locality of fishing points in the same fishing field because we occasionally encountered differences in sex ratios in different fishery transports (fishing points) on the same day. Nevertheless, our current data suggest that females persist in monoandrous mating system despite there is a significant change in OSR.
When considering a paradoxical hypothesis, where females tolerate copulations in accordance with concurrently changing OSR in the mating field, they would suffer from extremely frequent copulation opportunities, leading to excessive copulation-associated risks such as predation, infection, and injury (Marian 2012). Consequently, we assume that extremely male-biased OSR conditions do not allow chaste females to remate in favor of a cost–benefit trade-off. In this context, the rare occurrence of polyandrous mating in this species poses a fascinating question regarding the evolution and maintenance of squid mating systems.
In this study, we attempted time-resolved estimations of OSR dynamics by considering the polyandry and monoandry mating regimes as “time-in” and time-out” periods, respectively (Clutton-Brock and Parker 1992). As a result, we found two different decline curves (gradual and exponential) with time in the theoretical disciplines of extremely opposite and fixed mating regimes (Fig. 2C, 2D). These simulations would be of great interest when considering how the temporal OSR dynamics, which also occurs in many other animals, affects the degree of polyandry.
Because no apparent differences in morphology or maturation status were observed between virgin and mated females in a population collected on the same day. (Supplemental Fig. 2), future studies should identify mating signals, such as bioluminescent mating calls (Seidou et al. 1990; Kubodera et al. 2007; Burford and Robison 2020), that can transmit female’s receptivity to their mates.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
29 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00227-023-04216-1
References
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164. https://doi.org/10.1006/anbe.2000.1446
Bateman AJ (1948) Intra-sexual selection in drosophila. Heredity (edinb) 2:349–368. https://doi.org/10.1038/hdy.1948.21
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175:436–446. https://doi.org/10.1086/650727
Burford BP, Robison BH (2020) Bioluminescent backlighting illuminates the complex visual signals of a social squid in the deep sea. Proc Natl Acad Sci U S A 117:8524–8531. https://doi.org/10.1073/pnas.1920875117
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Davies NG, Gardner A (2018) Monogamy promotes altruistic sterility in insect societies. R Soc Open Sci 5:172190. https://doi.org/10.1098/rsos.172190
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, New Jersey
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223. https://doi.org/10.1126/science.327542
Firman RC (2011) Polyandrous females benefit by producing sons that achieve high reproductive success in a competitive environment. Proc Biol Sci 278:2823–2831. https://doi.org/10.1098/rspb.2010.2791
Guevara-Fiore P, Skinner A, Watt PJ (2009) Do male guppies distinguish virgin females from recently mated ones? Anim Behav 77:425–431. https://doi.org/10.1016/j.anbehav.2008.10.018
Hanlon RT, Maxwell MR, Shashar N (1997) Behavioral dynamics that would lead to multiple paternity within egg capsules of the squid Loligo pealei. Biol Bull 193:212–214. https://doi.org/10.1086/BBLv193n2p212
Hayashi S (1995) Fishery biological studies of firefly squid, Watasenia scintillans, (Berry) Toyama Bay. Bull Toyama Pref Fish Res Inst. 7:1–128
Head ML, Lindholm AK, Brooks R (2008) Operational sex ratio and density do not affect directional selection on male sexual ornaments and behavior. Evolution 62:135–144. https://doi.org/10.1111/j.1558-5646.2007.00277.x
Hughes WO, Oldroyd BP, Beekman M, Ratnieks FL (2008) Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320:1213–1216. https://doi.org/10.1126/science.1156108
Iwata Y, Munehara H, Sakurai Y (2005) Dependence of paternity rates on alternative reproductive behaviors in the squid Loligo bleekeri. Mar Ecol Prog Ser 298:219–228
Janicke T, Morrow EH (2018) Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol Lett 21:384–391. https://doi.org/10.1111/ele.12907
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc 75:21–64. https://doi.org/10.1017/s0006323199005423
Klug H, Heuschele J, Jennions MD, Kokko H (2010) The mismeasurement of sexual selection. J Evol Biol 23:447–462. https://doi.org/10.1111/j.1420-9101.2009.01921.x
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948. https://doi.org/10.1111/j.1420-9101.2008.01540.x
Komers PE, Brotherton PN (1997) Female space use is the best predictor of monogamy in mammals. Proc Biol Sci 264:1261–1270. https://doi.org/10.1098/rspb.1997.0174
Kubodera T, Koyama Y, Mori K (2007) Observations of wild hunting behaviour and bioluminescence of a large deep-sea, eight-armed squid, Taningia danae. Proc Biol Sci 274:1029–1034. https://doi.org/10.1098/rspb.2006.0236
Kvarnemo C (2018) Why do some animals mate with one partner rather than many? A review of causes and consequences of monogamy. Biol Rev Camb Philos Soc 93:1795–1812. https://doi.org/10.1111/brv.12421
Kvarnemo C, Ahnesjo I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408. https://doi.org/10.1016/0169-5347(96)10056-2
Lode T, Holveck MJ, Lesbarreres D, Pagano A (2004) Sex-biased predation by polecats influences the mating system of frogs. Proc Biol Sci 271(Suppl 6):S399-401. https://doi.org/10.1098/rsbl.2004.0195
Manning A (1962) A sperm factor affecting the receptivity of drosophila melanogaster females. Nature 194:252–253. https://doi.org/10.1038/194252a0
Marian JEAR (2012) A model to explain spermatophore implantation in cephalopods (Mollusca: Cephalopoda) and a discussion on its evolutionary origins and significance. Biol J Linn Soc 105:711–726. https://doi.org/10.1111/j.1095-8312.2011.01832.x
Markow TA (2002) Perspective: female remating, operational sex ratio, and the arena of sexual selection in drosophila species. Evolution 56:1725–1734. https://doi.org/10.1111/j.0014-3820.2002.tb00186.x
Matsumura K, Miyatake T, Yasui Y (2021) An empirical test of the bet-hedging polyandry hypothesis: female red flour beetles avoid extinction via multiple mating. Ecol Evol 11:5295–5304. https://doi.org/10.1002/ece3.7418
Murai R, Shiomi M, Yoshida M-a, Tomano S, Iwata Y, Sugai K, Hirohashi N (2021) All the spermatangia on a female were implanted by single-pair copulation in giant squid Architeuthis dux. Deep Sea Res Part I Oceanogr Res Pap 175:103585. https://doi.org/10.1016/j.dsr.2021.103585
Naud M-J, Hanlon RT, Hall KC, Shaw PW, Havenhand JN (2004) Behavioural and genetic assessment of reproductive success in a spawning aggregation of the Australian giant cuttlefish, Sepia apama. Anim Behav 67:1043–1050. https://doi.org/10.1016/j.anbehav.2003.10.005
Naud MJ, Sauer WH, McKeown NJ, Shaw PW (2016) Multiple mating, paternity and complex fertilisation patterns in the chokka squid loligo reynaudii. PLoS One 11:e0146995. https://doi.org/10.1371/journal.pone.0146995
Nigmatullin CM, Arkhipkin AI, Sabirov RM (1995) Age, growth and reproductive biology of diamond-shaped squid Thysanoteuthis rhombus (Oegopsida: Thysanoteuthidae). Mar Ecol Prog Ser 124:73–87
Pitnick S (1993) Operational sex ratios and sperm limitation in populations of Drosophila pachea. Behav Ecol Sociobiol 33:383–391. https://doi.org/10.1007/BF00170253
Plesnar-Bielak A, Sychta K, Gaczorek TS, Palka JK, Prus MA, Prokop ZM (2020) Does operational sex ratio influence relative strength of purging selection in males versus females? J Evol Biol 33:80–88. https://doi.org/10.1111/jeb.13547
Reynolds JD (1996) Animal breeding systems. Trends Ecol Evol 11:68–72. https://doi.org/10.1016/0169-5347(96)81045-7
Rosenthal GG, Ryan MJ (2022) Sexual selection and the ascent of women: mate choice research since darwin. Science 375:eabi6308. https://doi.org/10.1126/science.abi6308
Ruther J, Thal K, Blaul B, Steiner S (2010) Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signalling. Anim Behav 80:1035–1040. https://doi.org/10.1016/j.anbehav.2010.09.008
Sato N, Kasugai T, Munehara H (2014) Female pygmy squid cryptically favour small males and fast copulation as observed by removal of spermatangia. Evol Biol 41:221–228. https://doi.org/10.1007/s11692-013-9261-4
Sato N, Tsuda SI, Nur EAM, Sasanami T, Iwata Y, Kusama S, Inamura O, Yoshida MA, Hirohashi N (2020) Rare polyandry and common monogamy in the firefly squid, Watasenia Scintillans. Sci Rep 10:10962. https://doi.org/10.1038/s41598-020-68006-1
Seidou M, Sugahara M, Uchiyama H, Hiraki K, Hamanaka T, Michinomae M, Yoshihara K, Kito Y (1990) On the three visual pigments in the retina of the firefly squid, Watasenia scintillans. J Comp Physiol A 166:769–773. https://doi.org/10.1007/BF00187321
Stockley P (1997) Sexual conflict resulting from adaptations to sperm competition. Trends Ecol Evol 12:154–159. https://doi.org/10.1016/s0169-5347(97)01000-8
Taylor ML, Price TA, Wedell N (2014) Polyandry in nature: a global analysis. Trends Ecol Evol 29:376–383. https://doi.org/10.1016/j.tree.2014.04.005
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual Selection and the Descent of Man, 1871–1971. Aldine, Chicago, IL, pp 136–179
Tumulty J, Morales V, Summers K (2014) The biparental care hypothesis for the evolution of monogamy: experimental evidence in an amphibian. Behav Ecol 25:262–270. https://doi.org/10.1093/beheco/art116
Uller T, Olsson M (2008) Multiple paternity in reptiles: patterns and processes. Mol Ecol 17:2566–2580. https://doi.org/10.1111/j.1365-294X.2008.03772.x
Wedell N (2005) Female receptivity in butterflies and moths. J Exp Biol 208:3433–3440. https://doi.org/10.1242/jeb.01774
Weir LK, Grant JW, Hutchings JA (2011) The influence of operational sex ratio on the intensity of competition for mates. Am Nat 177:167–176. https://doi.org/10.1086/657918
Whiteman EA, Cote IM (2004) Monogamy in marine fishes. Biol Rev Camb Philos Soc 79:351–375. https://doi.org/10.1017/s1464793103006304
Xochipiltecatl D, Baixeras J, Cordero CR (2021) Atypical functioning of female genitalia explains monandry in a butterfly. PeerJ 9:e12499. https://doi.org/10.7717/peerj.12499
Young RL, Ferkin MH, Ockendon-Powell NF, Orr VN, Phelps SM, Pogany A, Richards-Zawacki CL, Summers K, Szekely T, Trainor BC, Urrutia AO, Zachar G, O’Connell LA, Hofmann HA (2019) Conserved transcriptomic profiles underpin monogamy across vertebrates. Proc Natl Acad Sci U S A 116:1331–1336. https://doi.org/10.1073/pnas.1813775116
Acknowledgements
We thank Dr. Osamu Inamura, Mr. Satoshi Kusama and other members of Uozu aquarium for useful information about W. scintillans. We thank anonymous reviewers for their comments. This study was supported by Kakenhi (#21K06333 to N.H.) and the faculty of Life and Environmental Sciences in Shimane Univ. (N.H. and M.N.E.A.)
Funding
Japan Society for the Promotion of Science, #21K06333,Noritaka Hirohashi.
Author information
Authors and Affiliations
Contributions
MNEA performed experiments, analyzed data and wrote the manuscript. NH designed experiments, analyzed data and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
No specific ethical approval was necessary for this study.
Additional information
Responsible Editor: H.J. Hoving.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article Fig. 2D is incorrectly appeared some labels are missing and labels on the Y-scale are displaced.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alam, M.N.E., Hirohashi, N. Persistence of a highly monoandrous mating system despite an extremely male-biased operational sex ratio in the firefly squid Watasenia scintillans. Mar Biol 170, 52 (2023). https://doi.org/10.1007/s00227-023-04204-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04204-5