Abstract
Merobenthic octopods like Octopus vulgaris undergo a transitional period between the planktonic and benthic phases, known as settlement stage. In this work, three sub-stages (“pre-settlement”, “settlement” and “post-settlement”) have been defined based on morphological, anatomical, and behavioural changes. At the end of the planktonic phase advanced paralarvae are transparent with 65–80 chromatophores, iridophores covering eyes and digestive system, Kölliker organs, circular pupils, ~ 20 suckers per arm (spa), and mantle length (ML) bigger than total length (TL; ML/TL > 60%). The “pre-settlement” sub-stage (ML/TL from 65 to 55%, ~ 20–25 spa) is marked by the onset of clinging reflexes, where the pre-settlers touch the walls and bottom of the tank and start crawling clumsily. Morphologically, they are transparent with increased chromatophores along the arms and iridophores around the eyes and head. During the “settlement” sub-stage (~ 55–48% ML/TL, ~ 25–35 spa) there is an exponential increase of chromatophores in the dorsal area of head and mantle, and the settlers show strong negative phototaxis, crawling for shelter when disturbed. The skin is still transparent but new chromatic cells (leucophores) develop and Kölliker organs are almost lost. During the “post-settlement” sub-stage (~ 48–40% ML/TL, > 35 spa) the post-settlers display very fast and coordinated movements, have horizontal pupils, and develop the “eye-bar”. The chromatic cells keep increasing exponentially, giving a pale appearance to the skin. The beginning of the benthic phase in O. vulgaris juveniles is marked by the presence of skin sculptural components (papillae) and a complex display of chromatic, postural, and cryptic patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transition from paralarva to juvenile in cephalopods does not involve a proper metamorphosis as found in many other marine invertebrates (Boletzky 1974). The changes occurring are subtle and concern the variation in growth rates, body proportions or the structure and function of specific organs (Robin et al. 2014). However, these changes are markedly different depending on the mode of life of the post-embryonic stages within the family Octopodidae: holobenthic and merobenthic. The holobenthic strategy requires the production of few large eggs resulting in well-developed, benthic, adult-like hatchlings that share the same habitat with adults. Octopodid species producing eggs > 10–12% of adult mantle length fall within this category (Boletzky 2003). These hatchlings are considered as juveniles since they are large, resemble the adults and actively use their arms as a mode of locomotion and food search (Villanueva and Norman 2008). The merobenthic strategy, on the other hand, corresponds to the production of numerous small eggs that hatch into swimming planktonic hatchlings, with low survival rates. Only octopodids producing eggs smaller than 8–10% of adult mantle length are merobenthic (Boletzky 2003). The hatchlings are not adult-like, characterized by short arms, a swimming behaviour based in jet propulsion and translucent bodies with few chromatophores in the ventral zone, called paralarvae. This merobenthic strategy is represented by up to 70 species within the family Octopodidae (see Table 1, Villanueva and Norman 2008), and it is considered as the ancestral state in benthic octopuses (Ibáñez et al. 2014).
The term paralarva was defined as “a cephalopod of the first post-hatching growth stage that is pelagic in near-surface waters during the day and that has a distinctively different mode of life from that of older conspecific individuals” by Young and Harman (1988). Under this definition, only the merobenthic species will have paralarvae, and therefore only in this group of octopods would be possible to identify, characterize and quantify the changes that occur during the settlement stage, a complex transition between the planktonic and the benthic phases. Merobenthic species do have profound changes that have been previously considered as true metamorphosis (Packard 1985), and one of these remarkable changes concerns the skin. The skin of planktonic paralarvae is almost transparent with only 65–80 mesodermal black pigment-filled chromatophores, or founder chromatophores, of which eight appear under the dorsal mantle covering the digestive gland (Packard 1985). During settlement, chromatophore number increases exponentially and new chromatic cells (iridocytes and leucophores) develop specially in the dorsal area, which will help them for camouflaging on the seafloor (Messenger 2001).
Mantle length is nearly double the arm length at the planktonic phase, but this ratio clearly diminishes during settlement owed to positive allometric arm growth (Villanueva 1995). When arms attain the length of the mantle, the advanced paralarvae switch to bottom life progressively contacting the substratum (Villanueva 1995). There are no skin sculptural components, apart from the Kölliker organs, in planktonic paralarvae (Nixon and Mangold 1996; Villanueva et al. 2021), which are common features in benthic juveniles, essential for camouflage and communication (Messenger 2001). Other morphological changes associated with settlement include the loss of Kölliker organs, the loss of the “lateral line system” and the loss of the oral denticles of the beaks (Villanueva and Norman 2008; Franco-Santos et al. 2014). Before settlement, the planktonic octopodids swift their swimming existence with a new mode of locomotion that requires the coordinated action of the arms and suckers: crawling. The onset of this behaviour was defined as a “pre-settlement reflex” by Villanueva (1995) or “clinging” by Dan et al. (2021). Late paralarvae of O. sinensis in captivity showed diurnal clinging and nocturnal swimming, likely related with minimizing the exposure to visual predators (Dan et al. 2021).
Despite being one of the main transitions in merobenthic octopus life cycle, the settlement stage has been scarcely addressed owed to two main facts: the technical difficulties in finding these organisms on the field and the lack of standardized protocols for rearing the paralarvae in captivity until settlement. The only works describing some morphological changes occurring in advanced paralarvae and early juveniles of O. vulgaris collected in the wild correspond to those of Rees (1950), Packard (1985), Nixon and Mangold (1996), and Roura et al. (2019), while for Octopus sinensis d´Orbigny, 1834 Sakaguchi et al. (1999) and Dan et al. (2021, 2022) are the only works. The growth of Octopus “vulgaris” during the planktonic phase and after settlement shown by Nixon and Mangold (1996) was based on the data obtained by Itami et al. (1963) in Japan. However, it is important to point out that the octopod cultivated by Itami and collaborators was not O. vulgaris, but O. sinensis (Gleadall 2016). This western Pacific octopod is raised at higher temperatures from 23 to 26.7 ºC and has a shorter planktonic phase < 25 days (Itami et al. 1963; Okumura et al. 2005; Dan et al. 2021) than O. vulgaris that is raised at 21–23 ºC and starts settling after 45 days (Villanueva 1995; Iglesias et al. 2004; Carrasco et al. 2006).
Few works have succeeded producing benthic juveniles from planktonic paralarvae in captivity (O. vulgaris: Villanueva 1995; Iglesias et al. 2004; Carrasco et al. 2006; O. sinensis: Itami et al. 1963; Okumura et al. 2005; Dan et al. 2021; Robsonella fontaniana (d'Orbigny, 1834): Uriarte et al. 2010). Of these, Uriarte and collaborators studied the morphological changes in R. fontaniana for up to 120 days during the planktonic and benthic phases, while Dan et al. (2021) focussed on the behavioural changes in swimming, clinging and shelter use during the onset of the settlement phase in O. sinensis from 10 to 28 days old; as well as the morphological changes during the first 100 days after hatching (Dan et al. 2022). Works made in captivity with O. vulgaris have gone through the settlement to adulthood (Iglesias et al. 2004; Carrasco et al. 2006; De Wolf et al. 2011), but no reference to the changes occurring during this transitional stage were described apart from indicating the pre-settlement reflexes found in the late paralarvae (Iglesias et al. 2004; Carrasco et al. 2006). The only work that described morphological and behavioural changes during the settlement stage stopped at 60 days (Villanueva 1995), evidencing that this transitional period is a complex process with numerous and profound changes that needs to be addressed in detail.
In this work we studied two generations of O. vulgaris reared in captivity, describing for the first time the major changes undergone between the planktonic and benthic phases, during the settlement stage. Three different sub-stages were defined based on the main morphological, behavioural and ecological changes observed during this transition from the pelagic to the benthic mode of life.
Methods
Two generations of O. vulgaris juveniles were analysed in this study. The first generation (experiment #1) was obtained from mature egg strings of a wild female that was monitored with scuba diving for two months in the Ría de Vigo (NW Spain). Paralarvae obtained from these eggs were raised in the laboratory and the second generation (experiment #2) was obtained from the surviving animals of experiment #1. In detail, six well-developed egg strings (stage XX, Deryckere et al. 2020) were transported to the Instituto de Investigaciones Marinas de Vigo (IIM-CSIC) in five L recipients with natural sea water in dark conditions. Transport lasted two hours and the eggs were maintained in a flow-through tank (20 × 20 × 30 cm) filled with seawater at 18 ºC and salinity 36. Egg strings were tied from sections of plastic pipe and immersed in the tank with enough horizontal current to keep the egg strings moving gently. After five days paralarvae hatched and the experiment #1 begun.
The paralarvae (DML = 2.44 ± 0.04, n = 10) were reared at a density of five paralarvae/L in 50 L dark green cylindrical fibre tanks provided with filtered seawater (1 µm) and a central outlet with 500-µm mesh size. An open water system with 150% renovation per day was used with mean water temperature 19 ºC (18.1–20.5), salinity 35.4 (34.8–36.2) and a 14:10 h light cycle provided with LED lights. The bottom of the tanks was syphoned every day. Live diet consisted of sub-adult Artemia salina (1–3 mm TL) at a concentration of 0.1–0.05 ind/mL, cultivated at 25 ºC with a phytoplankton mix. During settlement adult A. salina was offered as prey together with small fragments of frozen food. After settlement frozen food was offered daily, until around five months when animals were fed five times a week. The food rations were calculated as 20% of the octopus biomass estimated for each tank. At day 90 juveniles were placed in three glass tanks of 30 L at a density of 0.5 ind/L in an open water system with 200% renovation per day, mean water temperature 18 ºC (17.8–18.3), salinity 35.7 (34.8–36.2) and a 14:10 h light cycle provided with LED lights. The bottom of the tanks was syphoned every day and prey remains were removed and weighted. Tanks were transparent with black plastic covering the sides, to allow recording the behaviours displayed during the settlement phase.
At day 153, the surviving specimens from experiment #1 were transported in 30 L tanks to the facilities of Estación de Ciencias Marinas de Toralla (ECIMAT, Universidad de VIGO). Subadults and adults were maintained in the laboratory in 400 L fibreglass tanks, at a density of 5–10 kg/m3, with an open water system with 400% renovation per day at 18 ºC. One refuge per octopus—40 cm long PVC pipes 35 cm in diameter—was placed inside the tanks. The tanks were cleaned every day, and food remains weighted daily. Once the females from experiment #1 started laying eggs (around 15 months) they were isolated in individual 400 L tanks with 400% renovation and 18 ºC. The PVC pipes were used as dens, were females spawned for 15–25 days between May and June 2019. Egg strings were attached by the females on the upper side of the pipe, constantly cleaned, and oxygenated by gentle water jets from the female’s syphon. After 56 days the paralarvae started to hatch (DML = 2.41 ± 0.06, n = 10) and a new culture experiment (experiment #2 or second generation) was settled in similar conditions to the first one but reducing paralarval density to four specimens/L and temperature to 18 ºC. For the settlement stage in experiment #2, late paralarvae/early juveniles were placed in six grey tanks of 100 L in an open water system with 200% renovation per day, mean water temperature 18 ºC (17.8–18.3), salinity 35.7 (34.8–36.2) and 14:10 h light cycle provided with LED lights. The bottom of the tanks was syphoned every day, prey remains removed and weighted.
Meristic data was obtained in both experiments to the nearest 0.05 mm using a stereomicroscope (Nikon SMZ800) coupled to an image analysis software (NIS-Elements) from fresh and ethanol preserved paralarvae/juveniles: mantle length (ML) / total length (TL) ratio, as well as number of suckers per arm. A percentage of shrinkage in mantle length (9.74%) caused by fixation was considered for those individuals stored in 70% ethanol (Villanueva 1995). Fresh weights were obtained to the nearest 0.01 g with a portable scale in alive animals that were collected with a plastic pipette, weighted in a glass and then returned to the tanks from day 45 onwards. All the individuals were weighted at the fourth month in both experiments. An interocular area in the head (defined by the 6 dorsal founder chromatophores above and between the eyes) was used as a proxy to quantify chromatophore genesis during settlement and visualized with a regression between the number of chromatophores and suckers per arm (spa). Regressions on number of suckers per arm and ML/TL during the first 120 days were used to identify the different settlement sub-stages. Body changes (like the chromatogenesis, leucophore observation, horizontalization of the pupil, appearance of dermal sculpturing or papillae) were photographed and recorded throughout the settlement phase. Cephalopod life cycles are divided into several phases (embryonic, paralarval, juvenile, sub-adult and adult), which are markedly different from each other in terms of morphology, ethology or habitat and separated by distinct transitions (Vidal, EAG personal communication). These phases can be, in turn, divided into stages, like the embryonic phase in O. vulgaris that is divided in 20 different stages (Deryckere et al. 2020).
This work was carried in two different projects (AQUOPUS and OCTOBLUE) with the aim to increase the survival and enhance the zootechnical aspects of the culture of O. vulgaris in captivity and, therefore, experiments do not fall under Directive 2010/63/EU of the European Parliament and of the Council, of 22 September 2010 on the protection of animals used for scientific purposes. Nonetheless, the experimental procedures were supervised and approved by an ethics committee at the different institutions (IIM for AQUOPUS and University of Vigo for OCTOBLUE projects). The authors minimized the number of animals euthanized and maximized the sampling of animals that were moribund and appropriate methods for anaesthesia and euthanasia were used. Specimens were anaesthetised by immersion in a 1.5% ethanol-seawater solution at room temperature (18 ºC) for one minute. Afterwards, the specimens were immersed in 2% MgCl2 at 8 ºC for 5 min and finally immersed in 3.5% MgCl2 filtered seawater solution at 8 ºC for 10 min as a way of euthanasia (Fiorito et al. 2015).
Results
Octopus paralarvae turn into fully benthic juveniles in less than three months at a temperature of 18–19 ºC. The paralarvae hatch with three uniserial suckers per arm and start to add suckers from day 6 onwards in two rows at the terminal tip of the arm in a proximodistal direction. Figure 1 shows the incorporation of suckers and the decrease in ML/TL ratio during the first four months of life in O. vulgaris. During the planktonic phase, (Fig. 2a) the arms of the paralarvae start to grow and the ML/TL decreases from an initial 75 to < 60%, a figure that can be used as a proxy to mark the end of the planktonic phase, which is around day 45 when the paralarvae have ~ 20 suckers per arm (ranging from 10 to 27).
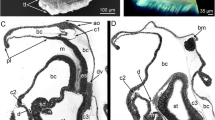
Planktonic phase and settlement stage in Octopus vulgaris. a Planktonic paralarva 22 days-old showing dermal iridescence produced by the bristles of the Kölliker organs (inside of the circle) scattered through the surface of the skin on mantle and arms. b Pre-settler 48 days-old with new iridophores along the ventral side of the arms and head (arrows) displaying iridescence. c Ventral view at the arms of a 62 days-old O. vulgaris settler during the settlement or “hiding” sub-stage, showing the reduced number of Kölliker organs embedded in the skin (arrows inside of the box). d The same specimen as in c, showing the onset of the chromatogenesis on the dorsal area of the head and mantle and the eyelid
At this moment, the paralarvae have a cuttlefish-like appearance, swimming with the arms pointing downwards. Late paralarvae (~ 35‒45 days old) swim with very precise movements, shifting directions with ease, and stopping effectively without impacting the tank walls. When they are illuminated from outside of the tank the numerous iridocytes developing in the skin reflect the light giving a silvery/copper look to the paralarvae (Fig. 2b, arrows).
The shift from the planktonic environment of the paralarvae to the benthic mode of life of the juveniles takes around 30–45 days, which is known as the settlement stage. Three different sub-stages can be distinguished during this stage based on behavioural, anatomic, and morphologic changes: the pre-settlement or “tactile” (~ 45–60 days), the settlement or “hiding” (~ 60–75 days) and the post-settlement or “ninja” sub-stages (~ 75–90 days) (Table 1, Fig. 3).
Chromatophore genesis in Octopus vulgaris paralarvae during the settlement stage, which is divided in three different sub-stages, as indicated at the top of the graph. An area defined by the position of 6 founder chromatophores between the eyes in the dorsal side of the head (blue polygon in pre-settlement sub-stage) was used to count the new chromatophores arising in the spaces between extant ones
During the pre-settlement or “tactile” sub-stage (~ 45–60 days), the transparent pre-settlers begin to switch between swimming and crawling, attaching to the bottom and walls of the tank in a clumsy way, i.e. with non-coordinated movements of the arms. During this sub-stage numerous attacks were observed in which the pre-settlers captured the brine shrimp against the tank wall and then returned to swim to feed on them. Similarly, when the pre-settlers were disturbed while attached to any surface, they returned swimming displaying a very intense red colour, rather than hiding or crawling. Numerous pre-settlers were also observed during this stage with the arms attached to the water–air interface. Morphologically, ML/TL ratio varied from 65 to 55% and they have ~ 20–25 suckers per arm (Fig. 1, Table 1). The number of chromatophores has increased along the arms (Fig. 2b), which start filled with yellow pigments that gradually become darker (melanophores). There are no new chromatophores in the dorsal area of the head, apart from the founder chromatophores (Fig. 3). The fresh weight of pre-settlers ranged between 80 and 120 mg and the ML ranged from 4.8 to 5.7 mm (Table 1).
The settlement or “hiding” sub-stage (~ 60–75 days) can be identified by the reclusive behaviour of the settlers within the refuges provided. At this point they displayed better arm coordination, using them to crawl and hide inside of the shelters. When disturbed outside of the shelter they do not swim but crawl and use jet scape (only going backwards) looking for dark zones of the tank. When disturbed in the shelter they direct water fluxes to the origin of disturbance and change from transparent to dark red (Fig. 4a). Most of the time they are inside of the shelters (Fig. 4b), except at night when they cautiously look for food nearby. At this point, the length of the arms equals that of the mantle (~ 55–48% ML/TL), and they have ~ 25–35 suckers per arm (Table 1, Figs. 1 and 2c, d). Chromatophore genesis starts to speed up in the dorsal area of the whole body during this stage that can be observed by the exponential increase in chromatophores in the dorsal area of the head (Fig. 3). The musculature and skin are transparent and Kölliker organs can still be observed imbibed in the skin (Figs. 2c, d). During this sub-stage, a loss of fresh weight was observed, ranging from 70 to 150 mg, and the ML recorded ranged from 5.7 to 6.5 mm.
Morphological changes in Octopus vulgaris from the settlement sub-stage to the benthic phase. a 69 days-old settler with 32 spa and the dorsal chromatophores expanded giving an intense red look just before hiding under the shelter. b 80 days-old post-settler with 37 spa hiding in a bivalve shell, showing the horizontal pupil and the chromatophores on the eyelid. c and d 92 day-old benthic juveniles with 41 and 44 spa, respectively, showing the papillae above the eye, with the horizontal pupil and the “eye-bar”. The musculature is no longer transparent, and the skin is pale owed to the development of leucophores. At this point, they are capable of camouflaging
The post-settlement or “ninja” sub-stage (~ 75–90 days) can be identified from a behavioural point of view by the highly coordinated movements of the post-settlers that can move in any direction with very fast and precise movements. This sub-stage is marked by a profuse production of chromatophores (Fig. 3), iridocytes and leucophores that gives the early juveniles a faint pale look. The increased number of chromatophores at the end of this sub-stage allows them to start displaying basic camouflage. Despite the pupil is circular during the planktonic phase and pre-settlement sub-stage, from ~ 80 days (> 35 suckers per arm, ~ 48–40% ML/TL) the pupil starts to develop a horizontal pupillary response, an adaptation to a benthic mode of life (Fig. 4b). Another feature is the pigmentation of the eyelid, which is responsible for the creation of the “eye-bar” (Fig. 4b), but at this sub-stage, it does not cover the full length of the eye as in benthic juveniles (Fig. 4c). The post-settlers are very voracious and skilled hunters from now on, able to catch prey larger than themselves. A sharp increase in weight was observed during this sub-stage with specimens ranging from 140 to 240 mg fresh weight.
Finally, in the benthic phase, the juveniles keep developing pigmented cells and are fully capable of camouflaging. They also develop the neural control of the skin musculature to create skin sculptural components like ocular papillae (appendicular-shaped skin protrusion) and dorsal papillae (Fig. 4c). From a behavioural point of view, the juveniles display two main cryptic postures when moving outside of the shelter. The most common during the first month of benthic life is a compressed posture that resembles a “stone” (first observed at day 88), where the juveniles have all the arms packed against the body and use the 4th pair of arms to move in short pulses (Fig. 5). This body pattern is very similar to the “zebra crouch” described in Packard and Sanders (1971), but without the colour transverse stripes (chevron pattern). The other pattern, less common than the “stone”, is the flamboyant posture (sensu Packard and Sanders 1971) that resembles a detached “alga” (Fig. 5). This body pattern was observed for the first time at day 92 when the juveniles collected for sampling were returned into the tank. They adopt this deceiving body pattern to swim through the water column until they reach the bottom, where the display is maintained for a while, usually turning into “stones” to look for shelter. They start to do the “cleaning manoeuvre” (first day observed 93 days-old) and seem to interact with other conspecifics with “passing clouds”, changing the colour unilaterally and doing sucker displays (both behaviours defined in Packard and Sanders 1971). Octopus juveniles are voracious predators that accept and ingest unfrozen food with ease.
Schematic diagram showing the major changes occurring during the transition from the planktonic to the benthic phases in Octopus vulgaris and the three different sub-stages (in bold) that comprise the settlement stage: pre-settlement (tactile), settlement (hiding) and post-settlement (ninja) sub-stages. Increase of number of suckers per arm (blue line) and ML/TL ratio (green line) from planktonic paralarvae to benthic juveniles reared in captivity through the first four months of life. The shadows surrounding the lines represent the standard deviation for each variable. The main changes are represented as colour arrows whose direction represent the first or last time these were detected (like the Kölliker organs)
In the first year of experiments, the average fresh weight of the surviving juveniles was 2710 ± 680 mg at day 120 (1690–6120 mg; n = 8). However, in the second year an increased dispersion was observed in the weights sampled at day 118 (average 380 mg, 100–2600 mg, n = 81).
Discussion
In this work, we have shown that settlement represents the first stage of the benthic phase since the pre-settlers start their transition to the benthos. As such, it marks the transition between the planktonic and benthic environments and the end of the true planktonic phase (Table 1, Fig. 5). Furthermore, we have defined for the first time three distinct sub-stages within the settlement stage and their associated morphological, anatomical, and behavioural adaptations. The duration of the different phases (planktonic and benthic) and the three sub-stages defined for the settlement stage (pre-settlement, settlement, and post-settlement) described herein are the result of the specific rearing conditions followed in this study and are by no means fixed values in terms of days. It is expected that paralarvae reared at higher temperatures will have shorter planktonic phases and start the settlement earlier, since somatic growth is largely increased with higher temperatures during the planktonic phase, when cephalopods grow exponentially (Forsythe 2004). Villanueva (1995) obtained settled specimens of O. vulgaris from day 47–54 at 21.2 ºC (from 19 to 23 ºC), Iglesias et al. (2004) from day 40 at 22.5 ºC (from 19.6 to 22.9 ºC), and Carrasco et al. (2006) observed the first pre-settlement reflexes from day 40 at 21.2 ºC, and by day 52 they were observed crawling (from 19.3º to 22.6 ºC). Settled O. vulgaris had between 5.7 and 7.5 mm ML (Villanueva 1995; Carrasco et al. 2006), which agrees with the post-settlers defined in this work (Table 1), despite the fact of being cultured at lower temperatures. The onset of the tactile response or “clinging” behaviour in Octopus sinensis pre-settlers start at smaller sizes (5.2–6 mg dry weight, Dan et al. 2020; 16 suckers per arm, Dan et al. 2021), but are settled at similar sizes (5.7–7 mm ML and 21–27 suckers per arm, Itami et al. 1963). In the present work, we did not observe day/night differences during the pre-settlement sub-stage as it was observed with O. sinensis in captivity, where pre-settlers alternate diurnal clinging/crawling with nocturnal swimming. This behaviour was observed until 12.2–15 mg dry weight (~ 85–105 mg fresh weight, considering that dry weight is around seven times lower), when the settlers stop swimming and exhibit strong negative phototaxis and reclusive behaviour, both characteristics of the settlement sub-stage defined herein.
Fresh weight at the pre-settlement sub-stage was the best descriptor to predict survival through the settlement stage. Fresh weight is easy to sample, it does not require anaesthesia, and minimise the handling stress caused to the octopus. Counting suckers and measuring ML in alive individuals requires anaesthesia and may not be accurate because the tip of the arms is coiled and get attached to the crystal, thus complicating the sampling and increasing the handling stress. Our observations suggest that animals > 110–120 mg are better prepared for surviving the major changes occurring during settlement, irrespective of age. Octopuses that display pre-settlement reflexes at weights < 100 mg have fewer chances to survive the settlement stage, where they need to hunt in the bottom. This new hunting field, completely different from the water column, requires a new set of skills that involves the development of certain brain lobes to coordinate the movements of arms and suckers (Nixon and Mangold 1996). Until these neural networks are developed, the pre-settlers are not very skilled benthic hunters and are too heavy to swim and so, the energy expenditure is high. This resulted in ~ 10–15% weight loss during the pre-settlement and settlement sub-stages, which coincides with the observations made by Villanueva (1995) where growth in length and weight slowed during the pre-settlement sub-stage and fell to their minimum rate during settlement.
A non-growth phase has been observed in juveniles of Octopus maya Voss & Solís, 1966 during the first 10 days post-hatching (Moguel et al. 2010). Despite being an holobenthic species, early O. maya go through a transition period characterized by changes in morphology, physiology and behaviour named “post-hatching” stage where they adapt to the benthic environment / juvenile phase. This post-hatching stage would be somehow equivalent to the pre-settlement sub-stage defined in this work, since O. maya hatchlings show necto-benthic behaviour and the arms are proportionally shorter than the mantle (equivalent to ML/TL < 50% shown in this study). Holobenthic species like O. bimaculoides Pickford and McConnaughey 1949 and O. maya have juveniles that hatch with 70–121 mg (Forsythe and Hanlon 1988; Ibarra-García et al. 2018; Briceño et al. 2010), which is very similar to the fresh weights recorded for the pre-settlers during the pre-settlement sub-stage (80–120 mg). The variability in fresh weight recorded at day 120 in this study, apart from being a consequence of the different number of specimens analysed, can be attributed to the rearing conditions, since the first experiment was carried at 19 ºC and the second at 18 ºC. Temperature could be the main factor driving these differences in growth (Forsythe 2004), as well as the tiny differences of size at hatching (Pecl et al. 2004), since small differences in initial weight are amplified through time in the same way for all individuals, (i.e. despite growing at the same rate, Briceño et al. 2010). The intrinsic variability observed in size-at-age data reflects individuals experiencing different conditions (like food availability, temperature variations), different metabolisms and or owed to their different abilities to hunt or process the ingested food (Forsythe and Hanlon 1988).
Obvious morphological changes during growth are characterised by discontinuities in relative growth that highlight crucial limits in stages of development, and the first discontinuity seems to coincide with the transition from paralarva to juvenile (Young and Harman 1988). These changes in growth have been documented in squid families including changes in body proportions in the Cranchiidae (Voss 1980) or in merobenthic octopods like Octopus sinensis (Dan et al. 2022), where two different phases were described between the planktonic and benthic phases in O. sinensis settlement based on morphological measurements (total length, mantle length, arm length, number of suckers per arm and body dry weight). At this point, it is important to remember that we used the terms “phase” and “stage” differently from previous authors, which have used these two terms indistinctly. The term phase corresponds to the periods that divide the life cycle of a cephalopod, which are markedly different from each other in terms of morphology, ethology or habitat and separated by distinct transitions (Vidal, EAG personal communication). Most cephalopod life cycles can be divided in the following five phases: embryonic, paralarval (not present in holobenthic octopods), juvenile, sub-adult and adult. These phases can be divided into stages, as it occurs with the embryonic phase of O. vulgaris (Deryckere et al. 2020), or with the juvenile phase, whose settlement stage is divided in three different sub-stages.
Settlement stage: a transition between planktonic and benthic lifestyles
A wide range of marine invertebrates have a merobenthic lifecycle that includes planktonic larval and benthic adult phases. The transition between these morphologically and ecologically distinct phases is known as metamorphosis and typically occurs when the competent larva detects an environmental cue via species-specific sensory system (Jackson et al. 2002). The morphogenetic cues are species-specific and range from food sources, microbial films, conspecifics, or particular benthic substrata (reviewed in Zimmer and Butman 2000). This ability to discriminate and respond to signals associated with different benthic substrata apparently ensures that larvae settle in habitats suitable for juvenile growth and survival. The transition from the planktonic to the benthic lifestyle in merobenthic cephalopods is a complex process rarely seen in the wild driven by poorly understood factors. Although the factors that may be acting during the settlement stage are difficult to evaluate and far from the aim of this work, it is likely that certain chemical cues present in the benthos may act as a trigger inducing the changes described during the settlement (Table 1, Fig. 5). Such contact with an inductive environmental cue would occur during the pre-settlement sub-stage, when O. vulgaris pre-settlers start to get in contact with the benthos, before transitioning from the pelagic to the benthic habitats. What is apparent is that the functional changes in morphology, anatomy and behaviour required to transform transparent planktonic paralarvae into fully benthic juveniles with coloured skin designed for camouflaging and cryptic concealment are very complex.
The morphological changes include the development of a very complex skin with innumerable chromatic components (chromatophores, iridophores/reflector cells and leucophores), associated epithelial musculature and motoneurons to control them all (Packard 1985). When O. vulgaris settles, the rate of chromatophore genesis into the empty dorsal mantle field rapidly overtakes the rate of recruitment into the ventral that results in higher densities of smaller chromatophores dorsally than ventrally. Packard (1985) mentioned that “the dorsal spurt in chromatophore genesis at the end of the planktonic phase is so dramatic as to hint at something like metamorphosis. It is as if the skin were waiting for its owner to settle on the sea floor before bringing out the fine-grain dress that is going to serve for the rest of its life and replace the coarse-grain set of extrategumental spots (on the surface of the viscera) that served during the transparent planktonic phase”.
The anatomical changes include the distal growth of the arms and the incorporation of new suckers. The patterns of ontogenic allometry suggest that during the first 20 days, growth is concentrated in the mantle area of the paralarvae and later shifts to the arms (Villanueva 1995), resulting in a decrease of the ML/TL ratio (Figs. 1 and 5). Naef (1923) reported Kölliker organs on a benthic juvenile measuring 10 mm ML collected in the Bay of Naples. Some authors suggest that the loss of Kölliker organs might be involved in the appearance of the first dermal papillae and buoyancy (Naef 1923), others that they may form the generative centres of the patches in the skin (Packard and Hochberg 1977), and more recently, that everted Kölliker organs in paralarvae may increase drag and improve passive buoyancy and their birefringent properties may be of help for camouflage during daytime (Villanueva et al. 2021). From our experience, we agree that these organs are adaptations for the pelagic environment, which may help in reducing the sinking rate. There are far more Kölliker organs covering the skin of the planktonic paralarvae (Fig. 2a) than dermal papillae in juveniles and adults. Moreover, as the paralarvae start the settlement stage the number of Kölliker organs clearly diminishes and the few ones left are embedded by the new skin (as shown in Fig. 2c), and totally disappear from the skin of the benthic juveniles. This also suggest that these organs may not be involved in the creation of papillae or the patches in the skin, but more studies are needed to contrast it.
In the intersection between morphological/anatomical and behavioural changes is the development of the nervous system, both central and peripherical, responsible for the new set of skills (e.g. crawling, camouflaging and body patterning) that “appear” throughout the settlement stage. Skin patterning results from neural control of radial muscles that expand the pigment sac of each chromatophore (Hanlon 1999). At the pre-settlement and settlement sub-stages, there is no specific patterning, and they can only shift from transparent to dark red (Fig. 4a), as it occurs during the planktonic phase. Specific patterning like the eye-bar (Fig. 4b, c) results from the selective neural excitation of groups of chromatophores, and it happens from the post-settlement sub-stage into the benthic phase, when the skin has developed numerous chromatophores and their corresponding nervous connections. Concurrently, the new musculature developing in the skin enables settled octopuses to create texture in the skin, like the ocular or dorsal papillae (Fig. 4c). These new sets of skills are reflected in the development of specific areas of the brain like the brachial, vertical, subvertical, subfrontal and optical lobes (Nixon and Mangold 1996). Furthermore, the brachial lobes are also involved in the control of the new suckers and their new functions like prey search, olfaction, and tactile responses (Nixon and Mangold 1996). The development of the brachial lobes is responsible for the coordinated movements needed for crawling during the post-settlement sub-stage that is characterized by “ninja” movements, which are very fast and precise movements that require complex neural control. Complicated body patterns of juvenile octopus like the “alga” or the “stone” (Fig. 5) result from the interaction of chromatic, textural, postural and locomotor responses that seem very basic at the end of the post-settlement sub-stage and become very precise at the beginning of the benthic phase.
Summarizing, the settlement stage in O. vulgaris does not imply a major modification of the body plan like in other molluscs (e.g. gastropods, bivalves), but requires profound modifications at morphological, anatomical and behavioural levels. The settlement stage can be divided in three different sub-stages based on the modifications shown in Table 1, which prepare the young octopods to live in a new environment and for a benthic lifestyle. Further research is needed to determine the environmental cues that drive this pelago-benthic transition, as well as the genetic and physiological basis of the changes observed.
Data availability
All the data are shown in the manuscript.
References
Boletzky SV (1974) The larvae of cephalopoda: a review. Thalass Jugosl 10(1/2):45–76
Boletzky SV (2003) Biology of early life stages in cephalopod molluscs. Adv Mar Biol 44:143–203
Briceño F, Mascaró M, Rosas C (2010) GLMM-based modelling of growth in juvenile Octopus maya siblings: does growth depend on initial size? ICES J Mar Sci 67(7):1509–1516. https://doi.org/10.1093/icesjms/fsq038
Carrasco JF, Arronte JC, Rodríguez C (2006) Paralarval rearing of the common octopus, Octopus vulgaris (Cuvier). Aquac Res 37:1601–1605. https://doi.org/10.1111/j.1365-2109.2006.01594.x
Dan S, Takasugi A, Iwasaki H, Shibasaki S, Oka M, Hamasaki K (2020) Ontogenic change in the vertical swimming of East Asian common octopus Octopus sinensis paralarvae under different water flow conditions. Aquat Ecol 54:795–881. https://doi.org/10.1007/s10452-020-09777-7
Dan S, Shibasaki S, Takasugi A, Takeshima S, Yamazaki H, Ito A, Hamasaki K (2021) Changes in behavioural patterns from swimming to clinging, shelter utilization and prey preference of East Asian common octopus Octopus sinensis during the settlement process under laboratory conditions. J Exp Mar Biol Ecol 539:151537. https://doi.org/10.1016/j.jembe.2021.151537
Dan S, Kamei Y, Takeshima S, Yamashita K, Hamasaki K (2022) Stepwise changes in morphology during the settlement process in a merobenthic octopus, Octopus sinensis, raised in the laboratory. Invertebr Biol 141(1):1–11. https://doi.org/10.1111/ivb.12358
De Wolf T, Lenzi S, Lenzi F (2011) Paralarval rearing of Octopus vulgaris (Cuvier) in Tuscany, Italy. Aquac Res 42:1406–1414. https://doi.org/10.1111/j.1365-2109.2010.02756.x
Deryckere A, Styfhals R, Vidal EAG, Almansa E, Seuntjens E (2020) A practical staging atlas to study embryonic development of Octopus vulgaris under controlled laboratory conditions. BMC Dev Biol 20:7. https://doi.org/10.1186/s12861-020-00212-6
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’Angelo L, Ludovic D et al (2015) Guidelines for the care and welfare of cephalopods in research—a consensus based on an initiative by CephRes, FELASA and the Boyd group. Lab Anim 49(2 Suppl):1–90. https://doi.org/10.1177/0023677215580006
Forsythe JW (2004) Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice. Mar Freshw Res 55:331–339
Forsythe JW, Hanlon RT (1988) Effect of temperature on laboratory growth, reproduction and life span of Octopus bimaculoides. Mar Biol 98:369–379
Franco-Santos RM, Iglesias J, Domingues PM, Vidal EAG (2014) Early beak development in Argonauta nodosa and Octopus vulgaris (Cephalopoda: Incirrata) paralarvae suggests adaptation to different feeding mechanisms. Hydrobiologia 725(1):69–83. https://doi.org/10.1007/s10750-013-1721-4
Gleadall IG (2016) Octopus sinensis d’Orbigny, 1841 (Cephalopoda: Octopodidae): valid species name for the commercially valuable East Asian common octopus. Spec Div 21(1):31–42. https://doi.org/10.12782/sd.21.1.031
Hanlon RT (1999) Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on Indo-Pacific coral reefs, with a method of quantifying crypsis from video tapes. Biol J Linn Soc 66(1):1–22. https://doi.org/10.1006/bijl.1998.0264
Ibáñez CM, Peña F, Pardo-Gandarillas MC, Méndez MA, Hernández CE, Poulin E (2014) Evolution of development type in benthic octopuses: holobenthic or pelago-benthic ancestor? Hydrobiologia 725(1):205–214. https://doi.org/10.1007/s10750-013-1518-5
Ibarra-García LE, Mazón-Suástegui JM, Rosas C, Tovar-Ramírez D, Bárcenas-Pazos G, Civera-Cerecedo R, Campa-Córdova AI (2018) Morphological and physiological changes of Octopus bimaculoides: from embryo to juvenile. Aquaculture 497:364–372. https://doi.org/10.1016/j.aquaculture.2018.07.069
Iglesias J, Otero JJ, Moxica C, Fuentes L, Sánchez FJ (2004) The completed life cycle of the octopus (Octopus vulgaris, Cuvier) under culture conditions: paralarval rearing using Artemia and zoeae, and first data on juvenile growth up to 8 months of age. Aquac Int 12:481–487
Itami K, Izawa Y, Maeda S, Nakai K (1963) Notes on the laboratory culture of Octopus larvae. Bull Jpn Soc Sci Res 29(6):514–520
Jackson D, Leys SP, Hinman VF, Woods R, Lavin MF, Degnan BM (2002) Ecological regulation of development: induction of marine invertebrate metamorphosis. Int J Dev Biol 46:679–686
Messenger JB (2001) Cephalopod chromatophores: neurobiology and natural history. Biol Rev 76(4):473–528
Moguel C, Mascaró M, Avila-Poveda OH, Caamal-Monsreal C, Sánchez A, Pascual C, Rosas C (2010) Morphological, physiological and behavioral changes during post-hatching development of Octopus maya (Mollusca: Cephalopoda) with special focus on the digestive system. Aq Bio 9(1):35–48. https://doi.org/10.3354/ab00234
Naef A (1923) Die cephalopoden. Systematik Fauna Flora Golfes Neapel 35:1–863
Nixon M, Mangold K (1996) The early life of Octopus vulgaris (Cephalopoda: Octopodidae) in the plankton and at settlement: a change in lifestyle. J Zool 239(2):301–327
Okumura S, Kurihara A, Iwamoto A, Takeuchi T (2005) Improved survival and growth in Octopus vulgaris paralarvae by feeding large type Artemia and pacific sand eel, Ammodytes personatus: Improved survival and growth of common octopus paralarvae. Aquaculture 244(1–4):147–215. https://doi.org/10.1016/j.aquaculture.2004.11.044
Packard A (1985) Sizes and distribution of chromatophores during post-embryonic development in cephalopods. Vie Et Milieu 35(3/4):285–298
Packard A, Hochberg FR (1977) Skin patterning in Octopus vulgaris and other genera. In: Nixon M, Messenger JB (eds) The biology of cephalopods, Symposia of the zoological society of London, vol 38. Springer, pp 191–231
Packard A, Sanders GD (1971) Body patterns of Octopus vulgaris and maturation of the response to disturbance. Anim Behav 19(4):780–790. https://doi.org/10.1016/S0003-3472(71)80181-1
Pecl GT, Steer MA, Hodgson KE (2004) The role of hatchling size in generating the intrinsic size-at-age variability of cephalopods: extending the forsythe hypothesis. Mar Freshw Res 55:387–394. https://doi.org/10.1071/MF03153
Rees WJ (1950) The distribution of Octopus vulgaris Lamarck in British waters. J Mar Biol Assoc United Kingdom 29(2):361–378. https://doi.org/10.1017/S0025315400055417
Robin JP, Roberts M, Zeidberg L, Bloor I, Rodriguez A, Briceño F, Downey N, Mascaró M, Navarro M, Guerra A, Hofmeister J, Barcellos DD, Lourenço SAP, Roper CFE, Moltschaniwskyj NA, Green CP, Mather J (2014) Transitions during cephalopod life history: the role of habitat, environment, functional morphology and behaviour. Adv Mar Biol 67:361–437. https://doi.org/10.1016/B978-0-12-800287-2.00004-4
Roura Á, Amor M, González ÁF, Guerra Á, Barton ED, Strugnell JM (2019) Oceanographic processes shape genetic signatures of planktonic cephalopod paralarvae in two upwelling regions. Progr Oceanogr 170:11–27. https://doi.org/10.1016/j.pocean.2018.10.005
Sakaguchi H, Hamano T, Nakazono A (1999) Occurrence of planktonic juveniles of Octopus vulgaris in Eastern lyo-Nada of the Seto lnland Sea. Japan Bull Jpn Soc Fish Oceanogr 63(4):181–187
Uriarte I, Hernández J, Dorner J, Paschke K, Farias A, Crovetto E, Rosas C (2010) Rearing and growth of the octopus Robsonella fontaniana (Cephalopoda: Octopodidae) from planktonic hatchlings to benthic juveniles. Biol Bull 218(2):200–210. https://doi.org/10.1086/BBLv218n2p200
Villanueva R (1995) Experimental rearing and growth of planktonic Octopus vulgaris from hatching to settlement. Can J Fish Aquat 52(12):2639–2650
Villanueva R, Norman MD (2008) Biology of the planktonic stages of benthic octopuses. Oceanogr Mar Biol 46:105–202
Villanueva R, Coll-Lladó M, Bonnaud-Ponticelli L, Carrasco SA, Escolar O, Fernández-Álvarez F et al (2021) Born with bristles: new insights on the Kölliker’s organs of octopus skin. Front Mar Sci 8:1–19. https://doi.org/10.3389/fmars.2021.645738
Voss NA (1980) A generic revision of the Cranchiidae (Cephalopoda: Oegopsida). Bull Mar Sci 30:365–412
Young RE, Harman RF (1988) Larva, paralarva and subadult in cephalopod terminology. Malacologia 29(1):201–207
Zimmer RK, Butman CA (2000) Chemical signalling processes in the marine environment. Biol Bull 198(2):168–187
Acknowledgements
We thank Dr. Ángel González for scientific assistance during both projects and the technical assistance of Lourdes Nieto, Rubén Chamorro and Xoán Martínez from IIM-CSIC. We are grateful to Damián Costas, Noelia Costoya, Arantxa Martínez, Victoriano Álvarez, Micael Vidal, Alberto García, Roberto Gómez, Enrique Poza, Rosana Rodríguez, Sergio González, Juán José Rodríguez, María Pérez and Alberto Álvarez for their technical assistance at ECIMAT. We would also like to thank Dr. Jesús Troncoso (Universidad de Vigo) and Dr. Pablo Sánchez (Universidad de Alicante). Special thanks to José Irisarri and Jade Irisarri for their underwater support. We thank the fruitful comments of Dr. Erica Vidal and two anonymous reviewers.
Funding
This study was financed by Armadora Pereira S.A. and CDTI funding to develop the projects AQUOPUS (IDI-20170704, 2017–2019) and OCTOBLUE (IDI-20190479, 2019–2021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AR and AC. The first draft of the manuscript was written by AR and all authors commented on posterior versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical standards
This study was financed by Armadora Pereira S.A. and CDTI funding to develop the projects AQUOPUS (IDI-20170704, 2017–2019, Instituto de Investigaciones Marinas de Vigo, IIM-CSIC) and OCTOBLUE (IDI-20190479, 2019–2021, ECIMAT, Universidad de Vigo). This work was carried with the aim to increase the survival and enhance the zootechnical aspects of the culture of O. vulgaris in captivity and, therefore, experiments do not fall under Directive 2010/63/EU of the European Parliament and of the Council, of 22 September 2010 on the protection of animals used for scientific purposes. Nonetheless, the experimental procedures (culture density, prey density, water quality, tank designs) were supervised and approved by an ethics committee at the different institutions (IIM for AQUOPUS and University of Vigo for OCTOBLUE projects).
Additional information
Responsible Editor: E.A.G. Vidal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roura, A., Castro-Bugallo, A. & Martínez-Pérez, M. The settlement stage in the common octopus Octopus vulgaris Cuvier, 1797: a complex transition between planktonic and benthic lifestyles. Mar Biol 170, 53 (2023). https://doi.org/10.1007/s00227-023-04188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04188-2