Abstract
Anthropogenic climate change has increased the frequency and intensity of extreme weather events, such as marine heatwaves (MHW). They exert a strong influence over the structure and functioning of marine ecosystems, considering temperature is one of the most critical environmental factors affecting marine life. Additionally, intertidal habitats are ecologically challenging ecosystems, and inhabiting organisms need to possess the necessary mechanisms to adapt to the periodic fluctuations in physical characteristics across tidal cycles. To assess the effect of simulated MHWs (categories I and IV) and low tide conditions (emersion) on the early development of the common cuttlefish Sepia officinalis, the development time and hatching success were evaluated, as well as the antioxidant enzymatic machinery, lipid peroxidation, HSP70 and total ubiquitin concentrations. Embryonic development time decreased significantly with temperature, but tidal emersion held no significant impact on development time or hatching success. Superoxide dismutase activity levels were significantly increased with temperature but lowered under emersion conditions. Glutathione-S-transferase activity significantly increased with temperature, while glutathione peroxidase activity was significantly enhanced under emersion. Catalase activity, lipid peroxidation, HSP70 content and total ubiquitin content were not affected by any of the treatments. These findings suggest that while development time is greatly conditioned by temperature, S. officinalis embryos are remarkably resilient to emersion conditions. Moreover, the simulated marine heatwaves did not elicit any sub-lethal oxidative stress-related effects, suggesting that such temperatures were still within the optimum range of the cuttlefish thermal window of aerobic performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past centuries, human-induced climate change has affected ecosystems worldwide, with significant implications for their services (Chiabai et al. 2018; Gulev et al. 2021; Redlin and Gries 2021). Anthropogenic greenhouse gas forcing, in particular, has been deemed the main factor influencing the increase of seawater surface temperatures (SSTs), with future SSTs projected to increase by up to 5.7 °C above pre-industrial values by the end of the century (Eyring et al. 2021; Gulev et al. 2021; Lee et al. 2021). Along with long-term warming, the frequency and intensity of extreme temperature events have been increasing as a byproduct of anthropogenic climate change (Perkins et al. 2012). Marine heatwaves (MHWs) are an example of these climatic extremes, defined as prolonged discrete warm water events characterized by their duration, intensity and spatial extent (Hobday et al. 2016, 2018). MHWs strongly influence marine ecosystems by affecting the development time, metabolism, survival and reproduction of organisms, and as a consequence of rising greenhouse gases emissions, the intensity and duration of such events have increased by 54% since the last century, being expected to continue to rise in the future (Oliver et al. 2018, 2019).
Alongside, intertidal environments represent some of the most challenging environments in the coastal marine systems, subject to rapid and periodic fluctuations in physical characteristics across tidal cycles (Helmuth et al. 2006; Teixeira et al. 2013). Low tides expose resident organisms to thermal stress, aerial emersion and desiccation, all of which influence their physiological state, growth and reproduction (Aguilera and Rautenberger 2011; Freire et al. 2011). As such, shallow water and intertidal organisms must display the necessary adaptive strategies to successfully thrive in such dynamic habitats (Teixeira et al. 2013). Two relevant mechanisms involved in dealing with these adversities are the heat shock response (HSR) and the anti-oxidative system (Dong et al. 2008; Teixeira et al. 2013; Axenov-Gribanov et al. 2014; Hawkins et al. 2016). The HSR prevents the unfolding of protein structures as a result of temperature fluctuations, hypoxia or excessive irradiation, and is characterized by the synthesis of heat shock proteins (HSPs) (Tomanek 2010; Yamashita et al. 2010; Logan and Somero 2011). The anti-oxidative system includes a group of antioxidant enzymes that act towards the detoxification of reactive oxygen species (ROS), and oxygen derivates that are overproduced under the environmental fluctuations typical of intertidal conditions (Lesser 2011). Toxic effects of ROS are indicative of oxidative stress and range from deleterious effects on proteins and DNA to a mechanism of cellular injury termed lipid peroxidation (LPO), which targets membrane-associated lipids and is quantified by assessing the levels of the terminal product malondialdehyde (MDA) (Abele and Puntarulo 2004; Lesser 2006; Bardaweel et al. 2018; Ighodaro and Akinloye 2018). The enzymes acting against ROS include superoxide dismutase (SOD), which converts the superoxide radical O2− into hydrogen peroxide (H2O2); catalase (CAT) and glutathione peroxidase (GPx), which prevent the accumulation of H2O2 in cells; and glutathione-S-transferase (GST), which deactivates secondary metabolites originating from oxidative stress (Abele and Puntarulo 2004; Lesser 2006; Belcheva and Chelomin 2011; Teixeira et al. 2013; Rosa et al. 2014, 2016; Ighodaro and Akinloye 2018).
The common cuttlefish (Sepia officinalis) is an ecologically and commercially important cephalopod species (Wang et al. 2003; Jereb and Roper, 2005; Jereb et al 2015). It is a semelparous organism characterized as having an active nekton-benthic lifestyle and hatchlings with direct development (Guerra 2006). S. officinalis is predominantly found on sandy and muddy substrates of the continental shelf, from the shoreline (2–3 m depth) to depths of approximately 200 m (Jereb and Roper, 2005; Guerra 2006; Jereb et al 2015). Previous studies have shown that S. officinalis possesses remarkable abiotic tolerance, not only to variations in salinity but also environmental fluctuations in ocean O2 partial pressure (pO2) and CO2 partial pressure (pCO2) (Feely et al. 2008; Melzner et al. 2013; Rosa et al. 2013; Hu 2016; Moura et al. 2019). Consequently, it has a wide distributional range, covering the North West coast of Africa, the Mediterranean Sea and the Eastern North Atlantic (Guerra 2006). The temperature range of S. officinalis spans from 10 to 30 °C, which is a key factor in mediating the reproductive processes of the species, as individuals undergo seasonal migrations from deeper to shallower waters where mating and spawning occur (Reid et al. 1998; Guerra 2006; Pierce et al. 2010; Bonnaud et al. 2013). Eggs are laid at depths rarely deeper than 40 m, and embryos develop within egg capsules, which act as protection against desiccation and as a barrier to gases diffusion (Boletzky 1986; Reid et al. 1998; Bonnaud et al. 2013).
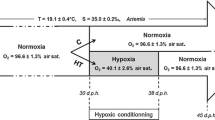
To the best of our knowledge, there are no studies addressing the biological effects of realistic MHW conditions on S. officinalis early ontogeny. This study aims to fill this knowledge gap and to bring a novel insight about our understanding on how cephalopod early stages are affected by tidal emersion—an abiotic characteristic of the shallow waters where cuttlefish spawning can take place (Fig. 1).
Methods
Egg collection and laboratory acclimation
During early October 2020, recently spawned egg clusters of common cuttlefish (S. officinalis) were collected during low tide in the Sado estuary (west coast of Portugal; 38°29′18.42″N; 8°53′15.12″W; Fig. 1). Subsequently, eggs (N = 720) were transported (under controlled conditions) to Laboratório Marítimo da Guia (MARE-ULisboa, Cascais, Portugal). Upon arrival, eggs from different clutches were evenly distributed across eighteen rectangular-shaped experimental tanks (n = 40 per tank), to control for the developmental stage. Three semi-open life support systems (LSS, 92.4 L total volume each) housed 6 experimental tanks (5L volume) each, being supplied with mechanically filtered (down to 5 µm) and UV-sterilized natural seawater (NSW). Photoperiod was maintained according to prevailing natural conditions (15 h/9 h, light/dark cycle), via T8 LED overhead illumination. The LSS were equipped with biological (ouriço®, Fernando Ribeiro Lda, Portugal) and mechanical (protein skimmer Reef SkimPro, TMC Iberia, Portugal) filtration. Water temperature was independently controlled by a custom-made digital STC-3000 temperature-controlling system. Upon demand, temperature was increased through submerged digital thermostats (V2Therm, 200 W, TMC Iberia, Portugal) or decreased using seawater chillers (Hailea, HC-250A, Guangdong, China). Temperature, pH, salinity and dissolved oxygen (DO) were monitored daily, using a portable pH/mV meter (pHenomenal® pH 1100 H, VWR), a digital refractometer (HI98319, Hanna Instruments) and a portable DO meter (DO 220 M, VWR), respectively. Total ammonia, nitrite and nitrate levels were monitored daily and kept below detectable levels. Laboratory acclimation was performed during a period of 8 days, so that the eggs are exposed to experimental conditions for at least two-thirds of their embryogenesis.
Experimental exposure
After the acclimation period, the eggs were randomly distributed across six experimental treatments (two treatments per LSS, each treatment comprising three replicates each; Supplemental Figure 1):
-
(i)
Control I (Control temperature, Immersion);
-
(ii)
Control E (Control temperature, Periodic Emersion);
-
(iii)
HW1I (MHW category I scenario, Immersion);
-
(iv)
MHW1E (MHW category I scenario, Periodic Emersion);
-
(v)
MHW4I (MHW category IV scenario, Immersion) and
-
(vi)
MHW4E (MHW category IV scenario, Periodic Emersion).
A thirty-year (1989–2019) dataset for SST in Sado estuary (Setúbal, Portugal) was acquired from the program Climate Data Record (CDR) of the National Oceanic and Atmospheric Administration (NOAA) (Banzon et al. 2016). The R package heatwaveR (Schlegel & Smit 2018) was used to determine the average duration (12 days), the maximum temperatures registered (19.7 and 23.8 °C, corresponding to a MHW categories I and IV, respectively), and the climatology (control) values (average of 18.2 °C). This package calculates and displays MHWs according to the definition by Hobday et al. (2018), wherein a moderate MHW (category I) occurs as SSTs surpass the 90th percentile compared with local historical climatology, and an extreme MHW (category IV) occurs as SSTs exceed four times that threshold (the respective projections for the different experimental treatments are shown in Fig. 2).
For the immersion scenarios (n = 3, Control I, MHW1I and MHW4I), replicate tanks were left submerged throughout the entire experimental period. Regarding the periodic emersion scenarios (n = 3, Control E, MHW1E and MHW4E) exposure to simulated high/low tide environment was performed via periodic oscillations (two high-low tide cycles per day) in seawater level, accomplished through an automatic control system (Profilux 3.1 N, GHL, Kaiserslautern, Germany) connected to height submerged pumps, which created and controlled high/low tide conditions on a daily basis. The emersion period followed a semi-diurnal tide cycle, characteristic of the Portuguese coast, which emerges cuttlefish during three hours, twice a day. LSS specifications and abiotic control were performed as previously described (i.e., laboratory acclimation). The eggs were exposed to experimental conditions for 30 days. Upon hatching, development time from collection of the eggs from the estuary was registered, and hatching success was calculated as the number of live hatchlings divided by the total number of fertilized eggs.
Biochemical analyses
Sample preparation
After the 30-day exposure, cuttlefish were collected upon hatching (Control I, n = 89; Control E, n = 97; MHW1 I, n = 94; MHW1 E, n = 94; MHW4 I, n = 101; MHW4 E, n = 90), transferred to 2-mL Eppendorfs, instantly frozen at − 80 °C and stored for biochemical analyses. Hatchling samples were homogenized in 3 mL of phosphate-buffered saline solution (PBS) (pH 7.4) (140 mM NaCl, 3 mM KCl, 10 mM disodium phosphate (Na2HPO4), 2 mM potassium phosphate (KH2PO4)), using an electrical tissue grinder (Warsley, Techn Germany). The homogenates were divided by two 1.5 mL microtubes, all of which were centrifuged (15 min at 10,000×g, at 4 °C) (VWR, Hitachi Koki Co., Ltd). Subsequently, the supernatant was transferred to 1.5 mL microtubes and frozen – (20 °C) until further analyses. Enzymatic activity and protein concentration were measured according to the supernatant fraction of the samples. In subsequent assays, each sample was run in duplicate.
Total protein content
To measure the concentration of total protein per sample, the method described by Bradford (1976) was used and optimized for 96-well microplates (Greiner Bio-One, Germany). A calibration curve was built using bovine serum albumin (BSA) (Sigma-Aldrich, USA) as a standard. The stock solution (4.0 mg/mL) was prepared in PBS and then serial dilutions were performed to obtain a standard curve (0–4.0 mg/mL). To carry out the assay, 20 µL of protein standard or sample were added to 180 µL of Bradford reagent in each well of 96-well microplates (Greiner Bio-One, Austria). The absorbance at 595 nm was recorded using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). The results were used to express the analyzed biomarkers per mg of total protein.
Glutathione S-transferase (GST) activity
GST activity was determined according to the method described by Habig et al. (1974) and adapted for 96-well microplates (Greiner Bio-One, Germany). In this assay, 1-chloro-2,4-dinitrobenzene (CDNB) was used as substrate, and enzymatic activity was measured according to the formation of a conjugate between CDNB and reduced L-glutathione (GSH). A reagent mix solution was prepared, consisting of 20 mM GSH (Sigma-Aldrich, USA), 100 mM CDNB solution (Sigma-Aldrich, USA) and 9.8 mL of PBS. To perform the assay, 180 µL of the substrate solution were added to each well of a 96-well microplate (Greiner Bio-One, Austria), followed by adding 20 µL of GST standard or sample. The absorbance at 340 nm was recorded every minute, for 6 min, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). The variation in absorbance per minute (ΔA340/min) was determined for each sample, and the reaction rate was determined using the CDNB molar extinction coefficient of 0.0053 µM−1 cm−1.
Glutathione peroxidase (GPx) activity
GPx activity was determined according to the method described by Lawrence and Burk (1976) and adapted for 96-well microplates (Greiner Bio-One, Germany). An assay buffer [50 mM KH2PO4 buffer (pH 7.4) (Sigma-Aldrich); 5 mM ethylenediaminetetraacetic acid (EDTA) (Riedel-Haën)] and a co-substrate mixture [4 mM sodium azide (NaN3) (Sigma-Aldrich); 1 mM beta nicotinamide adenine dinucleotide phosphate (β-NADPH) (Sigma-Aldrich); 4 U/mL glutathione reductase (Sigma-Aldrich, USA); 4 mM GSH (Sigma-Aldrich, USA)] were prepared. To perform the assay, assay buffer (120 µL) was added to each well, followed by 50 µL of the co-substrate mixture and 20 µL of sample or standard. To start the reaction, 20 µL of a cumene hydroperoxide solution (Sigma-Aldrich, USA) was added to each well, and the microplates were lightly shaken. The absorbance (340 nm) was read at 25 °C, for 6 min, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). Variations in absorbance per minute (ΔA340/min) were estimated for each sample. To determine GPx activity, the molar extinction coefficient (3.73 mM−1 cm−1) for β-NADPH was used.
Superoxide dismutase (SOD) activity
SOD activity was determined according to the procedure by Sun et al. (1988), adapted for 96-well microplates. To perform the assay, 200 µL of buffer (50 mM KH2PO4 buffer (pH 8.0), 10 µL of 3 mM EDTA (Riedel-Haën)), 10 µL of 3 mM xanthine solution (Sigma-Aldrich), 10 µL of 0.75 mM nitroblue tetrazolium (NBT) (Sigma-Aldrich) and 10 µL of sample were added to each well of a 96-well microplate (Greiner, Bio-One, Austria). Next, 10 µL of 100 mU xanthine-oxidase (XOD) was added to each well to initiate the reaction. The absorbance at 550 nm was read at 25 °C, for 15 min, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). Variations in absorbance per minute (ΔA550/min) were estimated for each sample, and the enzyme inhibition percentage (normalized by total protein) was determined.
Catalase (CAT) activity
To determine CAT activity, the method followed Beers and Sizer (1952), adapted for 96-well microplates. A solution of 50 mM KH2PO4 buffer containing H2O2 [0.0036% (w/w)] (Sigma-Aldrich, USA) was prepared. To perform the assay, 7 µL of sample and 193 µL of the buffer-H2O2 solution were added to each well of a 96-well microplate (Greiner, Bio-One UV-Star, Austria). The absorbance at 240 nm was read at 25 °C, at 42-s intervals, for a total of 2 min and 48 s, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). Variations in absorbance per minute (ΔA240/min) were estimated for each sample. To determine CAT activity, the molar extinction coefficient for H2O2 of 0.04 µM−1 cm−1 was used.
Lipid peroxidation (LPO)
LPO was measured by quantifying MDA concentration, following the method described by Ohkawa et al. (1979) (Pannunzio and Storey 1998) and adapted to 96-well microplate (Greiner, Bio-One, Austria). To perform the assay, 5 µL of sample were added to each microtube, followed by 45 µL of PBS, 12.5 µL of 8.1% sodium dodecyl sulfate (SDS) (Merck), 93.5 µL of 20% trichloroacetic acid (TCA) (Panreac), 93.5 µL of 1% thiobarbituric acid (TBA) (Sigma-Aldrich, USA) and 50.5 µL of milli-Q water. The microtubes were vortexed for 1 min, and the caps were pierced. Each microtube was incubated (100 °C) for 10 min, using a digital thermoblock (D130 Series, Labnet International). After the incubation, the microtubes were briefly placed on ice to cool. An additional 62.5 µL of milli-Q water was added to each microtube. Subsequently, 150 µL (in duplicate) was added to microplate wells. The absorbance was read (530 nm) using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). To quantify lipid peroxidation in samples, a 1 µM MDA stock solution (Merck) was used to construct a calibration curve (0–0.1 µM).
Heat shock protein (HSP70) content
HSP70 content was determined through a method of indirect Enzyme-Linked Immunosorbent Assay (ELISA), as described by Njemini et al. (2005). To carry out the assay, 100 µL of standard or sample was added to each well of a 96-well microplate (Greiner, Bio-One, Microlon, High Binding Austria), and the microplates were left to incubate overnight at 4 °C. After the incubation, the microplates were washed three times with PBS-Tween-20 (PanReac AppliChem, ITW Reagents). After preparing a 1% BSA blocking solution in PBS, 200 µL of it was added to each well, and an incubation of 2 h at room temperature took place. The microplates were washed as mentioned previously. The primary antibody solution (anti-HSP70, Acris Antibodies) (0.5 µg/mL) was prepared by performing a dilution in PBS with BSA (1%). Then, 100 µL of the primary antibody solution was added to each well, after which the microplates were left to incubate for 90 min at 37 °C. The microplates were washed again, and 100 µL of a conjugated secondary antibody solution (anti-mouse IgG, Fc specific, alkaline phosphatase-conjugated, Sigma-Aldrich, USA) were added to each well after the proper dilution (1:1000) of the solution in BSA (1%). The microplates were, once again, incubated for 90 min at 37 °C and washed afterwards. Following the previous step, 100 µL of alkaline phosphatase substrate solution (100 mM NaCl (Panreac, Spain), 100 mM Tris–HCl (Sigma-Aldrich, USA), 50 mM MgCl2 (Sigma-Aldrich, USA), 27 mM p-nitrophenyl phosphate, disodium salt hexahydrate (PnPP) (pH 8.5) (Sigma-Aldrich, USA)) were added to each microplate well, after which they were left to incubate for 30 min at room temperature. This step was followed by the addition of 100 µL of stop solution (3 M NaOH) (Panreac, Spain) to each well. The absorbance was read, at 405 nm, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). To determine the HSP70 concentration in each sample, a calibration curve (0–2 µg/mL) was prepared.
Total ubiquitin (Ub) content
Ubiquitin content was determined by an indirect ELISA, as described by Pegado et al. (2020). To perform the assay, 100 µL of standard or sample was added to each well of a 96-well microplate (Greiner, Bio-One, Microlon, High Binding Austria). Microplates were left to incubate overnight at 4 °C, after which they were washed three times with PBS-Tween-20 (PanReac AppliChem, ITW Reagents). Following the previous step, 200 µL of a 1% BSA blocking solution prepared in PBS was added to each well, and an incubation of 2 h at room temperature took place. Afterwards, microplates were washed as mentioned previously. A primary antibody solution (Ub antibody (P4D1): sc-8017, Santa Cruz Biotechnology) (0.5 µg/mL) was prepared by performing a dilution in PBS with BSA(1%). Next, 100 µL of the primary antibody solution was added to each microplate well, after which they were left to incubate for 90 min at 37 °C. The microplates were washed again, and 100 µL of a conjugated secondary antibody solution (anti-mouse IgG, Fc specific, alkaline phosphatase-conjugated, Sigma-Aldrich, USA) was added to each well after diluting (1:1000) the solution in BSA (1%). The microplates were incubated for 90 min at 37 °C and washed once again as described previously. Then, 100 µL of alkaline phosphatase substrate solution (100 mM NaCl (Panreac, Spain), 100 mM Tris–HCl (Sigma-Aldrich, USA), 50 mM MgCl2 (Sigma-Aldrich, USA), 27 mM PnPP (pH 8.5) (Sigma-Aldrich, USA)) were added to each well, and the microplates were left to incubate for 30 min at room temperature. This step was followed by the addition of 100 µL of stop solution (3 M NaOH) (Panreac, Spain) to each well. The absorbance was measured, at 405 nm, using a microplate reader (BioTek Synergy HTX multi-mode reader, USA). To quantify total ubiquitin content per sample, a calibration curve (0–0.8 µg/mL) was prepared using standard purified ubiquitin (Ubiquitin–Proteasome Biotechnologies).
Statistical analyses
To infer the influence of treatments on both stress-related biomarkers and development time, linear mixed-models (glmmTMB, from the package ‘glmmTMB’) (LMM) with a Gaussian distribution were employed. Treatments were set as a factor with 6 levels, and replicates as a random factor with 18 levels (3 replicates per treatment). Type II Wald chi-square tests were performed on every model, to evaluate the effect of the different treatments on all response variables. Model residuals were analyzed for assumptions of normality and homoscedasticity. Outliers consisting of values superior to 10 times the value of the upper quartile were removed. Significance levels were set at p < 0.05, and p-values were adjusted via Tukey corrections. An analysis was performed to determine the effects of emersion and MHWs on survival, and a Cox proportional hazards regression model (‘coxph’, from the package ‘survival’) was fitted to successful hatchings (0/1) and embryonic development time. Temperature was set as a three-level factor and emersion as a two-level factor (co-variates). The assumptions of proportional hazards of the ‘coxph’ models (proportional hazards, no over-influential observations and linearity of co-variates) were not held (Schoenfeld test, represents scaled Schoenfeld residuals over time). Subsequently, a ‘survdiff’ function was fitted, followed by post hoc multiple comparisons (‘pairwise_survdiff’). Statistical analyses were done in RStudio (version 2021.09.1).
Results
Development time and survival
Cuttlefish exhibited a significantly shorter embryonic development under increased temperatures (Control vs. MHW1 condition, LMM d.f. = 555, t ratio = 4.138, p = 0.0001; Control vs. MHW4 condition, LMM t ratio = 11.033, p < 0.0001). On the other hand, tidal-related treatments had no significant effect on development time (Wald chi-squared test, d.f. = 1, χ2 = 3.15, p = 0.0762) (Fig. 3; Supplemental Table 1). Hatching success significantly increased in the MHW1 (Cox Model, p = 0.0078) and MHW4 (p < 0.0001) when compared to control conditions (Fig. 3). Hatching success increased under immersion, but an opposite trend was observed under emersion. However, tidal emersion had no significant effect on hatching success (Cox Model, p = 0.2).
Development time of cuttlefish (Sepia officinalis) embryos under the different treatments (relative to the day of the first hatching) (A), and cumulative proportions of hatched eggs under different marine heatwave (MHW) (B) and immersion (C) treatments. Boxplots illustrate the upper and lower quartile, and medians are displayed by an inside bar. Outliers outside the inter-quartile range are displayed by black dots, individual observations are displayed through gray dots. MHW I marine heat wave category I, MHW IV marine heat wave category IV, E emersion, I immersion
Biochemical responses
SOD activity levels increased significantly from the control to the MHW4 conditions (LMM, d.f. = 519, t ratio = − 2.438, p = 0.04), and were observed to significantly decrease when exposed to tidal emersion (Wald chi-squared test, d.f. = 1, χ2 = 9.56, p = 0.0020) (Fig. 4). CAT activity levels showed no significant differences among temperature and tidal-related treatments (Wald chi-squared test, d.f. =2, χ2 = 0.62, p = 0.7325 and d.f. =1, χ2 = 0.01, p = 0.9345, respectively). There were no significant differences in GPx activity levels among MHW treatments (Wald chi-squared test, d.f. = 2, χ2 = 1.66, p = 0.4367). However, they were significantly increased by tidal-related treatments (Wald chi-squared test, d.f. =1, χ2 = 3.99, p = 0.046). There was a significant increase in the activity of GSTs under MHW1 when compared to Control (LMM, d.f. = 550, t ratio = − 3.261, p = 0.0149) and MHW4 (t ratio = 5.907, p < 0.0001). Yet, no significant changes were observed among tidal-related treatments (Wald chi-squared test, d.f. = 1, χ2 = 1.05, p = 0.3058).
SOD (A), CAT (B), GPx (C) and GST (D) activity levels in cuttlefish (Sepia officinalis) embryos under the different treatments. Boxplots illustrate the upper and lower quartile, and medians are displayed by an inside bar. Outliers outside the inter-quartile range are displayed by black dots, individual observations are displayed through gray dots. MHW I marine heat wave category I, MHW IV marine heat wave category IV, E emersion, I immersion
LPO levels in S. officinalis embryos, expressed as MDA concentration, are shown in Fig. 5. No significant variations in LPO were observed among all treatments (Wald chi-squared test, d.f. = 2, χ2 = 2.31, p = 0.3144 and d.f. = 1, χ2 = 0.00, p = 0.9807 for MHW and tidal-related treatments, respectively). HSP70 levels revealed no significant differences under the different MHW scenarios and tidal-related treatments (Wald chi-squared test, d.f. = 2, χ2 = 0.04, p = 0.9794 and d.f. = 1, χ2 = 1.15, p = 0.2837, respectively). A similar trend was observed in Ub levels (Wald chi-squared test, d.f. = 2, χ2 = 2.49, p = 0.2877 and d.f. = 1, χ2 = 0.77, p = 0.3787, respectively).
MDA (A), HSP70 (B) and Ub (C) levels in cuttlefish (Sepia officinalis) embryos under the different treatments. Boxplots illustrate the upper and lower quartile, and medians are displayed by an inside bar. Outliers outside the inter-quartile range are displayed by black dots, individual observations are displayed through gray dots. MHW I marine heat wave category I, MHW IV marine heat wave category IV, E emersion, I immersion
Discussion
Development time and survival
In the present study, the development time of S. officinalis embryos decreased under warming conditions (Fig. 3A). In fact, elevated temperatures are known to promote increased metabolic rates in ectotherms (Moran and Woods 2007; Rosa and Seibel 2008; Pimentel et al. 2012), and faster early development (Pimentel et al. 2012; Rosa et al. 2013). Concomitantly, there is a drop in pO2 levels within eggs, which has also been established as a trigger for early hatching in embryos (Moran and Woods 2007; Rosa et al. 2013).
On other hand, periodic exposure to emersion had no significant impact on development time, showing that S. officinalis early stages are tolerant to the effects of air exposure. In intertidal habitats, air exposure is a major stressor to inhabiting organisms, leading to hypoxia and thermal stress (Aguilera and Rautenberger 2011; Teixeira et al. 2013). Yet, S. officinalis intertidal spawning grounds (such as those in Sado estuary; Fig. 1) are characterized by significant daily (and seasonal) fluctuations in key abiotic factors (e.g., temperature, salinity, UV radiation), which might confer the required phenotypic tolerance to the simulated emersion periods. It is also worth noting that, in Sado estuary, S. officinalis spawning not only occur during spring and summer, but can also extend until late Autumn (Rosa, pers. observation); such extended spawning period also highlights the great phenotypic plasticity of S. officinalis early life stages.
Concomitantly, hatching rates increased with temperature during immersion (Fig. 3; Supplemental Table 1). Such results may suggest that the temperature conditions during MHW1 and MHW4 are still within the optimum thermal range for aerobic performance. According to the oxygen- and capacity-limited thermal tolerance (OCLTT) hypothesis, the range of active thermal tolerance is limited by the “pejus” temperatures, and warming or cooling beyond these leads to a decrease in the ability to perform aerobically, compromising growth and survival (Pörtner and Knust 2007; Pörtner et al. 2017). Therefore, MHW1 and MHW4 with immersion conditions did not seem to exceed such physiological thresholds. Moreover, emersion itself had no significant impact on hatching success (Fig. 3C), highlighting again the high tolerance of cuttlefish embryos to such environmental conditions.
Biochemical responses
MHW conditions led a significant increase in SOD activity (Fig. 4), which might be expected because higher temperatures lead to a higher mitochondrial activity, thus creating more ROS that are catalyzed by SOD (Lesser 2006; Rosa et al. 2012; Wang et al. 2018; Mariana and Badr 2019). Other studies have also shown that O2− production increases under exposure to other-type of stressors, namely xenobiotics and pollutants, reinforcing SOD as a relevant antioxidant defense (Lesser 2006; Caeiro et al. 2017). Considering that production of ROS directly relates to the concentration of O2, the significant decreases in SOD activity observed in embryos exposed to air (Fig. 4) suggest a reduced production of the enzyme’s substrate as a result of lower oxygen availability for aerobic metabolism (Lesser 2006; Teixeira et al. 2013). The activity of CAT, the enzyme that completes the ROS-detoxifying chain reaction initiated by SOD, was not influenced by either the MHW or the emersion treatments (Fig. 4), further reinforcing the tolerance of embryos to oxidative stress-inducing conditions. GPx activity increased significantly when embryos were subjected to emersion (Fig. 4). This is in accordance with previous research, as the subsequent reoxygenation state drives the formation of H2O2, which GPx removes from cells as a means to oxidize GSH (Lesser 2006; Rosa et al. 2014). The few changes in enzymatic activity observed in the present study may also suggest that embryos possess other means of maintaining homeostasis, such as alterations of general energetic metabolism and a high tolerance to hypoxia (Wolf et al. 1985; Pimentel et al. 2012; Rosa et al. 2013). Hypoxia-tolerant organisms can downregulate their metabolism into a hypometabolic state, thus balancing their ATP demand and supply pathways, allowing them to survive under short-term hypoxic conditions (Rosa and Seibel 2008, 2010; Rosa et al. 2013; Teixeira et al. 2013).
The present study also showed no significant changes in MDA levels among treatments (Fig. 5). The low levels of MDA, and thus LPO, can be indicative of a low amount of oxidative stress or of the antioxidant system working properly. Similar to MDA, HSP70 and Ub also showed no significant changes among treatments (Fig. 5). All these biochemical findings corroborate the idea of a great phenotypic plasticity of S. officinalis early life stages. This is also supported by the high number of influential data points, which reflect high inter-individual variability. Interestingly, outliers in biochemical responses were consistently from the same individuals, suggesting that they exhibit high intrinsic variation in antioxidant responses. They also support the notion that when certain abiotic stressors are frequently encountered (here in the dynamic intertidal zones), the organisms develop a type of desensitization against such stressor(s), thus leading to a less drastic biological response or even an absence of it (Reid et al. 1998).
Summing up, S. officinalis is an ecologically and commercially important species that is considered a viable alternative resource in the context of the ongoing decline in finfish stocks, and this study showed S. officinalis early life stages are well adapted to fluctuating tidal conditions and MHWs. Nonetheless, it is worth noting that MHWs are expected to increase in frequency, duration and intensity in the near future (Hobday et al. 2016, 2018). Therefore, the impacts of repeated and longer extreme temperature events should be investigated. More research on predicting how the common cuttlefish will adapt (or not) in the oceans of tomorrow of paramount importance.
Data availability
Data will be made available upon request.
Code availability
Data will be made available upon request.
References
Abele D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A Mol Integr Physiol 138:405–415. https://doi.org/10.1016/j.cbpb.2004.05.013
Aguilera J, Rautenberger R (2011) Oxidative stress tolerance strategies of intertidal macroalgae. Oxidative Stress in Aquatic Ecosystems. John Wiley & Sons, Ltd, Chichester, pp 58–71
Axenov-Gribanov DV, Bedulina DS, Shatilina ZM, Lubyaga YA, Vereshchagina KP, Timofeyev MA (2014) A cellular and metabolic assessment of the thermal stress responses in the endemic gastropod Benedictia limnaeoides ongurensis from Lake Baikal. Comp Biochem Physiol B Biochem Mol Biol 167:16–22. https://doi.org/10.1016/j.cbpb.2013.09.006
Banzon V, Smith TM, Chin TM, Liu C, Hankins W (2016) A long-term record of blended satellite and in situ sea-surface temperature for climate monitoring, modeling and environmental studies. Earth Syst Sci Data 8:165–176. https://doi.org/10.5194/essd-8-165-2016
Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, Alsalamat HA, Bashatwah RM (2018) Reactive oxygen species: the dual role in physiological and pathological conditions of the human body. Eurasian J Med 50:193–201. https://doi.org/10.5152/eurasianjmed.2018.17397
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Belcheva NN, Chelomin VP (2011) Glutathione S-Transferase activity in marine invertebrates from Peter the Great Bay in the Sea of Japan. Russ J Mar Biol 37:62–68. https://doi.org/10.1134/S1063074011010056
Boletzky SV (1986) Encapsulation of cephalopod embryos—A search for functional correlations. Am Malacol Bull. https://doi.org/10.5860/choice.48-6651
Bonnaud L, Franko D, Vouillot L, Bouteau F (2013) A study of the electrical polarization of Sepia officinalis yolk envelope, a role for Na+/K+-ATPases in osmoregulation? Commun Integr Biol. https://doi.org/10.4161/cib.26035
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Caeiro S, Vaz-Fernandes P, Martinho AP, Costa PM, Silva MJ, Lavinha J, Matias-Dias C, Machado A, Castanheira I, Costa MH (2017) Environmental risk assessment in a contaminated estuary: an integrated weight of evidence approach as a decision support tool. Ocean Coast Manag 143:51–62. https://doi.org/10.1016/j.ocecoaman.2016.09.026
Chiabai A, Quiroga S, Martinez-Juarez P, Higgins S, Taylor T (2018) The nexus between climate change, ecosystem services and human health: towards a conceptual framework. Sci Total Environ 635:1191–1204. https://doi.org/10.1016/j.scitotenv.2018.03.323
Dong Y, Miller LP, Sanders JG, Somero GN (2008) Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull 215:173–181. https://doi.org/10.2307/25470698
Eyring V, Gillett NP, Achuta Rao KM, Barimalala R, Barreiro Parrillo M, Bellouin N, Cassou C, Durack PJ, Kosaka Y, McGregor S, Min S, Morgenstern O, Sun Y (2021) Human influence on the climate system. In: Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change.
Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B (2008) Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320:1490–1492. https://doi.org/10.1126/science.1155676
Freire CA, Welker AF, Storey JM, Storey KB, Hermes-Lima M (2011) Oxidative stress in estuarine and intertidal environments (temperate and tropical). Oxidative Stress in Aquatic Ecosystems. John Wiley & Sons, Ltd, Chichester, pp 41–57
Guerra A (2006) Ecology of Sepia officinalis. Vie Milieu 56:97–107
Gulev SK, Thorne PW, Ahn J, Denterner FJ, Domingues CM, Gerland S, Gong D, Kaufman DS, Nnamchi HC, Quaas J, Rivera JA, Sathyendranath S, Smith SL, Trewin B, von Schuckmann K, Vose RS (2021) Changing state of the climate system. In: Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change.
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hawkins SJ, Evans AJ, Firth LB, Genner MJ, Herbert RJH, Adams LC, Moore PJ, Mieszkowska N, Thompson RC, Burrows MT, Fenburg PB (2016) Impacts and effects of ocean warming on intertidal rocky habitats. In: Laffoley D, Baxter JM (eds) Explaining Ocean Warming: Causes, Scale, Effects and Consequences. Gland, IUCN, Switzerland, pp 147–176
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404. https://doi.org/10.1146/annurev.ecolsys.37.091305.110149
Hobday AJ, Alexander LV, Perkins SE, Smale DA, Straub SC, Oliver ECJ, Benthuysen JA, Burrows MT, Donat MG, Feng M, Holbrook NJ, Moore PJ, Scannell HA, Sen Gupta A, Wernberg T (2016) A hierarchical approach to defining marine heatwaves. Prog Oceanogr 141:227–238. https://doi.org/10.1016/j.pocean.2015.12.014
Hobday AJ, Oliver ECJ, Sen GA, Benthuysen JA, Burrows MGD MT, Holbrook NJ, Moore PJ, Thomsen TW MS (2018) Categorizing and naming marine heatwaves. Oceanography. https://doi.org/10.1007/s00376-014-4097-0
Hu MY (2016) Effects of C02 driven ocean acidification on ontogenetic stages of the cutllefisii Sepia officinalis. Vie Milieu 66:57–63
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 54:287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Jereb P, Roper CFE (2005) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes, Rome, p 262
Jereb P, Allcock AL, Lefkaditou E, Piatkowski U, Pierce GJ (2015) Cephalopod biology and fisheries in Europe: II. Species Accounts. ICES Cooperative Research Report, Copenhagen, p 360
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958. https://doi.org/10.1016/0006-291X(76)90747-6
Lee J-Y, Marotzke J, Bala G, Cao L, Corti S, Dunne JP, Engelbrecht F, Fischer E, Fyfe JC, Jones C, Maycock A, Mutemi J, Ndiaye O, Panickal S, Zhou T (2021) Future global climate: scenario-based projections and near-term information. In: climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change.
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278. https://doi.org/10.1146/annurev.physiol.68.040104.110001
Lesser MP (2011) Oxidative stress in tropical marine ecosystems. Oxidative Stress in Aquatic Ecosystems. John Wiley & Sons, Ltd, Chichester, pp 7–19
Logan CA, Somero GN (2011) Effects of thermal acclimation on transcriptional responses to acute heat stress in the eurythermal fish Gillichthys mirabilis (Cooper). Am J Physiol Integr Comp Physiol 300:R1373–R1383. https://doi.org/10.1152/ajpregu.00689.2010
Mariana SA, Badr G (2019) Impact of heat stress on the immune response of fishes. J Surv Fish Sci 5:149–159
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160:1875–1888. https://doi.org/10.1007/s00227-012-1954-1
Moran AL, Woods HA (2007) Oxygen in egg masses: interactive effects of temperature, age and egg-mass morphology on oxygen supply to embryos. J Exp Biol 210:722–731. https://doi.org/10.1242/jeb.02702
Moura É, Pimentel M, Santos CP, Sampaio E, Pegado MR, Lopes VM, Rosa R (2019) Cuttlefish early development and behavior under future high CO2 conditions. Front Physiol 10:1–10. https://doi.org/10.3389/fphys.2019.00975
Njemini R, Demanet C, Mets T (2005) Comparison of two ELISAs for the determination of Hsp70 in serum. J Immunol Methods 306:176–182. https://doi.org/10.1016/j.jim.2005.08.012
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oliver ECJ, Donat MG, Burrows MT, Moore PJ, Smale DA, Alexander LV, Benthuysen JA, Feng M, Sen Gupta A, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Straub SC, Wernberg T (2018) Longer and more frequent marine heatwaves over the past century. Nat Commun 9:1–12. https://doi.org/10.1038/s41467-018-03732-9
Oliver ECJ, Burrows MT, Donat MG, Sen Gupta A, Alexander LV, Perkins-Kirkpatrick SE, Benthuysen JA, Hobday AJ, Holbrook NJ, Moore PJ, Thomsen MS, Wernberg T, Smale DA (2019) Projected marine heatwaves in the 21st century and the potential for ecological impact. Front Mar Sci 6:1–12. https://doi.org/10.3389/fmars.2019.00734
Pannunzio TM, Storey KB (1998) Antioxidant defenses and lipid peroxidation during anoxia stress and aerobic recovery in the marine gastropod Littorina littorea. J Exp Mar Bio Ecol 221:277–292. https://doi.org/10.1016/S0022-0981(97)00132-9
Pegado MR, Santos CP, Pimentel M, Cyrne R, Sampaio E, Temporão A, Röckner J, Diniz M, Rosa R (2020) Lack of oxidative damage on temperate juvenile catsharks after a long-term ocean acidification exposure. Mar Biol 167:1–10. https://doi.org/10.1007/s00227-020-03770-2
Perkins SE, Alexander LV, Nairn JR (2012) Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys Res Lett 39:1–5. https://doi.org/10.1029/2012GL053361
Pierce GJ, Allcock L, Bruno I, Bustamante P, González Á, Guerra Á, Jereb P (2010) Cephalopod biology and fisheries in Europe. ICES Cooperative Research Report No. 303
Pimentel MS, Trübenbach K, Faleiro F, Boavida-Portugal J, Repolho T, Rosa R (2012) Impact of ocean warming on the early ontogeny of cephalopods: a metabolic approach. Mar Biol 159:2051–2059. https://doi.org/10.1007/s00227-012-1991-9
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97. https://doi.org/10.1126/science.1135471
Pörtner HO, Bock C, Mark FC (2017) Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol 220:2685–2696. https://doi.org/10.1242/jeb.134585
Redlin M, Gries T (2021) Anthropogenic climate change: the impact of the global carbon budget. Theor Appl Climatol 146:713–721. https://doi.org/10.1007/s00704-021-03764-0
Reid SG, Bernier NJ, Perry SF (1998) The adrenergic stress response in fish: control of catecholamine storage and release. Comp Biochem Physiol Part C Pharmacol Toxicol Endocrinol 120:1–27. https://doi.org/10.1016/S0742-8413(98)00037-1
Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci 105:20776–20780. https://doi.org/10.1073/pnas.0806886105
Rosa R, Seibel BA (2010) Metabolic physiology of the Humboldt squid, Dosidicus gigas: implications for vertical migration in a pronounced oxygen minimum zone. Prog Oceanogr 86:72–80. https://doi.org/10.1016/j.pocean.2010.04.004
Rosa R, Pimentel MS, Boavida-Portugal J, Teixeira T, Trübenbach K, Diniz M (2012) Ocean warming enhances malformations, premature hatching, metabolic suppression and oxidative stress in the early life stages of a keystone squid. PLoS ONE. https://doi.org/10.1371/journal.pone.0038282
Rosa R, Trubenbach K, Repolho T, Pimentel M, Faleiro F, Boavida-Portugal J, Baptista M, Lopes VM, Dionísio G, Leal MC, Calado R, Pörtner HO (2013) Lower hypoxia thresholds of cuttlefish early life stages living in a warm acidified ocean. Proc R Soc B Biol Sci 280:1–7. https://doi.org/10.1098/rspb.2013.1695
Rosa R, Lopes AR, Pimentel M, Faleiro F, Baptista M, Trübenbach K, Narciso L, Dionísio G, Pegado MR, Repolho T, Calado R, Diniz M (2014) Ocean cleaning stations under a changing climate: biological responses of tropical and temperate fish-cleaner shrimp to global warming. Glob Change Biol 20:3068–3079. https://doi.org/10.1111/gcb.12621
Rosa R, Ricardo Paula J, Sampaio E, Pimentel M, Lopes AR, Baptista M, Guerreiro M, Santos C, Campos D, Almeida-Val VMF, Calado R, Diniz M, Repolho T (2016) Neuro-oxidative damage and aerobic potential loss of sharks under elevated CO2 and warming. Mar Biol 163:119. https://doi.org/10.1007/s00227-016-2898-7
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Teixeira T, Diniz M, Calado R, Rosa R (2013) Coral physiological adaptations to air exposure: heat shock and oxidative stress responses in Veretillum cynomorium. J Exp Mar Bio Ecol 439:35–41. https://doi.org/10.1016/j.jembe.2012.10.010
Tomanek L (2010) Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol 213:971–979. https://doi.org/10.1242/jeb.038034
Wang J, Pierce GJ, Boyle PR, Denis V, Robin JP, Bellido JM (2003) Spatial and temporal patterns of cuttlefish (Sepia officinalis) abundance and environmental influences—A case study using trawl fishery data in French Atlantic coastal, English Channel and adjacent waters. ICES J Mar Sci 60:1149–1158
Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217:1915–1928. https://doi.org/10.1083/jcb.201708007
Wolf G, Verheyen E, Vlaeminck A, Lemaire J, Decleir W (1985) Respiration of Sepia officinalis during embryonic and early juvenile life. Mar Biol 90:35–39. https://doi.org/10.1007/BF00428212
Yamashita M, Yabu T, Ojima N (2010) Stress protein HSP70 in fish. Aqua-BioSci Monogr 3:111–141. https://doi.org/10.5047/absm.2010.00304.0111
Funding
This study was funded by the project VALPRAD (MAR-01.04.02-FEAMP-0007) within the framework of MAR2020 program. The authors also acknowledge funding from Fundação para a Ciência e Tecnologia (FCT) under the strategic project UIDB/04292/2020 granted to MARE, project LA/P/0069/2020 granted to the Associate Laboratory ARNET, project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HBm, and the researcher contract (DL57/2016/CP1479/CT0023) granted to TR.
Author information
Authors and Affiliations
Contributions
RR, VML and JRP conceptualized the study. JC, VML, EO, MC and JRP collected and analyzed the data. RR and MD provided supervision. All authors contributed to and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
All the procedures were approved by the FCUL Animal Welfare Committee (ORBEA FCUL) and the Portuguese General-Directorate for Food and Veterinarian Contacts (DGAV) of the Portuguese Government, according to National (Decreto-Lei 113/2013) and the EU legislation (Directive 2010/63/EU) on the protection of animals used for scientific purposes (within the framework of MAR2020— MAR-01.04.02-FEAMP-0007).
Additional information
Responsible Editor: E. A.G. Vidal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coelho, J., Court, M., Otjacques, E. et al. Effects of tidal emersion and marine heatwaves on cuttlefish early ontogeny. Mar Biol 170, 3 (2023). https://doi.org/10.1007/s00227-022-04150-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04150-8