Abstract
With the aim of maintaining or obtaining good environmental status in the Northeast Atlantic and northern Mediterranean Sea, the European Marine Strategy Framework Directive (EU-MSFD) came into force in 2008. All EU Member States (MS) have the task of assessing the current state of their adjacent waters to guarantee sustainable use of marine resources and healthy ecosystems for the current and future generations. Although the MSFD has been implemented for over a decade, there are still gaps in the assessment of various ecologically and commercially important marine species, such as cephalopods. Here we describe the status of cephalopods, a key component of marine ecosystems and a valuable fishing resource, within the MSFD. To do so, we review how MS deal with this group in their reporting and identify and explain the gaps in the cephalopod assessment. We describe the main challenges including the limited data and the rarity of dedicated surveys on cephalopods. However, we argue that cephalopods can be partially integrated into the EU-MSFD assessment, illustrating the current opportunities and future possibilities of their integration into the MSFD, mainly using Descriptors 1–4.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global change involves a variety of different human pressures including climate warming (rising sea temperatures), ocean acidification, invasive species and resource use such as fishing, sand mining and energy generation, all of which affect marine ecosystems worldwide (Oesterwind et al. 2016). To manage the different anthropogenic pressures in European Union seas, diverse directives have been implemented, including the European Marine Strategy Framework Directive (EU-MSFD).
The EU-MSFD aims to protect and preserve the marine environment and its natural resources. It came into force in 2008 and was implemented in 2010 to form the environmental pillar of the future maritime policy of the European Union (EU Directive 2008/56/EC). With the intention of introducing an ecosystem-based management of biodiversity, habitats and resources, this directive focuses on obtaining or maintaining good health of marine ecosystems and the sustainable use of the marine environment by current and upcoming generations (Morato et al. 2016). The overall goal was to maintain or achieve this ‘Good Environmental Status’ (GES) by 2020, something which has not so far been achieved for most indicators and areas.
The EU-MSFD process started in 2012 and involves a 6-year cycle with the following three main phases: (i) reporting the current environmental status (performed in 2012 and 2018), (ii) setting up monitoring programmes to assess progress in achieving GES (conducted in 2014) and (iii) setting up programmes of measures to deliver the objectives of the MS (performed in 2016) and reporting on implementation progress (performed in 2018) (COM/2020/259). As the second cycle started in 2018, the GES assessment in support of the updated assessment guidance is due in 2024. The description of GES is based on the initial assessment from 2012 and refers to the following 11 qualitative descriptors: D1—biodiversity, D2—non-indigenous species, D3—commercial fish and shellfish, D4—food webs, D5—eutrophication, D6—sea-floor integrity, D7—hydrography, D8—contaminants in environment, D9—contaminants in seafood, D10—marine litter, D11—energy/noise (EU Directive 2008/56/EC). A number of criteria and indicators have been developed for both biodiversity-related and pressure descriptors. However, these criteria and indicators are constantly being supplemented and revised so that their number and nature change continually. In 2010, the Commission Decision 2010/477/EU named 29 associated criteria and 56 indicators for the different descriptors. Seven years later, the number of criteria had increased to 42 (Commission Decision 2017/848/EU).
Even if the MSFD considers all European marine waters, the assessments (and monitoring and measures) can be subdivided into subareas by the MS (EU Directive 2008/56/EC). Until now the requirements of the MSFD have not yet been met following a holistic approach and various publications illuminate the weak points on different aspects of the MSFD (e.g. Santos and Pierce 2015; Probst et al. 2016; Borja et al. 2021; Tsiamis et al. 2021). Furthermore, some large and/or important groups of species are still not (or insufficiently) considered, including keystone groups or species, which are, in this case, species which have an important impact on the ecosystem or are important commercial resources.
One of these key groups is the Cephalopoda. Most cephalopods are opportunistic predators (e.g. Lipinski 1992) with a fast growth rate and a short lifespan. Their semelparous lifecycle mainly ends after 1–2 years and only a few species live longer (Mangold and Boletzky 1988). Their short lifespan enables them to quickly adapt to changing environments and they might also benefit from overfishing of commercial finfish stocks (Caddy and Rodhouse 1998; Balguerías et al. 2000; Hunsicker et al. 2010), due to decreasing predation pressure, less food competition and likely due to ‘free’ ecological niches, that can quickly be occupied by cephalopods as opportunists and r-strategists. Many cephalopods display typical r-selected features such as a high production to biomass ratio (Clarke 1996b; Smale 1996) and a high number of eggs (Cuccu et al. 2013; Laptikhovsky 2000). However, the life-cycles of some cephalopods also show K-selected features such as a relatively low number of eggs (e.g. in the octopus Eledone cirrhosa; Regueira et al. 2013), parental egg or brood care (e.g. in octopuses and some deep-sea squids like Gonatus onyx and Bathyteuthis berryi; Mangold and Boletzky 1973; Seibel et al. 2000; Bush et al. 2012) as well as a large body size (e.g. in the squid Dosidicus gigas; Argüelles et al. 2008). Furthermore, because cephalopods have a strong influence on other trophic levels (TLs) and, therefore, play a structuring role in their food webs, they can be defined as keystone species according to Paine (1995). The importance of a species in its community or ‘Keystoneness’ can be ranked by an index (Hurlbert 1997; Coll et al. 2013). For example, cephalopods are the group with the third highest ‘Keystoneness Index’ in the Azores, after pelagic sharks and toothed whales (Morato et al. 2016), and have a relatively high ‘Keystoneness’ in other marine waters (Coll et al. 2013; Smith et al. 2014), which emphasizes their importance in the ecosystem and consequently the need to include cephalopods into the MSFD. Various studies show significant changes in the occurrence and abundance of cephalopods from year to year (in addition to within-year changes associated with the life cycle) (Doubleday et al. 2016), while others illustrate impressive range expansions which affect regional biodiversity (D1) (Chen et al. 2006; Zeidberg and Robinson 2007; van der Kooij et al. 2016; Moura et al. 2020; Oesterwind and Schaber 2020; Oesterwind et al. 2022).
However, not all range expansions are natural. Many established non-indigenous marine species (NIS) are registered in the European Union, comprising 824 taxa including five cephalopod species (Tsiamis et al. 2018). The bioregions most heavily affected by introduced species in general include the Mediterranean Sea and the Northeast Atlantic (Hewitt and Campbell 2010), where the local community structure, biodiversity and ecosystem functions are influenced by the direct and indirect impacts caused by these species (Thomsen et al. 2015). Besides negative effects such as the reduction in native biodiversity and ecosystem shifts, there might also be benefits from NIS, such as the replacement of lost ecological functions and the strengthening of biogenic complexity (Kleitou et al. 2021). A recent study describes 13 non-indigenous cephalopod species for the Mediterranean Sea, which entered the region over the last few decades, most likely by the following three different routes: the natural pathway Strait of Gibraltar, the human-constructed pathway Suez Canal or through other anthropogenic pathways like shipping (Bello et al. 2020). Consequently, cephalopods should also be considered in the NIS assessment within the MSFD (D2).
Fisheries landings of cephalopods are increasing in parts of the world (Arkhipkin et al. 2015; Doubleday et al. 2016), but in some species/areas, there is an opposite trend in cephalopod catches (Ospina-Alvarez et al. 2021), likely due to unsustainable fisheries (Katağan et al. 2015). For example, the catch biomass of Octopoda in the Aegean Sea showed a strong decrease between 2001 and 2013. Katağan et al. (2015) attributed this to unsuitable policies such as non-optimal closed seasons in cephalopod fisheries, along with weak controls on cephalopod fisheries. In European waters, catching cephalopods in large-scale fisheries is largely unregulated, but not all fluctuations have been attributed to fishing, as there have also been significant decreases associated with changing environmental conditions (e.g. Sobrino et al. 2020). However, the combination of high fishing pressure and the semelparous life cycle of cephalopods, with only a single spawning event before they die, make them a potentially vulnerable resource for both targeted and bycatch fisheries. Around 17 of nearly 70 cephalopod species inhabiting European marine waters are commercially relevant (Jereb et al. 2015; Katağan et al. 2015). Cephalopods are fished in many areas of the EU, where their commercial landings show both increasing (Caddy and Rodhouse 1998; Arkhipkin et al. 2015) and decreasing trends, depending on the area and species fished (Ospina-Alvarez et al. 2021). These cephalopod species should be considered in the assessment under D3 (status of commercial fish and shellfish) and are also potentially relevant for D9 (contaminants in seafood) as they are able to accumulate contaminants in their tissues (Bustamente et al. 2002).
In food webs, cephalopods exert top-down pressure on lower TLs but also have a bottom-up function due to their role as major prey for some marine mammals and predatory fishes, depending on their life stage and size. For example, cephalopods are a major food source for sperm whales, dolphins, seals and some elasmobranchs (Clarke 1996a, 1996b; Katağan et al. 2015). They also play an important role in the diet of some teleosts (Bello 1991; Daly et al. 2001; Battaglia et al. 2013). Changes in fish and/or cephalopod abundances have already caused a community shift towards squid-dominated food webs in some places (Caddy and Rodhouse 1998; Balguerías et al. 2000; Hunsicker et al. 2010; Doubleday et al. 2016). Logically, cephalopods play an important key role in marine coastal and open sea food webs and should therefore be integrated in MSFD D4 (food webs).
In summary, one should expect that, in accordance to the MSFD, information on cephalopods is reported under D1, D2, D3, D4 and D9. Of course, other Descriptors including eutrophication and noise might also have a possible impact on the state of cephalopods, though the relationship between pressure and state is still unclear. However, some studies on acoustic exposure in cephalopods (Guerra et al. 2011; André et al. 2011; Solé et al. 2013a, b, 2017, 2022) do already exist. Nevertheless, we will review the current integration of cephalopods within the MSFD focusing on D1–D4 and D9 but will also deal with D11 (energy/noise). The aim of this review is to illustrate the gaps and to provide some preliminary suggestions on how to deal with this keystone group in future MSFD reporting.
State-of-the-art: cephalopods within the MSFD
Until now, only 23 EU–MS were obliged to complete MSFD reporting due to their geographical location and access to marine waters. Furthermore, in addition to MS without a coastline, those with a lack of cephalopods in their marine areas (e.g. Baltic and Black Sea) are not committed to reporting on cephalopods. This leaves 16 EU-MS for which it could be assumed that they would include cephalopods in their reporting. However, our results show that only a few even mentioned cephalopods in their MSFD reporting (Table 1). The non-reporting of cephalopods in the MSFD usually occurs without any justification (Dupont et al. 2014a, b, c, d, e).
Within the first reporting cycle (2012), the Netherlands reported that only an insignificant number of cephalopods occurs in their national waters (Dupont et al. 2014f). Germany stated that no methods for the analysis and evaluation of the cephalopod species group have currently been developed (Dupont et al. 2014g) and the UK justified the reporting gap citing insufficient information and data on cephalopods in their waters and stated that further development may be required (Dupont et al. 2014h).
For the initial assessment of cephalopods under D3, Portugal described the stock development using secondary indicators (for an explanation see section ‘Descriptor 3’) for D3C1 and D3C3 (Dupont et al. 2014i) and included cephalopod species in D1, indicator 1.6 (pelagic habitat conditions) and the states of two functional groups (Cochrane et al. 2010) were assessed: Coastal/shelf cephalopods and deep-sea cephalopods. Although no verdict regarding the GES of cephalopods was made, it was assumed to be achieved in Portuguese waters. Spain included cephalopods under D9 and assessed the heavy metal concentration of seven species from the Spanish Atlantic waters (Dupont et al. 2014j) and three species from the western Mediterranean Sea. All of them had concentrations below the respective reference values for Cd, Hg and Pb (Dupont et al. 2014j). France and Italy also included cephalopods in D9. The contamination by hazardous substances in cephalopods for the French Mediterranean region was described as moderate (Dupont et al. 2014d), whereas Dupont et al. (2014k) provided no information on such levels in cephalopods in Italian waters. The remaining countries ignored cephalopods in their reports for the contaminants in seafood Descriptor, so that it seems that countries where more cephalopods are eaten, take this descriptor more seriously.
Within the second reporting cycle in 2018, the integration of cephalopods had only changed slightly and around half of the 16 MS still ignored this species group. However, most MS which have not carried out a cephalopod assessment have justified their non-reporting. Denmark, Netherlands and Sweden designated cephalopods in their waters as ‘not relevant’ for assessment (Banfi et al. 2021a, b, c). Croatia justified its non-reporting by the lack of monitoring, available data and methodological standards, while Cyprus argued that developed indicators are missing (Banfi et al. 2021d, e). Ireland excused the non-reporting by the lack of biological information and data collection systems (Anonymous 2020). Germany illustrated the trends in abundance within D1, but without any evaluation (BMU 2018). Italy, Malta, Slovenia and Spain considered cephalopods under D1 in relation to different criteria (see Table 1) (Banfi et al. 2021f, g). The contamination of cephalopods (D9) was assessed in the second cycle (2018) by two MS. Spain focused on heavy metals (Banfi et al. 2021g), whereas Belgium focused on PAH and dioxins (Banfi et al. 2021h). Portugal included cephalopods within D1 and D3 in the second reporting cycle and used criteria D1C2, D1C3 (for more information about criteria see Descriptor sections), D3C1 and D3C3, following Commission Decision 2017/848/EU (about criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment) for their status reporting. Poor GES for biodiversity of coastal/shelf cephalopods was reported, mainly due to the low biomass of Alloteuthis spp. in recent years but increasing biomass trends for three cephalopod species were also reported by Portugal in D3 (Ministerio do Mar 2020). Thus, to our best knowledge, out of 16 MS, only Portugal described the status of cephalopods in D3. Meanwhile, the International Council for the Exploration of the Sea (ICES) already advises the inclusion of five cephalopod species (Illex coindetii, Illex illecebrosus, Loligo vulgaris, Octopus vulgaris and Sepia officinalis) in the reference list of D3 species (ICES 2020a) (although I. illecebrosus can be ignored in European waters as it most likely does not occur (Oesterwind et al. 2020)).
Where a reason was provided by MS to exclude cephalopods within their reporting, most MS argue that the lack of data is the primary reason (COM/2020/259). However, many standardized fisheries research surveys report cephalopods, particularly the commercially important species. While such data are insufficient to determine absolute abundance of cephalopods, for various reasons, these data allow comparisons of species distribution, relative abundance, size distribution, age distribution, and size at maturity (by sex) of different cephalopod species, as well as comparisons between years and seasons (Pierce et al. 1998; Krstulović Šifner et al. 2011; Oesterwind et al. 2015; van der Kooij et al. 2016). Furthermore, commercial landings data also exist for many regions, although these are often at higher taxonomic level and therefore would often be insufficient for a species-specific assessment in many cases. However, even if these studies might not provide species-specific datasets over decades, they allow the description of the current status and, in some areas, can indicate changes in MSFD-relevant aspects like species distribution and abundance, reproduction and phenology (e.g. Pierce et al. 2005; Oesterwind et al. 2020, 2022).
As an example, the ICES Working Group on Cephalopod Fisheries and Life History (WGCEPH) analyses the landings data and fishery research survey data (in those species that are commercially important for D3) by regions, species groups (at different taxonomic levels) and fishing effort, to describe the trend in indices of biomass and/or abundance. These exercises demonstrate the potential of using existing data, but also highlight the data gaps (e.g. ICES 2020b). In the following sections, we discuss the current and potential future possibilities to integrate cephalopods into the MSFD using the existing and publicly available data, along with any limitations and knowledge gaps. As an overview we have listed descriptors that can already be addressed in Table 2, including those indicators which can already be used to assess status (marked in bold) and additional potential indicators for cephalopod assessment.
Descriptor 1 (biodiversity)
According to Commission Decision 2010/477/EU, the objective under D1 is as follows: "Habitat quality and occurrence, and species distribution and abundance, are consistent with prevailing physiographic, geographic and climatic conditions."
Different indicators or methods to assess fish species might serve as a useful starting point for potential application to cephalopods. Among them are trophodynamic, climatic (Fortibuoni et al. 2017), pressure and state indicators that can be used for assessing GES (Lynam and Mackinson 2015). In the latest MSFD assessment, D1 contained five relevant criteria for cephalopods, the first four of which relate directly to the individual species (Commission Decision 2017/848/EU). These include species mortality rate (D1C1) (Table 2), which is mainly relevant for species considered at risk due to bycatch and discarding; species population abundance (D1C2) (Table 2), which is important for non- or less commercial species with a relevant ecological role in the ecosystem and not assessed in D3; species demographic characteristics (D1D3) (Table 2); and species distributional range and pattern (D1C4) (Table 2). The last criterion (D1C5) relates to the habitat, which has to meet all the necessary requirements for the life stages of a species. These indicators are intended for use to assess the status of cephalopods directly and as a constituent part of marine communities.
While mortality data are very rare for cephalopods and fishing mortality is, in most cases, documented at higher taxonomic level (in so far as it is meaningful for species which usually die when 1–2 years old regardless of whether they have been fished), data about species population abundance (D1C2), species demographic characteristics (D1D3), and species distributional range and pattern (D1C4) are, in many cases, provided by fishery research cruises and can be used at least for some species in some areas. Arkhipkin et al. (2021) describes the challenges for stock assessment of short-lived species, and exploratory stock assessments of the main European cephalopods have been carried out under the auspices of ICES WGCEPH based on the use of natural mortality (M) and fishing mortality (F), which can be estimated, for example, using a surplus production model in continuous time (SPiCT) (Larivain 2020). In addition, several assessments of various stocks of octopuses have been carried out in the Mediterranean, most recently using production models or related methods (Froese et al. 2018; Geraci et al. 2021; Tsikliras et al. 2021). The only routine stock assessment for a cephalopod takes place in the Western Asturias small-scale fishery for O. vulgaris, where it is required under the terms of Marine Stewardship Council (MSC) certification and is based on the depletion model described by Roa-Ureta et al. (2021).
Indices of relative species population abundance provide important information and are collated by the ICES WGCEPH (e.g. ICES 2020b) based on a data call to MS, and has already been used in various publications (e.g. van der Kooij et al. 2016; Oesterwind et al. 2022). Due to the short life cycle, and other reasons, most species show strong interannual fluctuations which require special attention during the analysis. Fishery research surveys also provide some information about species demographic characteristics, as, in many cases, length data of the survey catches are provided. However, due to the high plasticity of cephalopod life cycles and the occurrence of micro-cohorts (Guerra and Rocha 1994; Oesterwind et al. 2010), because within any given year hatching and recruitment may occur in several discrete ’pulses’ over an extended period of time or there may even be separate summer and winter breeding groups, interpretation of length data should be performed with caution.
Occurrence and distribution depend on natural factors such as habitat preferences, temperature, salinity and food availability, as well as seasonality of the life cycle, which applies to most species of commercial interest in Europe. Due to the high sensitivity of cephalopods to environmental conditions, a change in their distribution could indicate either significant natural change or man-made pressures, and these two are not easily disentangled. Understanding spatial distributions is an important aspect of developing management and conservation strategies (Planque et al. 2011). However, cephalopods are highly mobile species so, for most species, their geographic distribution can vary from year to year (Pierce et al. 1994). Another reason for distribution changes (in this case within years) in cephalopods is ontogenetic migrations during their life cycle. Such migrations occur when the range of a species is divided into spawning/nursery and feeding grounds (Nesis 1985). In a 6-step approach, Colloca et al. (2015) identified annual nursery areas for E. cirrhosa and I. coindetii by modeling the spatial distribution of recruits with the purpose of reconstructing the distribution of ‘density hot-spots’, regarded as more productive areas in the Mediterranean Sea. Within the Northeast Atlantic spawning, nursery and feeding grounds for Loligo vulgaris and Loligo forbesii were published recently (Laptikhovsky et al. 2022). Nursery grounds of coastal species in the European Mediterranean Sea are consistently protected from trawling fisheries (Colloca et al. 2015) due to a ban on use of this gear for three nautical miles of coastline or waters < 50 m depth (Council Regulation (EC) No 1967/2006), which is in contrast to the less consistent (or non-existent) protection of nursery areas for species with offshore recruitment. Last but not least, many knowledge gaps exist regarding habitat requirements in cephalopods, although studies are beginning to be performed to fill this gap (recently e.g. Barrett et al. 2021; Laptikhovsky et al. 2022). All-in-all, cephalopods can be integrated in D1, at least for some of the criteria, as described above.
Descriptor 2 (non-indigenous species)
To maintain or obtain GES within D2 requires “non-indigenous species introduced by human activities are at levels that do not adversely alter the ecosystems” (Commission Decision 2010/477/EU). In D2, one primary and two secondary criteria are relevant for cephalopods. According to Commission Decision 2017/848/EU, the primary criterion considers the number of NIS introduced into the area by human activity (D2C1) (Table 2). The secondary criteria consider the abundance and distribution of these species (D2C2) (Table 2), and the species and habitat affected by NIS (D2C3).
OSPAR (2018) listed three parameters (indicators) for the assessment of non-indigenous or cryptogenic species. These include the ‘new introductions’ parameter for the number of new introductions within the assessment area and assessment period (t), the ‘community abundance’ parameter showing the relative change in the total number of NIS between different assessment periods and the ‘dispersal’ parameter for the relative change in the proportion of monitoring locations (M) within which the species is found (OSPAR 2018). The list of new NIS requires understanding of the invasion process, as the species relevant to MSFD monitoring and future assessment are those related to human-mediated transmission. The status of NIS can be regularly monitored by existing fishing research cruise surveys, in which the sampling protocols have been improving in the last decade to assure good cephalopod identification. Where ship-based surveys cannot be executed, e.g. in Marine Protected Areas, and within hotspots or likely entry points for NIS, the use of scientific divers (Deidun et al. 2019) or other survey technologies will be helpful for some species. In addition to survey-based indicators, eDNA (Obst et al. 2020) and Citizen Science [e.g. Environmental NGO iSea project “Is it Alien to you? Share it!!!” (iSea 2022)] can be cost-effective tools to supplement routine monitoring, by filling knowledge gaps on the distribution and dynamics of marine bioinvasions, hence contributing to the assessment of GES within D2 of the MSFD (Giovos et al. 2019). Although none of the MS with non-indigenous cephalopods in their marine regions included them in their assessment, it seems that cephalopods could already be integrated in D2.
Descriptor 3 (commercial fish and shellfish)
The aim of D3 is that “populations of all commercially exploited fish and shellfish are within safe biological limits, exhibiting a population age and size distribution that is indicative of a healthy stock” (Commission Decision 2010/477/EU). The Commission Decision 2017/848/EU names three primary criteria for D3; fishing mortality rate (D3C1), spawning stock biomass (D3C2) and age and size distribution (D3C3) of commercial species (Table 2), which are usually derived from the analytical stock assessments required under the European Common Fishery Policy (CFP). The first two criteria aim to achieve maximum sustainable yield (MSY) of the commercially fished stocks. Thus, fishing mortality should be at or below the level (D3C1) and spawning stock biomass should at or above a level (D3C2) which can achieve MSY (Commission Decision 2017/848/EU). However, not all commercially exploited fish stocks in EU waters (and only one cephalopod stock) are routinely subject to full analytical assessments and, hence, for the non-assessed stocks, time series for fishing mortality (F) and spawning stock biomass (SSB) are generally not available, and the age structure is usually unknown. In addition, bycatch data for commercially exploited species are often still lacking. Another issue is that typical analytical assessment methods are developed for long-lived finfish stocks and many are not appropriate for cephalopod species, which possess 1- or 2-year life cycle (Pierce and Guerra 1994; Payne et al. 2006; Arkhipkin et al. 2021). Obviously, the European CFP is (or would be if cephalopods were included) faced with a special challenge in order to develop sustainable cephalopod fisheries management (Arkhipkin et al. 2021). While some authors point towards the advantages of using depletion methods to assess cephalopod stocks (e.g. Arkhipkin et al. 2021), other have favoured a simpler approach based on production models (e.g. Froese et al. 2018). The former requires intensive monitoring and (ideally) real-time assessment while the latter make the questionable assumption of a fixed carrying capacity—but have the advantage of rapidly generating simple indicators (e.g. F/FMSY, B/BMSY). As an alternative to analytical assessment and its indicators, the European Commission suggested the use of so-called ‘secondary indicators’ which can serve at least for some fish stocks as reliable indicators (Probst and Oesterwind 2014). These include the ‘Catch Per Unit Effort’ (CPUE) indices (Table 2), based in numbers or biomass, which are assumed to be proportional to stock size (King 2007), and can be based on data from commercial fisheries or fisheries-independent scientific surveys, though the latter are considered more reliable for estimating stock abundance (Harley et al. 2001). However, Probst and Oesterwind (2014) note that the quality of such indicators depends on the stock in question, as well as on the chosen metric. In the case of cephalopods, it might be that the patchy spatial distribution in some species (Pierce et al. 1998) and the difficulty of ensuring that surveys coincide with the same part of life cycle decrease the quality of the indicators. Nevertheless, the CPUE can also be modified to estimate recruitment, or SSB (Table 2). While the ICES WGCEPH already use the CPUE to describe general trends for different species in different regions (ICES 2020b), size-modified CPUE to assess spawning stock, for example, may require further refinement for cephalopods because, in many cases, the relationship between length and maturity stage is highly variable in cephalopod species and depends on sampling location, particularly latitude, and sampling season (Jackson and Yeatman 1996; Oesterwind et al. 2018), and because recruits and spawners may be present only in certain seasons.
Another secondary index to reflect fishing mortality is the Harvest Ratio (HR) (Table 2), which is the ratio between the biomass of commercial catches (or landings) and abundance indices (Piet et al. 2010). A two-stage biomass model has been developed to calculate HR for cephalopods in the English Channel (Gras et al. 2014; Alemany et al. 2017), but without a routine assessment in this area. To estimate the HR, reliable documentation of commercial landings is needed but this is somewhat lacking in cephalopod species because many species are fished as bycatch and landings are often reported at higher taxonomic level. Better sampling of the cephalopods landings by MS could address this issue.
Various length-based indicators exist to assess the demography of cephalopod stocks but, as mentioned in D1, high plasticity, a short life cycle and the possible existence of micro-cohorts might render the demography criterion useless. Furthermore, size-dependent indicators for D3 in fish are based on the concept that overfishing tends to result in the loss of the larger and older animals. This might make sense in long-lived animals but it is not as relevant in fished species with a short lifespan, non-overlapping generations (in many cases) and high plasticity where length depends not only on the life cycle stage but is also driven by environmental factors like oxygen and temperature (Pecl and Jackson 2008; Rosa et al. 2012; Rogov et al. 2019). A potentially useful length-based indicator for control purposes is the ‘small squid indicator’ (Table 2) because a catch with many small squids could be a sign of fishing in a recruitment or nursery area, which could result in overfishing and endanger the stock (Pierce et al. 2016). The seasonal variability of cephalopod sizes, however, can pose a challenge to using this indicator for GES assessment. Nevertheless, as Laptikhovsky et al. (2022) mentioned, as quarterly research cruises are timed consistently across the years, any massive changes in the sizes of the sampled population at a particular time of year might indicate that more investigations are necessary and could also point to climate-associated shifts. In addition to the assessment of commercially important species (D3), this size indicator is just as important for any non-commercial species in D1. In summary, the integration of cephalopods within D3 is partly possible for the main commercial species. However, the primary indicators mentioned above seem problematic for cephalopods due to the short life cycle and the general absence of routine assessment, and it might be better to develop a separate indicator approach for this group instead.
Descriptor 4 (food webs)
The focus in D4 is that “all elements of the marine food webs, to the extent that they are known, occur at normal abundance and diversity and levels capable of ensuring the long-term abundance of the species and the retention of their full reproductive capacity” (Commission Decision 2010/477/EU). According to Commission Decision 2017/848/EU, four criteria are related to D4, which are divided into two primary and two secondary criteria. The primary criteria are trophic guild diversity (D4C1) (Table 2) and abundance balance (D4C2). These two are complemented by the secondary criteria, size distribution of individuals (D4C3) and productivity (D4C4) (Table 2). As with D2, these indicators are related to ecological communities in marine ecosystems and cephalopods should not be neglected.
As indicators of community structure, trophic levels (TLs) are important for monitoring ecosystem changes (Joshi et al. 2016). Besides calibration, parameterization and validation of food web models, the estimation of TL is also used to calculate metrics and indicators of food web structure and human impact (Jennings and van der Molen 2015). A distribution change in a cephalopod species, for example, may lead to a change in its role as predator and prey and thus its trophic relationship in the food web (Lishchenko et al. 2021). Shannon et al. (2014) made a comparison between indicators based on TL, whereby landings-based TL indicators (e.g. TL of the landed catch) show the pressure on the system, whereas survey-based TL indicators show (potentially) unbiased state changes of the surveyed community. In addition, model-based TL indicators (based on Ecopath with Ecosim models) can facilitate the inclusion of poorly sampled species in ecosystem assessments (e.g. Gasalla et al. 2010; Coll et al. 2013). However, it should be noted that model-based indicators, which are based on equilibrium measures, have some difficulties with interannual variability in cephalopod biomass. As Christensen and Walters (2004) describe, assuming equilibrium for a reference year can lead to overly optimistic estimates of sustainable fishing mortality.
Biomass and productivity are related to the structural properties of food webs and can thus serve as proxies for ecosystem functioning (Zador et al. 2016). ICES (2014b) and Tam et al. (2017) have stated that the guild level biomass indicator is commonly used for fish, but should include guilds across all TLs. With this, both the food web condition and the relative stability of its main components could be assessed. Some predators or groups of species play a more important role in food webs than others and can thus be used as energy flow indicators for the whole system. The major predator–prey processes are measured using the productivity of these key predators (Rogers et al. 2010). To assess cephalopods using these indicators, high-quality and regularly updated fisheries landings and survey data time series from regional seas are required. Furthermore, information on regional and ontogenetically specific prey composition is something which is still lacking in many species and needs to be improved in future. Overall, cephalopods can partly be integrated in D4 on the basis of currently available data.
Descriptor 9 (contaminants in seafood) and Descriptor 11 (energy/noise)
According to Commission Decision 2010/477/EU, the aim for D9 is that “contaminants in fish and other seafood for human consumption do not exceed levels established by Community legislation or other relevant standards”. For D11, the following applies: “Introduction of energy, including underwater noise, is at levels that do not adversely affect the marine environment” (Commission Decision 2010/477/EU). One primary criterion is listed by Commission Decision 2017/484/EU for D9, which is contaminant levels in seafood (D9C1) (Table 2), whereas D11 has two primary criteria related to anthropogenic impacts on marine animals, concerning impulsive sound source levels (D11C1) and levels of continuous low-frequency sound (D11C2) (Commission Decision 2017/848/EU).
Unlike the ecologically and environmentally focused D1–D4, contaminants in seafood (D9) and introduction of energy and noise (D11) have a clear human focus and refer directly to the extent of human impact on the environment (O’Higgins 2016). Nevertheless, it is possible to use cephalopods as indicators for heavy metal pollution in the oceans. As Pierce et al. (2016) already describe, they could be used as an early warning of high environmental levels due to their accumulation of cadmium and PCBs in their tissues.
The impact of noise as underwater pollution and its impact on the surrounding environment are unclear in most cases. Therefore, information on the effects on most cephalopods is also lacking, although there are some documented effects on squid behavior (Fewtrell and McCauley 2012), on squid and cuttlefish statocyst development (Solé et al. 2013a, b, 2017, 2022) and on (giant) squid mortality (Guerra and González 2006; Guerra et al. 2011). In addition, the topographic conditions of an area, for example, great depths and steep slopes, or a large number of islands and islets play a major role in this assessment because they influence noise transmission in complex ways (Paramana et al. 2021). However, André et al. (2011) and Solé et al. (2013a, b, 2017, 2022) exposed several cephalopod species to low-frequency sounds (which is the main type of noise generated by coastal industry, marine traffic, geophysical surveys, offshore wind energy, etc.) and this resulted in permanent and substantial alterations of sensory hair cells of the statocysts of the individuals.
Case study
To illustrate how existing data from the ICES-DATRAS-database and EU Data Collection Framework (DCF) could be used to integrate cephalopods into the MSFD, we present some examples, with graphic representations and tables from which conclusions can be drawn for a first cephalopod assessment. The time analyses and associated trend analyses in our case study are not intended as a recommendation and can be adapted or modified. In addition, due to the Covid-pandemic in Europe, some cruises have not taken place or have been shortened, so that the data quality within the past 2 years seems to be limited, but this should increase again in further years.
Mortality rate (D1C1), fishing mortality (D3C1)
Landings data collected within the DCF provide an indication of fishing pressure on commercially important cephalopods. Landings tend to be highly variable across ICES management units.
A summary of the Northeast Atlantic fishing mortality trends in Ommastrephidae indicated by landings shows an increasing fishing mortality in all areas, when recent landings are compared with historical landings, and this also happens in most areas when compared with the recent period (Table 3). Interpreting the reasons for these trends is more difficult and abundances as well as fishing effort should be considered.
The summary trends in Loliginidae based on the landings show an increase in the North Sea, Western Iberia and Gulf of Cadiz and Azores, while landings decreased in Celtic Seas, English Channel and Bay of Biscay (Table 4). However, the Azores Loligo stock is considered to be reproductively isolated and independent of the European Shelf populations, while the latter population shows high genetic geneflow and connectivity amongst the constituent shelf regions (Brierley et al. 1995; Shaw et al. 1999; Göpel et al. 2022; Sheerin et al. 2022).
Population abundance (D1C2, D3C2)
The data of the standardized fishery surveys (available at DATRAS) can address abundance indicators. Except for the North Sea CPUE trend of Todaropsis eblanae, CPUEs in ommastrephids show an increasing trend within the recent years in the North Sea, Celtic Seas and Bay of Biscay, while CPUE values at the Gulf of Cadiz decreased for I. coindetii and T. eblanae (Table 5). However, as it is very typical that cephalopod CPUE fluctuates across years, especially for ommastrephid squids, ideally further analysis should be performed to determine whether the declining CPUE is part of its natural fluctuation, or not. Ommastrephid squids are known to show short-lived outbreaks, as seen in Norway, Scotland, and at Rockall Bank in recent decades. Even for very well-documented stocks like those of Illex argentinus and Todarodes pacificus, separating fishing and environmental influences on abundance is difficult (Sundet 1985; Hastie et al. 1994; Rodhouse et al. 2014).
Within the southern areas (Gulf of Cadiz, North-Western Iberia), all available data show an increasing trend for L. forbesii, L. vulgaris and Alloteuthis spp., while in the northern area, loliginids do not show a consistent trend in biomass among surveyed areas (Table 6). CPUE values for L. forbesii increased in the North Sea, while in the Celtic Seas both increasing and decreasing trends are observed, depending on the sub-area. A negative trend is also documented for Alloteuthis spp. in the Celtic Seas and North Sea in winter, while the CPUE of this taxon in the North Sea increased in summer. In the North Sea, CPUE trends of L. vulgaris are neutral or negative depending on the quarter. An exclusively decreasing trend for all considered loliginid species is shown by the Bay of Biscay data.
Species demographic characteristics (D1C3)
Although the frequency distribution of lengths of I. coindetii individuals in 2018/2019 fluctuated more than those in 2020/2021, they showed a similar trend (Fig. 1). Both show the highest frequency of individuals with dorsal mantle length of approx. 80 mm, with a decrease in frequency of subsequent size classes, followed by a renewed increase in larger length classes. An irregular drop in this length distribution, compared to previous years, could provide information on fishing pressure on a particular life stage of cephalopods, or, since survey timings tend to be invariable from year to year, could indicate a seasonal shift in abundance of the sampled demographic stages.
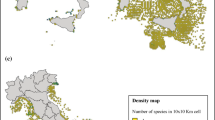
Distributional range and pattern (D1C4)
The same survey data set from DATRAS can provide some information about the geographic distribution and any changes in the distributional pattern on a regional scale. As shown in Fig. 2, a heatmap for the differences in CPUE of I. coindetii and L. forbesii in the North Sea can allow potential differences in distribution to be visualized and compared over a time period, always assuming that seasonal data collection remains the same. This may be used to tease apart natural variability from longer-term climate-associated impacts on both the seasonal timing of distribution and annual changes in spatial distribution.
Discussion
Our results show that not all MSFD criteria are currently suitable for cephalopods due to missing data and the very specific biology (fast and flexible lifecycles) in this group. Many cephalopods are key components in ecosystems (Coll et al. 2013) as well as being important commercial species, yet regular assessment comparable with commercial fish stocks does not exist in Europe (with the exception of the western Asturias octopus fishery, where assessment is required as part of the justification for MSC certification (Roa-Ureta et al. 2021)). Other countries around the world prove that an ecosystem approach to assessment and management, at least of commercially important cephalopod species, is quite possible (e.g. Arkhipkin et al. 2021). Therefore, we recommend including cephalopods within the MSFD instead of giving them little attention. Marchal et al. (2016) compares EU fisheries management with the innovative fisheries management of non-EU countries such as Australia, New Zealand and Iceland, whose approach is characterized by standard tools such as licensed commercial fishing vessels, assessment of key stocks and catch limitations through input controls. For instance, fisheries in the United States are regulated by law through the Magnuson–Stevens fishery conservation and management Act. The Act requires individual states to comply with various standards for fishing effort and management (NOAA 2007). Thus, each species, or species complex (e.g. all octopus species are grouped into a single assemblage) has its annual catch limit, which applies even to non-commercial species. For example, the overfishing limit (OFL, which corresponds to the stock’s MSY) of Enteroctopus dofleini in the Gulf of Alaska is calculated by multiplying the best available estimate of octopus biomass by the best estimate of their natural mortality (Ormseth and Conners 2018). However, the MSY concept is based on equilibrium points, which can be inappropriate for cephalopods due to the particular highly dynamic life cycle of semelparous populations, where the growth rate and their yield is not constant (Roa-Ureta et al. 2021). One way to assess the seasonal mortality of short-lived species is to use a surplus production model in continuous time (SPiCT), which has already been tested for an assessment of cuttlefish (Sepia officinalis) in the English Channel, though these assessments are expected to show large uncertainty in model outputs due to larger inter-annual fluctuations, as well as possible non-equilibrium between stock production and environmental carrying capacity, and they may require long time series with seasonal inputs (Larivain 2020). Ultimately, environmental covariates which predict recruitment success may enable assessments based on stock-production to be strengthened for cephalopods.
Most EU-MS justify their lack of reporting by citing insufficient data availability or non-harmonized assessment methods. This is partly valid, and in many regions, the available datasets might be insufficient to perform an optimal assessment, especially as reliable data at species level is problematic (Lishchenko et al. 2021). However, an evaluation at taxonomic family level for some criteria may be sufficient, for example to describe substantial changes in the cephalopod fauna in European waters, as presented in various published studies (e.g. Chen et al. 2006). MS have conducted standardized surveys in the Northeast Atlantic, such as the standardized International Bottom Trawl Survey (IBTS) and the Beam Trawl Survey (BTS), for almost 30 years (ICES 2009, 2012a). Similarly, in the Mediterranean Sea, the standardized International Bottom Trawl Survey (MEDITS) has been performed over a long time period. Consequently, a large amount of data already exist and data collection is ongoing in many areas, including long-term data series for many cephalopod species, particularly those of commercial importance. In addition, fisheries landings data are available and can be used to evaluate the level of fishing pressure, although taxonomic identification problems are even more obvious in these data. However, even if the quality of available datasets has to improve in future, survey and landings data sources are already used by ICES WGCEPH to describe the trends in landings, biomass and abundances, as shown in case studies presented in the present study. In addition to the development of particular metrics over time, such analyses can also be used to provide a certain degree of information about the status and trends of commercially exploited cephalopod groups. It should also be noted that, like many highly mobile marine organisms, due to their natural migratory behavior, cephalopods are not bounded inside the marine areas of a particular MS and, therefore, subregional monitoring programs are necessary for GES assessments. Indeed, Shephard et al. (2015) proposed that ICES working groups could help integrate the assessment of GES at a subregional level. We argue that, a non-harmonized assessment provides more information than no assessment, and will serve as initial baseline, which can be optimized in future. In particular, against the background of an increased interest in fisheries diversification to non-quota species, there is little fisheries management (with the exception of local management in small-scale cephalopod fisheries, e.g. for O. vulgaris) and almost no stock assessment in cephalopods, hence the need to include this species group in the MSFD framework is all the more appropriate.
For easier integration of cephalopods into an assessment within the MSFD, we have listed some indicators in Table 2. In addition to the indicators highlighted as serving existing criteria in the descriptors, additional ‘potential’ indicators are also listed, which might be used in future for a more comprehensive assessment of cephalopods within this framework (although these probably require further evaluation first). Moreover, there are some inconsistencies or difficulties within the indicators themselves, not all of which are due to the particular biology of cephalopods. The detection, assessment of abundance and distribution of non-indigenous or cryptogenic cephalopod species is important due to their potentially strong influences on native biodiversity. Indicators are available and immediately applicable (Table 2), but which species are included in the category NIS requires clarification. NIS (synonyms: alien, exotic, non-native, allochthonous) are species, subspecies or lower taxa which have been introduced intentionally or accidentally in new areas (past or present) by different anthropogenic vectors or natural means (Olenin et al. 2010). The European Commission itself named NIS as species introduced by human activities (Commission Decision 2017/848/EU). However, the exact meaning of anthropogenically influenced NIS could be interpreted in different ways. All cephalopod species are more or less powerful swimmers and highly mobile. Various squids undertake diurnal vertical migrations which result in temporal and spatial fluctuations in their distribution and contribute significantly to the transfer of energy, resources and nutrients between deep water and shallow water systems. Therefore, deep-sea studies should include the assessment of cephalopods as well (Kazanidis et al. 2020). Horizontal movements are also common, due to long feeding and spawning migrations, which can likewise lead species into new areas. In addition, squid and some octopus species have planktonic paralarvae which may travel with ocean currents. Migrations are common and can be extensive in ommastrephids, to a lesser extent in loliginids, and only exist to some extent in cuttlefish. I. coindetii in the North Sea, for example, apparently represents a new spawning stock, which migrated into this area within the past 10 years (Oesterwind et al. 2020). Thus, this species entered the North Sea through natural pathways but was probably only able to colonize this new area due to global warming and the associated rise in temperature in the North Sea, which is influenced by human activity. Whether this is considered a natural or human-mediated range shift is somewhat ambiguous under present definitions.
In relation to fishing mortality for D3 we could illustrate that some data exist but those data are at a higher taxonomic level and, therefore, give only a rough idea about fishing mortality within the group. The unknown fishery status of certain commercially exploited species, particularly in some areas, as well as the unknown spatial structure of commercially fished stock units generally, needs to be addressed (Lishchenko et al. 2021) in future. Also, analysis of suggested descriptors by taxonomic family is clearly inappropriate in some cases, e.g. our analysis shows that commercially targeted species within a taxon show contrasting trends in fishing mortality and CPUE abundance relative to historical means (e.g. Tables 3. 4). Hence, in this case, assessing the group obscures contrasting trends within the group members.
Finally, achieving the major targets of the EU-MSFD requires knowledge of the food web and prediction of the trophic consequences of major changes to it (Heiskanen et al. 2016; Lynam and Mackinson 2015; Hastie et al. 2009). Different datasets can present different information in this regard. For example, survey-based TL indicators represent unbiased state changes at the level of the surveyed community while catch-based TL indicators show the pressure on the system (Heiskanen et al. 2016). Various food web models exist but cephalopods are not included in many cases, most likely due to knowledge gaps in knowledge of prey composition (Gasalla et al. 2010) and total cephalopod biomasses. However, Mackinson and Daskalov’s (2007) North Sea ecosystem model, for example, lists cephalopod biomass, productivity, catchability and the role of cephalopods as prey for apex predators. Given that some of the current D4 indicators and criteria are borrowed from other descriptors, there should be no problem assessing cephalopods under this Descriptor.
As a first approach, it seems reasonable to calculate the existing criteria and to fill the indicator gaps with adapted fish indicators. While the calculation of various indicators is possible, they clearly need a sensible interpretation because they have not been adapted to the specific biology of cephalopods. The typical annual fluctuations in abundance and biomass, the sensitivity of the life cycle stages to environmental factors and the short life cycle of cephalopods complicate the interpretation of any changes. In a worst case, the substantial biological differences between cephalopods and finfish make some of the fish indicators unhelpful (Arkhipkin et al. 2021). Most importantly, annual or short-lived species show a seasonal cycle in terms of distribution, abundance and size composition. The seasonal variability, against a background of strong environmental forcing, must, therefore, be considered for any assessment with use of the corresponding indicators. Due to this high variability, it is difficult to determine or set baselines/threshold values/reference points. Possible strategies to overcome these difficulties are to identify a threshold range that encompasses the normal natural variations. Such an environmentally dependent threshold can be determined, for example, by modeling the threshold as a function of the relevant environmental parameters and recalculating the threshold, as needed, to assess GES. Since cephalopod populations are subject to natural fluctuations, it is advisable to focus on trends rather than status. If there is a sustained decreasing trend over a period of time, this is likely a sign of a negative impact. Responding to short-term declines, while they may be environmental, might be advisable in order to reduce fishing pressure on stocks with low biomasses in particular assessment areas.
In conclusion, there is a need for indicators to be adapted and additional indicators developed to evaluate the status of European cephalopods inside the MSFD. Certainly, better definitions of GES are needed and thresholds for GES are not yet available, when it comes to cephalopods. Furthermore, the species identification of cephalopods has already improved in some surveys but is still insufficient in some areas and for some families, and has to improve especially, in commercial landings (see Lishchenko et al. 2021) to fill the data gaps. The lack of routine assessment of almost all cephalopod stocks in European waters should also be addressed, for both MSFD assessment and fishery management (Lishchenko et al. 2021). Despite all of this, we advocate that, based on our case studies, a first integration of cephalopods into the MSFD framework is already possible and would provide useful preliminary information on their status.
Conclusions and outlook
To ensure GES in Europe within the MSFD, cephalopods must be considered in the assessment, as it is the case for fish, marine mammals and marine birds. Besides CPUE-based indicators, other methods can already be applied using the existing data collected from commercial fisheries and research surveys incorporating cephalopods. The indicators, which can already be implemented in the framework of MSFD, are listed in bold in Table 2. For a more comprehensive cephalopod assessment in future, further potential indicators are listed (non-bold in Table 2). The total catch, total biomass and population abundance of cephalopods, for example, can be measured within the framework of ICES Trawl surveys (IBTS, BTS and MEDITS) on the basis of CPUE (catch per tow, catch per time or catch per area) using standardized fishing gear. However, absolute abundance estimates depend on an appropriate survey design (e.g. locations, timing) and the fishing gear (Pierce et al. 1998), which is always size-selective, to a certain extent, as the entire size range of a species cannot be caught with one net size. Another consideration is that these are finfish surveys and cephalopods are only fished as bycatch. Nevertheless, the data collected in these surveys over decades, along with newly collected data in future, possibly involving tweaked data collection frameworks, can and should be used for an assessment using relative trend analyses of commercially fished cephalopod species. Furthermore, indicators can be developed on the basis of these data (including threshold values, e.g. following trends analysis) and implemented in the cephalopod assessment within the MSFD. Using current and long-term data, it should then be possible to define or determine GES of the corresponding cephalopod species (or, at the very minimum, for higher taxa, even if this has large disadvantages). Arkhipkin et al. (2021) have already discussed indicators for the assessment of cephalopods for management purpose in target fisheries and have shown the inutility and faulty advice arising from some methods. For instance, serious problems have been shown with using MSY to manage Octopus fisheries (Roa-Ureta et al. 2021). Nevertheless, it is important to start with some kind of assessment, for example, using the indicators and methods we have listed here. In addition to the adaptation of the potential indicators, which are similar to ICES fisheries assessment category three (ICES 2012b), where stocks are assessed on the basis of surveys or other trend indices (ICES 2015), more data are needed to increase the quality of the indicators. This includes, for example, the monitoring design, quality of species identification, stock discrimination (Lishchenko et al. 2021; Göpel et al. 2022; Sheerin et al. 2022), and survey timing in relation to the life cycle. Further enhancement of cephalopod assessment methods is also required, along with improved understanding of their trophic importance and ‘Keystoneness’ within ecosystem models (e.g. Gasalla et al. 2010; Coll et al. 2013).
Data availability
The datasets used for the case study are available in ICES DATRAS Database (https://datras.ices.dk/Data_products/Download/Download_Data_public.aspx) or by the corresponding author on reasonable request.
References
Alemany J, Rivot E, Foucher E, Vigneau J, Robin J-P (2017) A Bayesian two-stage biomass model for stock assessment of data-limited species: an application to cuttlefish (Sepia officinalis) in the English Channel. Fish Res 191:131–143. https://doi.org/10.1016/j.fishres.2017.03.010
André M, Solé M, Lenoir M, Durfort M, Quero C, Mas A, Lombarte A, van der Schaar M, López-Bejar M, Morell M, Zaugg S, Houégnigan L (2011) Low-frequency sounds induce acoustic trauma in cephalopods. Front Ecol Environ 9(9):489–493. https://doi.org/10.1890/100124
Anonymous (2020) Marine Strategy Framework Directive 2008/56/EC: Article 17 update to Ireland’s Marine Strategy Part 1: Assessment (Article 8), Determination of Good Environmental Status (Article 9) and Environmental Targets (Article 10). Prepared by the Department of Housing, Planning and Local Government
Argüelles J, Tafur R, Taipe A, Villegas P, Keyl F, Dominguez N, Salazar M (2008) Size increment of jumbo flying squid Dosidicus gigas mature females in Peruvian waters, 1989–2004. Prog Oceanogr 79:308–312. https://doi.org/10.1016/j.pocean.2008.10.003
Arkhipkin AI, Rodhouse PGK, Pierce GJ, Sauer W, Sakai M, Allcock L et al (2015) World Squid Fisheries. Rev Fish Sci Aquac 23(2):92–252. https://doi.org/10.1080/23308249.2015.1026226
Arkhipkin AI, Hendrickson LC, Payá I, Pierce GJ, Roa-Ureta RH, Robin J-P, Winter A (2021) Stock assessment and management of cephalopods: advances and challenges for short-lived fishery resources. ICES J Mar Sci 78(2):714–730. https://doi.org/10.1093/icesjms/fsaa038
Balguerías E, Quintero ME, Hernández-González CL (2000) The origin of the Saharan Bank cephalopod fishery. ICES J Mar Sci 57(1):15–23. https://doi.org/10.1006/jmsc.1999.0572
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021a) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Denmark. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021b) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—The Netherlands. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021c) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Sweden. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021d) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Croatia. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021e) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Cyprus. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021f) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Mediterranean Sea Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/Regional%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021g) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Spain. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Banfi P, Gea G, Labayle L, Landais D, Muro M, Moreira G, McNeill A, Thomas I (2021h) Article 12 technical assessment of the 2018 updates of Articles 8, 9 and 10—Belgium. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/National%20technical%20reports%20MSFD%202018.zip. Access: 29 July 2022
Barrett C, MacLeod E, Oesterwind D, Laptikhovsky V (2021) Ommastrephid squid spawning in the North Sea: oceanography, climate change and species range expansion. Sci Mar 85(1):49–56. https://doi.org/10.3989/scimar.05065.005
Battaglia P, Pedà C, Sinopoli M, Romeo T, Andaloro F (2013) Cephalopods in the diet of young-of-the-year bluefin tuna (Thunnus thynnus L. 1758, Pisces: Scombridae) from the southern Tyrrhenian Sea (central Mediterranean Sea). Ital J Zool 80(4):560–565. https://doi.org/10.1080/11250003.2013.837105
Bello G (1991) Role of Cephalopods in the diet of the swordfish, Xiphias gladius, from the eastern Mediterranean Sea. Bull Mar Sci 49(1–2):312–324
Bello G, Andaloro F, Battaglia P (2020) Non-indigenous cephalopods in the Mediterranean Sea: a review. Acta Adriat 61(2):113–134. https://doi.org/10.32582/aa.61.2.549
BMU (2018) Zustand der deutschen Nordseegewässer 2018. Aktualisierung der Anfangsbewertung nach § 45c, der Beschreibung des guten Zustands der Meeresgewässer nach § 45d und der Festlegung von Zielen nach § 45e des Wasserhaushaltsgesetzes zur Umsetzung der Meeresstrategie-Rahmenrichtlinie. Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit (BMU)
Borja A, Menchaca I, Garmendia JM, Franco J, Larreta J, Sagarminaga Y, Schembri Y, González R, Antón R, Micallef T, Camilleri S, Solaun O, Uriarte A, Uyarra MC (2021) Big insights from a small country: the added value of integrated assessment in the marine environmental status evaluation of Malta. Front Mar Sci 8:638232. https://doi.org/10.3389/fmars.2021.638232
Brierley AS, Thorpe JP, Pierce GJ, Clark MR, Boyle PR (1995) Genetic variation in the neritic squid Loligo forbesii (Myopsida: Loliginidae) in the Northeast Atlantic-Ocean. Mar Biol 122(1):79–86. https://doi.org/10.1007/BF00354316
Bush SL, Hoving HJT, Huffard CL, Robinson BH, Zeidberg LD (2012) Brooding and sperm storage by the deep-sea squid Bathyteuthis berryi (Cephalopoda: Decapodiformes). J Mar Biol 92(07):1629–1636. https://doi.org/10.1017/S0025315411002165
Bustamente P, Cosson R, Gallien I, Caurant F, Miramand P (2002) Cadmium detoxification process in the digestive gland of cephalopods in relation to accumulated cadmium concentrations. Mar Environ Res 53(3):227–241. https://doi.org/10.1016/S0141-1136(01)00108-8
Caddy JF, Rodhouse PGK (1998) Cephalopod and groundfish landings: evidence for ecological change in global fisheries? Rev Fish Biol 8(4):431–444. https://doi.org/10.1023/A:1008807129366
Chen CS, Pierce GJ, Wang J, Robin J-P, Poulard JC, Pereira J, Zuur AF, Boyle PR, Bailey N, Bearem DJ, Jereb P, Ragonese S, Mannini A, Orsi-Relini L (2006) The apparent disappearance of Loligo forbesii from the south of its range in the 1990s: trends in Loligo spp. Abundance in the Northeast Atlantic and possible environmental influences. Fish Res 78(1):44–54. https://doi.org/10.1016/j.fishres.2005.12.002
Christensen V, Walters CJ (2004) Ecopath with Ecosim: methods, capabilities and limitations. Ecol Model 172(2–4):109–139. https://doi.org/10.1016/j.ecolmodel.2003.09.003
Clarke MR (1996a) Cephalopods as prey III. Cetaceans. Philos Trans R Soc Lond B Biol Sci 351(1343):1053–1065. https://doi.org/10.1098/rstb.1996.0093
Clarke MR (1996b) The role of cephalopods in the world’s oceans: an introduction. Philos Trans R Soc Lond B Biol Sci 351(1343):979–983. https://doi.org/10.1098/rstb.1996.0088
Cochrane SKJ, Connor DW, Nilsson P, Mitchell I, Reker J, Franco J, Valavanis V, Moncheva S, Ekebom J, Nygaard K, Serrão Santos R, Naberhaus I, Packeiser T, van de Bund W, Cardoso AC (2010) Marine Strategy Framework Directive task group 1 report: biological diversity. JRC Scientific and Technical Reports. Publications Office, Luxembourg, p 111. ISBN 978-92-79-15650-2
Coll M, Navarro J, Olson RJ, Christensen V (2013) Assessing the trophic position and ecological role of squids in marine ecosystems by means of food-web models. Deep Sea Res Part II 95:21–36. https://doi.org/10.1016/j.dsr2.2012.08.020
Colloca F, Garofalo G, Bitetto I, Facchini IB, Grati F, Martiradonna A, Mastrantonio G, Nikolioudakis N, Ordinas F, Scarcella G, Tserpes G, Tugores MP, Valavanis V, Carlucci R, Fiorentino F, Follesa MC, Iglesias M, Knittweis L, Lefkaditou E, Lembo G, Manfredi C, Massutí E, Pace ML, Papadopoulou N, Sartor P, Smith CJ, Specicato MT (2015) The seascape of demersal fish nursery areas in the North Mediterranean Sea, a first step towards the implementation of spatial planning for trawl fisheries. PLoS ONE. https://doi.org/10.1371/journal.pone.0119590
COM/2020/259: report from the Commission to the European Parliament and the Council on the implementation of the Marine Strategy Framework Directive (Directive 2008/56/EC)
Commission Decision 2010/477/EU: Commission Decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters (notified under document C (2010) 5956) Text with EEA relevance
Commission Decision 2017/848/EU: Commission Decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU (Text with EEA relevance)
Council Regulation (EC) No 1967/2006: Council Regulation (EC) No 1967/2006 of 21 December 2006 concerning management measures for the sustainable exploitation of fishery resources in the Mediterranean Sea, amending Regulation (EEC) No 2847/93 and repealing Regulation (EC) No 1626/94
Cuccu D, Mereu M, Porcu C, Follesa MC, Cau AL, Cau A (2013) Development of sexual organs and fecundity in Octopus vulgaris Cuvier, 1797 from the Sardinian waters (Mediterranean Sea). Mediterr Mar Sci 14(2):270–277. https://doi.org/10.12681/mms.412
Daly HI, Pierce GJ, Santos MB, Royer J, Cho SK, Stowasser G, Robin J-P, Henderson SM (2001) Cephalopod consumption by trawl caught fish in Scottish and English Channel waters. Fish Res 52:51–64. https://doi.org/10.1016/S0165-7836(01)00230-2
Deidun A, Schembri Y, Dunlop S, Martin Anton R, Gonzalo Jemenez R, Borja A, Franco J, Garmendja JM, Larreta J, Menchaca I, Sagarminaga Y, Uriarte A, Valencia V, Ramos-Espla A (2019) Preliminary results from MSFD-prescribed national monitoring within maltese waters for descriptor 2 (NIS). Conference: proceedings of the 1st Mediterranean symposium on the non-indigenous species. Antalya, Turkey
Doubleday ZA, Prowse TAA, Arkhipkin A, Pierce GJ, Semmens J, Steer M, Leporati SC, Lourenço S, Quetglas A, Sauer W, Gillanders BM (2016) Global proliferation of cephalopods. Curr Biol 26(10):R406–R407. https://doi.org/10.1016/j.cub.2016.04.002
Dupont C, Belin A, Vermonden B, Moreira G (2014a) Article 12 Technical Assessment of the MSFD 2012 obligations—Belgium. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014b) Article 12 Technical Assessment of the MSFD 2012 obligations—Cyprus. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014c) Article 12 Technical Assessment of the MSFD 2012 obligations—Denmark. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014d) Article 12 Technical Assessment of the MSFD 2012 obligations—France. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014e) Article 12 Technical Assessment of the MSFD 2012 obligations—Ireland. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014f) Article 12 Technical Assessment of the MSFD 2012 obligations—The Netherlands. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014g) Article 12 Technical Assessment of the MSFD 2012 obligations—Germany. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014h) Article 12 Technical Assessment of the MSFD 2012 obligations—United Kingdom. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014i) Article 12 Technical Assessment of the MSFD 2012 obligations—Portugal. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014j) Article 12 Technical Assessment of the MSFD 2012 obligations—Spain. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Dupont C, Belin A, Vermonden B, Moreira G (2014k) Article 12 Technical Assessment of the MSFD 2012 obligations—Italy. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/implementation/pdf/national_reports.zip. Access: 29 July 2022
Fewtrell JL, McCauley RD (2012) Impact of air gun noise on the behaviour of marine fish and squid. Mar Pollut Bull 64(5):984–993. https://doi.org/10.1016/j.marpolbul.2012.02.009
Fortibuoni T, Giovanardi O, Pranovi F, Raicevich S, Solidoro C, Libralato S (2017) Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Front Mar Sci 4:33. https://doi.org/10.3389/fmars.2017.00033
Froese R, Winker H, Coro G, Demirel N, Tsikliras A, Dimarchopoulou D et al (2018) Status and rebuilding of European fisheries. Mar Policy 93:159–170. https://doi.org/10.1016/j.marpol.2018.04.018
Gasalla MA, Rodrigues AR, Postuma FA (2010) The trophic role of the squid Loligo plei as a keystone species in the South Brazil Bight ecosystem. ICES J Mar Sci 67(7):1413–1424. https://doi.org/10.1093/icesjms/fsq106
Geraci ML, Falsone F, Gancitano V, Scannella D, Fiorentino F, Vitale S (2021) Assessing cephalopods fisheries in the Strait of Sicily by using poor data modelling. Front Mar Sci 8:584657. https://doi.org/10.3389/fmars.2021.58465
Giovos I, Kleitou P, Poursanidis D, Batjakas I, Bernardi G, Crocetta F, Doumpas N, Laogirou S, Kampouris TE, Keramidas I, Langeneck J, Maximiadi M, Mitsou E, Stoilas V-O, Tiralongo F, Romanidis-Kyriakidis G, Xentidis N-J, Zenetos A, Katsanevakis S (2019) Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean. Biol Invasions 21:3707–3721. https://doi.org/10.1007/s10530-019-02083-w
Göpel A, Oesterwind D, Barrett C et al (2022) Phylogeography of the veined squid, Loligo forbesii, in European waters. Sci Rep 12:7817. https://doi.org/10.1038/s41598-022-11530-z
Gras M, Roel BA, Coppin F, Foucher E, Robin J-P (2014) A two-stage biomass model to assess the English Channel cuttlefish (Sepia officinalis L.) stock. ICES J Mar Sci 71(9):2457–2468. https://doi.org/10.1093/icesjms/fsu081
Guerra Á, González ÁF (2006) Severe injuries in the giant squid Architeuthis dux stranded after seismic explorations. Paper presented at Impacts of Seismic Survey Activities on Whales and Other Marine Biota, Dessau. http://www.umweltbundesamt.de
Guerra Á, Rocha F (1994) The life history of Loligo vulagris and Loligo forbesii (Cephalopoda: Loliginidae) in Galician waters (NW Spain). Fish Res 2(1–2):43–69. https://doi.org/10.1016/0165-7836(94)90095-7
Guerra Á, González ÁF, Pascual S, Dawe EG (2011) The giant squid Architeuthis: an emblematic invertebrate that can represent concern for the conservation of marine biodiversity. Biol Conserv 144(7):1989–1997. https://doi.org/10.1016/j.biocon.2011.04.021
Harley SJ, Myers RA, Dunn A (2001) Is catch-per-unit-effort proportional to abundance? Can J Fish Aquat Sci 58:1760–1722
Hastie LC, Joy JB, Pierce GJ, Yau C (1994) Reproductive biology of Todaropsis eblanae (Cephalopoda: Ommastrephidae) in Scottish waters. J Mar Biolog Assoc UK 74(2):367–382. https://doi.org/10.1017/S0025315400039394
Hastie LC, Pierce GJ, Wang J, Bruno I, Moreno A, Piatkowski U, Robin J-P (2009) Cephalopods in the north-eastern Atlantic: species, biogeography, ecology, exploitation and conservation. Oceanogr Mar Biol 47:111–190
Heiskanen A-S, Berg T, Uusitalo L, Teixeira H, Bruhn A, Kraus-Jensen D, Lynam CP, Rossberg AG, Korpinen S, Uyarra MC, Borja A (2016) Biodiversity in marine ecosystems—European developments towards robust assessments. Front Mar Sci. https://doi.org/10.3389/fmars.2016.00184
Hewitt CL, Campbell M (2010) The relative contribution of vectors to the introduction and translocation of marine invasive species. Australian Department of Agriculture, Fisheries and Forestry, Canberra, p 56
Hunsicker ME, Essington TE, Watson R, Sumaila UR (2010) The contribution of cephalopods to global marine fisheries: can we have our squid and eat them too? Fish Fish 11(4):421–438. https://doi.org/10.1111/j.1467-2979.2010.00369.x
Hurlbert SH (1997) Functional importance vs keystoneness: reformulating some questions in theoretical biocenology. Aust J Ecol 22(4):369–382. https://doi.org/10.1111/j.1442-9993.1997.tb00687.x
ICES (2009) Manual for the Offshore Beam Trawl Surveys, Revision 1.2, June 2009, Working Group on Beam Trawl Surveys, p 30
ICES (2012a) Manual for the international bottom trawl surveys. Series of ICES Survey Protocols. SISP 1-IBTS VIII
ICES (2012b) ICES implementation of advice for data-limited stocks in 2012 in its 2012 Advice. ICES CM 2012/ACOM 68, p 42. https://doi.org/10.17895/ices.pub.5322
ICES (2014a) Report of the Working Group on the Ecosystem Effects of Fishing Activities (WGECO), 8–15 April 2014a, Copenhagen, Denmark. ICES CM 2014a/ACOM:26
ICES (2014b) Report of the Workshop to develop recommendations for potentially use-full Food Web Indicators (WKFooWI), 31 March–3 April 2014b, ICES Headquarters, Copenhagen, Denmark. ICES CM 2014b\ACOM:48, p 75
ICES (2015) General context of ICES advice. ICES technical guidelines. Report. https://doi.org/10.17895/ices.advice.18667646.v1
ICES (2018) Report of the Working Group on the Ecosystem Effects of Fishing Activities (WGECO), 12–19 April 2018, San Pedro del Pinatar, Spain. ICES CM 2018/ACOM:27, p 69
ICES (2020a) EU request for advice on developing appropriate lists for Descriptor 3, commercially exploited fish and shellfish, for reporting by EU Member States under MSFD Article 17 in 2024. In: Report of the ICES Advisory Committee, ICES Advice 20. https://doi.org/10.17895/ices.advice.7646
ICES (2020b) Working Group on Cephalopod Fisheries and Life History (WGCEPH; outputs from 2019 meeting). ICES Sci Rep 2:46. https://doi.org/10.17895/ices.pub.6032
ICES (2023) Report of the ICES Working Group on cephalopod fisheries and life history. ICES Sci Rep 5(1)
iSea (2022) Is it Alien to you…. Share it!!!. https://isea.com.gr/activities/projects/citizen-science/is-it-alien-to-you-share-it/?lang=en. Access: 10 Sept 2022
Jackson GD, Yeatman J (1996) Variation in size and age at maturity in Photololigo (Mollusca: Cephalopoda) from the northwest shelf of Australia. Fish Bull 94:59–65
Jennings S, van der Molen J (2015) Trophic levels of marine consumers from nitrogen stable isotope analysis: estimation and uncertainty. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsv120
Jereb P, Allcock AL, Lefkaditou E, Piatkowski U, Hastie LC, Pierce G (2015) Cephalopod biology and fisheries in Europe: II. Species accounts. In: ICES Cooperative Research Report 325. https://doi.org/10.13140/RG.2.1.1081.616
Joshi A, Desai AY, Kumar J, Saroj J, Teheesen P (2016) Assessment of mean trophic level and prey–predator relationship. Int J Sci Environ Technol 5(3):1046–1056
Katağan T, Tokaç A, Beşiktepe Ş, Öztürk B (2015) The Aegean Sea marine biodiversity, fisheries, conservation and governance. Turkish Marine Research Foundation (TUDAV). Türk Deniz Araştırmaları Vakfı (Turkish Marine Research Foundation). ISBN: ISBN-978-975-8825-33-2
Kazanidis G, Orejas C, Borja A, Kenchington E, Henry L-A, Callery O, Carreiro-Silva M, Egilsdottir H, Giacomello E, Grehan A, Menot L, Morato T, Raganarsson SA, Rueda JL, Stirling D, Stratmann T, van Oevelen DA, Johnson D, Roberts JM (2020) Assessing the environmental status of selected North Atlantic deep-sea ecosystems. Ecol Indic 119(1):106624. https://doi.org/10.1016/j.ecolind.2020.106624
King M (2007) Fisheries biology, assessment and management. Blackwell Publishing, Oxford, p 382
Kleitou P, Crocetta F, Giakoumi S, Giovos I, Hall-Spencer JM, Kalogirou S, Kletou D, Moutopoulos DK, Rees S (2021) Fishery reforms for the management of non-indigenous species. J Environ Manag 280:111690. https://doi.org/10.1016/j.jenvman.2020.111690
Krstulović Šifner S, Peharda M, Vrgoč N, Isajlović I, Dadić V, Petrić M (2011) Biodiversity and distribution of cephalopods caught by trawling along the northern and central Adriatic Sea. Cah Biol Mar 52:291–302
Laptikhovsky V (2000) Fecundity of the squid Loligo vulgaris Lamarck, 1798 (Myopsida, Loliginidae) of northwest Africa. Sci Mar 64(3):275–278. https://doi.org/10.3989/scimar.2000.64n3275
Laptikhovsky V, Allcock L, Barnwall L, Barrett C, Cooke G, Drerup C, Firmin C, Lozach S, Ludwig KE, Oesterwind D, Petroni M, Robin J-P, Sheerin E, Power A-M, Pierce G (2022) Spatial and temporal variability of spawning and nursery grounds of Loligo forbesii and Loligo vulgaris squids in ecoregions of Celtic Seas and Greater North Sea. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsac128
Larivain A (2020) Stock assessment trials for the cuttlefish (Sepia officinalis) in the English Channel (7.de) using SPiCT: 33–34. In: ICES (2020) Workshop on Data-limited Stocks of Short-Lived Species (WKDLSSLS2). ICES Scientific Reports. 2:99. https://doi.org/10.17895/ices.pub.5984
Lipinski MR (1992) Cephalopods and the Benguela Ecosystem: trophic relationships and impact. S Afr J Mar Sci 12(1):791–802. https://doi.org/10.2989/02577619209504742
Lishchenko F, Perales-Raya C, Barrett C, Oesterwind D, Power AM, Larivain A, Laptikhovsky V, Karatza A, Badouvas N, Lishchenko A, Pierce GJ (2021) A review of recent studies on the life history and ecology of European cephalopods with emphasis on species with the greatest commercial fishery and culture potential. Fish Res 236(3):105847. https://doi.org/10.1016/j.fishres.2020.105847
Lynam CP, Mackinson S (2015) How will fisheries management measures contribute towards the attainment of good environmental status for the North Sea ecosystem? Glob Ecol Conserv 4(C):160–175. https://doi.org/10.1016/j.gecco.2015.06.005
Mackinson S, Daskalov G (2007) An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation. Science Series Technical Report, Cefas Lowestoft, vol 142, p 196
Maggi C, Lomiri S, Di Lorenzo B, d’Antona M, Berducci MT (2014) Environmental quality of Italian marine water by means of marine strategy framework directive (MSFD) descriptor 9. PLoS ONE 9(9):e108463. https://doi.org/10.1371/journal.pone.0108463
Mangold K, Boletzky SV (1973) New data on reproductive biology and growth of Octopus vulgaris. Mar Biol 19:7–12. https://doi.org/10.1007/BF00355414
Mangold K, Boletzky SV (1988) Mediterranean cephalopod fauna. In: Clarke MR, Trueman ER (eds) The mollusca. Paleontology and neontology of cephalopods 12. Academic Press Inc, San Diego, New York, Berkeley, Boston, London, Sydney, Tokyo, Toronto, pp 315–330. https://doi.org/10.1016/B978-0-12-751412-3.50025-5
Marchal P, Levring Andersen J, Aranda M, Fitzpatrick M, Goti L, Guyader O, Haraldsson G, Hatcher A, Hegland TJ, Le Floch P, Macher C, Malvarosa L, Maravelias CD, Mardle S, Murillas A, Rasmus Nielsen J, Sabatella R, Smith ADM, Stokes K, Thoegersen T, Ulrich C (2016) A comperative review of fisheries management experiences in the European Union and in other countries worldwide; Iceland, Australia, and New Zealand. Fish Fish 17(3):803–824. https://doi.org/10.1111/faf.12147
Ministerio do Mar (2020) Reavaliação do Estado Ambiental e Definição de Metas: Parte D, Subdivisão do Continente. Estratégia Marinha, Relatório do 2º ciclo. Ministério do Mar, República Portuguesa, p 458
Morato T, Lemey E, Menezes G, Pham CK, Brito J, Soszynski A, Pitcher TJ, Heymans JJ (2016) Food-web and ecosystem structure of the open-ocean and deep-sea environments of the Azores, NE Atlantic. Front Mar Sci. https://doi.org/10.3389/fmars.2016.00245
Moura T, Chaves C, Figueiredo I, Mendes H, Moreno A, Silva C, Vasconcelos RP, Azevedo M (2020) Assessing spatio-temporal changes in marine communities along the Portuguese continental shelf and upper slope based on 25 years of bottom trawl surveys. Mar Environ Res 160:105044. https://doi.org/10.1016/j.marenvres.2020.105044
Nesis KN (1985) Oceanic cephalopods, distribution, life forms and evolution. Nauka, Moscow
NOAA (2007). Magnuson-Stevens Fishery Conservation and Management Act, as amended through January 12, 2007. U.S. Department of Commerce, National Marine Fisheries Service, p 170. Available at: https://media.fisheries.noaa.gov/dam-migration/msa-amended-2007.pdf. Access: 04 Aug 2022
O’Higgins T (2016) Marine Strategy Framework Directive discovering data and information in the Celtic Seas. Report to the British Oceanographic Data Centre for the Celtic seas Partnership Project, p 37
Obst M, Exter K, Allcock AL, Arvanitidis C, Axberg A, Bustamante M et al (2020) A Marine Biodiversity Observation Network for Genetic Monitoring of Hard-Bottom Communities (ARMS-MBON). Front Mar Sci 7:572680. https://doi.org/10.3389/fmars.2020.572680
Oesterwind D, Schaber M (2020) First evidence of Illex coindetii (Vérany, 1839) in the Baltic Sea and the Kattegat. Thalassas Int J Mar Sci 36(4):143–147. https://doi.org/10.1007/s41208-019-00178-8
Oesterwind D, Hofstede R, Harley B, Brendelberger H, Piatkowski U (2010) Biology and meso-scale distribution patterns of North Sea cephalopods. Fish Res 106(2):141–150. https://doi.org/10.1016/j.fishres.2010.06.003
Oesterwind D, Piatkowski U, Brendelberger H (2015) On distribution, size and maturity of shortfin squids (Cephalopoda, Ommastrephidae) in the North Sea. Mar Biol Res 11(2):188–196. https://doi.org/10.1080/17451000.2014.894246
Oesterwind D, Rau A, Zaiko A (2016) Drivers and pressures—untangling the terms commonly used in marine science and policy. Open Access J Environ Manag 181:8–15. https://doi.org/10.1016/j.jenvman.2016.05.058
Oesterwind D, Barrett C, Bobowski BTC, Brunsch A, Laptikhovsky V, Visconti V (2018) Trial study about age reading of North Sea squids. Working Document in: ICES. 2019. Interim report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH), 5–8 June 2018, Pasaia, San Sebastian, Spain. ICES CM 2018/EPDSG:12, pp 188–194
Oesterwind D, Bobowski BTC, Brunsch A, Laptikhovsky V, van Hal R, Sell AF, Pierce GJ (2020) First evidence of a new spawning stock of Illex coindetii in the North Sea (NE-Atlantic). Fish Res. https://doi.org/10.1016/j.fishres.2019.105384
Oesterwind D, Barrett CJ, Sell AF, Núñez-Riboni I, Kloppmann M, Piatkowski U, Wieland K, Laptikhovsky V (2022) Climate change-related changes in cephalopod biodiversity on the North East Atlantic Shelf. Biodivers Conserv. https://doi.org/10.1007/s10531-022-02403-y
Olenin S, Alemany F, Cardoso AC, Gollasch S, Goulletquer P, Lehtiniemi M, McCollin T, Minchin D, Miossec L, Occhipinti Ambrogi A, Ojaveer H, Jensen KR, Stankiewicz M, Wallentinus I, Aleksandrov B (2010) Marine Strategy Framework Directive—task group 2 report: non-indigenous species. Publications Office, Luxembourg. https://data.europa.eu/doi/10.2788/87092
Ormseth OA, Conners ME (2018) Assessment of the Octopus Stock Complex in the Gulf of Alaska. Alaska Fisheries Science Center. https://repository.library.noaa.gov/view/noaa/20260
OSPAR (2016) CEMP guidelines. Common indicator: seal abundance and distribution (M3), Paris, Agreement 2016-11, p 20
OSPAR (2018) CEMP guidelines. Common indicator: changes to non-indigenous species communities (NIS3), Paris, Agreement 2018-04e, p 8
Ospina-Alvarez A, de Juan S, Pita P, Ainsworth GB, Matos FL, Pita C, Villasante S (2021) Main actors in the Cephalopod Global Trade Network: a graph theory analysis. Project: cephs and chefs—octopus, squid, cuttlefish, sustainable fisheries and chefs. https://doi.org/10.21203/rs.3.rs-721404/v1
Paine RT (1995) A conversation on refining the concept of keystone species. Conserv Biol 9:962–964. https://doi.org/10.1046/j.1523-1739.1995.09040962.x
Palma-Pedraza S, Sarrazin V, Clarke M, Stokes D (2020) Status of non-assessed fish species in Irish waters. Irish Fish Bull, No. 51. Marine Institute, Ireland
Paramana T, Karditsa A, Milatou N, Petrakis S, Megalofonou P, Poulos S, Dassenakis M (2021) MSFD in-depth knowledge of the marine environment as the stepping stone to perform marine spatial planning in Greece. Water 13(15):2084. https://doi.org/10.3390/w13152084
Payne AG, Agnew DJ, Pierce GJ (2006) Trends and assessment of cephalopod fisheries. Fish Res 78(1):1–3. https://doi.org/10.1016/j.fishres.2005.12.010
Pecl G, Jackson GD (2008) The potential impacts of climate change on inshore squid: biology, ecology and fisheries. Rev Fish Biol Fisheries 18:373–385. https://doi.org/10.1007/s11160-007-9077-3
Pierce GJ, Guerra A (1994) Stock assessment methods used for cephalopod fisheries. Fish Res 21(1–2):255–285. https://doi.org/10.1016/0165-7836(94)90108-2
Pierce GJ, Boyle PR, Hastie LC, Shanks AM (1994) Distribution and abundance of the fished population of Loligo forbesii in UK waters: analysis of fishery data. Fish Res 21(1–2):193–216. https://doi.org/10.1016/0165-7836(94)90104-X
Pierce GJ, Bailey N, Stratoudakis Y, Newton A (1998) Distribution and abundance of the fished population Loligo forbesii in Scottish waters: analysis of research cruise data. ICES J Mar Sci 55:14–33. https://doi.org/10.1006/jmsc.1997.0257
Pierce GJ, Zuur AF, Smith JM, Santos MB, Bailey N, Chen C-S, Boyle PR (2005) Interannual variation in life-cycle characteristics of the veined squid (Loligo forbesii) in Scottish (UK) waters. Aquat Living Resour 18:327–340. https://doi.org/10.1051/alr:2005037
Pierce GJ, Allcock L, Bruno I, Bustamante P, González À, Guerra À, Jereb P, Lefkaditou E, Malham S, Moreno A, Pereira J, Piatkowski U, Rasero M, Sánchez P, Santos MB, Santurtún M, Seixas S, Sobrino I, Villanueva R (2010) Cephalopod biology and fisheries in Europe. ICES cooperative research report 303, p 181. https://doi.org/10.17895/ices.pub.5414
Pierce GJ, Hastie LC, El Shanawany N, Fernandez J, Valavanis V, Robin J-P, Arkhipkin A, Santos MB, Ellis J, Roel B, Burns F, Greenstreet S (2016) Cephalopod indicators for the MSFD. Final Report to Defra on Contract No ME5311. Corpus ID: 53629536
Piet GJ, Albella AJ, Aro E, Farrugio H, Lleonart J, Lordan C, Mesnil B, Petrakis G, Push C, Radu G, Rätz H-J (2010) Marine Strategy Framework Directive—task group 3 report: commercially exploited fish and shellfish, Luxembourg. JRC Scientific and Technical Reports, p 82. https://data.europa.eu/doi/10.2788/83073
Planque B, Loots C, Petitgas P, Lindstrøm U, Vaz S (2011) Understanding what controls the spatial distribution of fish populations using a multi-model approach. Fish Oceanogr 20(1):1–17. https://doi.org/10.1111/j.1365-2419.2010.00546.x
Probst WN, Oesterwind D (2014) How good are alternative indicators for spawning-stock biomass (SSB) and fishing mortality (F)? ICES J Mar Sci 71:1137–1141. https://doi.org/10.1093/icesjms/fst207
Probst WN, Rau A, Oesterwind D (2016) A proposal for restructuring Descriptor 3 of the Marine Strategy Framework Directive (MSFD). Mar Policy 74:128–135. https://doi.org/10.1016/j.marpol.2016.09.026
Quincoces I, Arregi L, Basterretxea M, Galparsoro I, Garmendia JM, Martínez J, Rodríguez JG, Uriarte A (2011) Ecosistema bento-demersal de la plataforma costera vasca, información para su aplicación en la Directiva Marco de la Estrategia Marina europea. 18(4). AZTI-Tecnalia
Regueira M, González AF, Guerra A, Soares A (2013) Reproductive traits of horned octopus Eledone cirrhosa in Atlantic Iberian waters. J Mar Biol Assoc UK 93(06):1641–1652. https://doi.org/10.1017/S0025315413000118
Roa-Ureta RH, del Pino F-R, Acuña JL, Rivera A, González-Gil R, García-Flórez L (2021) Estimation of the spawning stock and recruitment relationship of Octopus vulgaris in Asturias (Bay of Biscay) with generalized depletion models: implications for the applicability of MSY. ICES J Mar Sci 78(6):2256–2270. https://doi.org/10.1093/icesjms/fsab113
Rodhouse PGK, Pierce GJ, Nichols OC, Sauer WHH, Arkhipkin AI, Laptikhovsky VV, Lipinski ML, Ramos J, Gras M, Kidokoro H, Sadayasu K, Pereira J, Lefkaditou E, Pita C, Gasalla M, Haimovici M, Sakai M, Downey N (2014) Environmental effects on cephalopod population dynamics: implications for management of fisheries. Adv Mar Biol 67C:99–234. https://doi.org/10.1016/B978-0-12-800287-2.00002-0
Rogers S, Casini M, Cury P, Heath M, Irigoien X, Kuosa H, Scheidat M, Skov H, Stergiou K, Trenkel V, Wikner J, Yunev O (2010) Marine Strategy Framework Directive—task group 4 report food webs. EUR 24343 EN. Publications Office, Luxembourg. JRC58110. ISBN: 978-92-79-15656-4. https://data.europa.eu/doi/10.2788/87659
Rogov M, Shcheptova E, Ippolitov AP, Seltser VB, Mironenko A, Pokrovsky BG, Desai BG (2019) Response of cephalopod communities on abrupt environmental changes during the early Aptain OAE1a in the Middle Russian Sea. Cretac Res 96:227–240. https://doi.org/10.1016/j.cretres.2019.01.007
Rosa R, Gonzalez L, Dierssen HM, Seibel BA (2012) Environmental determinants of latitudinal size-trends in cephalopods. Mar Ecol Prog Ser 464:153–165. https://doi.org/10.3354/meps09822
Santos M, Pierce GJ (2015) Marine mammals and good environmental status: science, policy and society; challanges and opportunities. Hydrobiologia. https://doi.org/10.1007/s10750-014-2164-2
Seibel B, Hochberg FG, Carlini D (2000) Life history of Gonatus onyx (Cephalopoda: Teuthoidea): deep-sea spawning and post-spawning egg care. Mar Biol 137(3):519–526. https://doi.org/10.1007/s002270000359
Shannon JL, Coll M, Bundy A, Gascuel D, Heymans JJ, Kleisner K, Lynam CP, Piroddi C, Tam J, Travers-Trolet M, Shin YJ (2014) Trophic level-based indicators to track fishing impacts across marine ecosystems. Mar Ecol Prog Ser 512:115–140. https://doi.org/10.3354/meps10821
Shaw PW, Pierce GJ, Boyle PR (1999) Subtle population structuring within a highly vagile marine invertebrate, the veined squid Loligo forbesi, demonstrated with microsatellite DNA markers. Mol Ecol 8:407–417. https://doi.org/10.1046/j.1365-294X.1999.00588.x
Sheerin E, Barnawall L, Abad E, Larivain A, Oesterwind D, Petroni M, Perales-Raya C, Robin JP, Sobrino I, Valeiras J, O’Meara D (2022) Multi-method approach shows stock structure in Loligo forbesii squid. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsac039
Shephard S, van Hal R, de Boois I, Birchenough SNR, Foden J, O’Connor J, Geelhoed SCV, Van Hoey G, Marco-Rius F, Reid DG, Schaber M (2015) Making progress towards integration of existing sampling activities to establish Joint Monitoring Programmes in support of the MSFD. Mar Policy 59:105–111. https://doi.org/10.1016/j.marpol.2015.06.004
Smale MJ (1996) Cephalopods as prey. IV. Fishes. Philos Trans R Soc Lond B Biol Sci 351(1343):1067–1081. https://doi.org/10.1098/rstb.1996.0094
Smith C, Papadopoulou N, Sevastou K, Franco A, Teixeira H, Piroddi C, Katsanevakis S, Fürhaupter K, Beauchard O, Cochrane S, Ramsvatn S, Feral J-P, Chenuil A, David R, Kiriakopoulou N, Zaiko A, Moncheva S, Stefanova K, Churilova T, Krvenko O (2014) Report on identification of keystone species and processes across regional seas. Report number: Deliverable 6.1, DEVOTES Project. 105 pp + 1 Annex. Project: DEVOTES: DEVelopment Of innovative Tools for understanding marine biodiversity and assessing. https://doi.org/10.1016/j.ecolind.2020.107148
Sobrino I, Rueda L, Pilar Tugores M, Burgos C, Cojan M, Pierce GJ (2020) Abundance prediction and influence of environmental parameters in the abundance of Octopus (Octopus vulgaris Cuvier, 1797) in the Gulf of Cadiz. Fish Res 221:105382. https://doi.org/10.1016/j.fishres.2019.105382
Solé M, Lenoir M, Durfort M, López-Bejar M, Lombarte A, Van Der Schaar M, André M (2013a) Does exposure to noise from human activities compromise sensory information from cephalopod statocysts? Deep Sea Res Part II 95:160–181. https://doi.org/10.1016/j.dsr2.2012.10.006
Solé M, Lenoir M, Durfort M, López-Bejar M, Lombarte A, André M (2013b) Ultrastructural damage of Loligo vulgaris and Illex coindetii statocysts after low frequency sound exposure. PLoS ONE 8(10):e78825. https://doi.org/10.1371/journal.pone.0078825
Solé M, Sigray P, Lenoir M, Van Der Schaar M, Lalander E, André M (2017) Offshore exposure experiments on cuttlefish indicate received sound pressure and particle motion levels associated with acoustic trauma. Sci Rep 7:45899. https://doi.org/10.1038/srep45899
Solé M, De Vreese S, Fortuño J-M, Van Der Schaar M, Sánchez AM, André M (2022) Commercial cuttlefish exposed to noise from offshore windmill construction show short-range acoustic trauma. Environ Pollut 312(9):119853. https://doi.org/10.1016/j.envpol.2022.119853
Sundet JH (1985) A short review on the biology and fishery of the squid Todarodes sagittatus. ICES
Tam JC, Link JS, Rossberg AG, Rogers SI, Levin PS, Rochet M-J, Bundy A, Belgrano A, Libralato S, Tomczak M, van der Wolfshaar K, Pranovi F, Gorokhova E, Large SI, Niquil N, Greenstreet SPR, Druon J-N, Lesutiene J, Johansen M, Preciado I, Patricio J, Palialexis A, Tett P, Johansen GO, Houle J, Rindorf A (2017) Towards ecosystem-based management: identifying operational food-web indicators for marine ecosystems. ICES J Mar Sci 74(7):2040–2052. https://doi.org/10.1093/icesjms/fsw230
Thomsen MS, Wernberg T, Schiel D (2015) Invasions by non-indigenous species. In: Crowe T, Frid C (eds) Marine ecosystems: human impacts on biodiversity, functioning and services (ecology, biodiversity and conservation). Cambridge University Press, Cambridge, pp 274–332. https://doi.org/10.1017/CBO9781139794763.010
Tsiamis K, Zenetosm A, Deriu I, Gervasini E, Cardoso AC (2018) The native distribution range of the European marine non-indigenous species. Aquat Invasions 13(2):187–198. https://doi.org/10.3391/ai.2018.13.2.01
Tsiamis K, Palialexis A, Connor D, Antoniadis S, Bartilotti C, Bartolo GA et al (2021) Marine Strategy Framework Directive Descriptor 2, non-indigenous species: delivering solid recommendations for setting threshold values for non-indigenous species pressure on European seas. JRC Science for Policy Report. https://doi.org/10.2760/035071
Tsikliras AC, Touloumis K, Pardalou A, Adamidou A, Keramidas I, Orfanidis GA, Dimarchopoulou D, Koutrakis M (2021) Status and exploitation of 74 un-assessed demersal fish and invertebrate stocks in the Aegean Sea (Greece) using abundance and resilience. Front Mar Sci 7:578601. https://doi.org/10.3389/fmars.2020.578601
Van der Kooij J, Engelhard GH, Righton DA (2016) Climate change and squid range expansion in the North Sea. J Biogeogr 43(11):2285–2298. https://doi.org/10.1111/jbi.12847
Whitfield AK (2016) Biomass and productivity of fishes in estuaries: a South African case study. J Fish Biol. https://doi.org/10.1111/jfb.13110
Zador SG, Holsman KK, Aydin KY, Gaichas SK (2016) Ecosystem considerations in Alaska: the value of qualitative assessments. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsw144
Zeidberg LD, Robinson BH (2007) Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. PNAS 104(31):12948–12950. https://doi.org/10.1073/pnas.0702043104
Acknowledgements
BTCB thanks the German Foundation ‘Evangelisches Studienwerk Villigst’ for funding the doctorate in the context of which this review was written. In this context, she would also like to thank Dr. Stefan Forster from the marine biology department at the University of Rostock, who made the application for the scholarship possible. We also thank ICES WGCEPH, where elements and contents of this review were discussed. Finally, we thank the anonymous reviewers for their comments that improved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the Begabtenförderungswerk Evangelisches Studienwerk Villigst e.V.
Author information
Authors and Affiliations
Contributions
BTCB and DO contributed to the study conception the design and wrote the first draft. AMP, AI, AM developed landings and survey tables, DO analysed length and geographic distribution for the case study. All authors reviewed and revised the first draft and gave final approval for submission. Finally, all authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethics approval
A corresponding declaration that all applicable international, national and/or institutional guidelines for the sampling, care and experimental use of organisms for the study have been followed and all necessary permissions have been obtained is accordingly not necessary.
Additional information
Responsible Editor: Ulrich Sommer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bobowski, B.T.C., Power, A.M., Pierce, G.J. et al. Cephalopods, a gap in the European Marine Strategy Framework Directive and their future integration. Mar Biol 170, 26 (2023). https://doi.org/10.1007/s00227-022-04148-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04148-2