Abstract
Efforts for the conservation of green turtles (Chelonia mydas) in the Chagar Hutang Turtle Sanctuary (CHTS), Redang Island, Malaysia, have been in place for over two decades. Here we propose that the recruitment of new mothers to this rookery has a significant influence on the recovery of nest abundance in the long term. A 2–3 years’ quasi-periodic oscillation in nest abundance was also observed. This fluctuation might have been a consequence of El Niño-Southern Oscillation (ENSO), which affects sea-surface temperature (SST) in the South China Sea (SCS) in the months following El Niño/La Niña events. To test these hypotheses, the number of clutches laid in the CHTS over a 24-year period was assessed via the Seasonal and Trend Decomposition by Loess (STL) algorithm. The long-term trend of nest abundance was shown to be dependent on the recruitment of new mothers, while a 2–3 years’ quasi-periodic oscillation in nest abundance showed a 2.5-year-lagged negative correlation to the Oceanic Niño Index (ONI) and a 2-year-lagged negative correlation to the SST data series from SCS shallow waters, including known foraging grounds of green turtles nesting in the CHTS. In summary, we demonstrated that La-Niña peaks by decreasing SST in the SCS for the subsequent semester promotes green turtle nesting after 2–3 years. These results highlight the influence of broad climatological fluctuations in the sea turtles’ life cycle and the importance of conservation measures to secure their entire home range and life stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After having experienced worldwide decline by the turn of the century (Seminoff 2004), some green turtle (Chelonia mydas, L.) populations have been showing signs of recovery in the Central North, East, and West Pacific Ocean (Chaloupka et al. 2008; Kittinger et al. 2013; Seminoff et al. 2015), the South and North Atlantic Ocean (Chaloupka et al. 2008; Weber et al. 2014; Silva et al. 2017; Blumenthal et al. 2021), the South-west Indian Ocean (Mortimer et al. 2020), and the Mediterranean Sea (Omeyer et al. 2021). This is a consequence of decades of conservation efforts (Mazaris et al. 2017), and the species’ resilience to climate change and sea-level rise (Patrício et al. 2019). In the South China Sea (SCS), however, despite increasing conservation efforts (Bjorndal et al. 1993; Hawkes et al. 2005), the population decline has been estimated to reach 90% at the turn of the century (Chan 2006). For example, among the Malaysian conservation programmes, only the Sabah Turtle Island Sanctuary, in northern Borneo, has successfully promoted population regrowth, after more than 40 years of its establishment (Chan 2006). Delayed maturity, migratory cycles that can reach thousands of kilometres, variation in occupied niches during different life stages, temperature-dependent development, and the extremely high mortality rate of juveniles are some of the factors that increase turtles’ susceptibility to various threats throughout their life, consequently affecting population growth (Heppell et al. 2003). Thus, evaluating the success of conservation programmes is essential to adjust strategies accordingly.
Moreover, sea turtles’ susceptibility to physical variations in their habitats makes it important to predict their response to climate variability, especially in the context of climate crisis and increases in meteorological extremes (Santidrián Tomillo et al. 2020). Limpus and Nicholls (1988, 2000) were the first to document a coupled oscillation between green turtles’ nesting abundance and El Niño oscillations at rookeries in the Great Barrier Reef (GBR), southwestern Pacific Ocean. The same phenomenon was later documented in Peru (Quiñones et al. 2010; Castro et al. 2012), the Seychelles (Mortimer 2012), and Malaysia and the Philippines (Chaloupka 2001). El Niño oscillations are known to affect sea-surface temperatures (SSTs), which influence green turtles’ main food source (Marbà and Duarte 2010) and re-migration intervals (Solow et al. 2002).
The present study investigated the relationship between abundance of nests and recruitment of new mothers at Chagar Hutang Turtle Sanctuary (CHTS), in Redang Island, Malaysia, to understand how conservation efforts are affecting green turtles’ population structure. It also investigated the influence of El Niño Southern Oscillation (ENSO) on the nesting phenology of green turtles reproducing at CHTS using the Oceanic Niño Index (ONI) and SST of the SCS as proxies for El Niño-related conditions to determine its effects on the annual oscillations of nest abundance.
More specifically, it was hypothesised that: (i) the annual proportion of recruited mothers had a significant correlation with the variation of nests at CHTS, such that if there was a general increasing trend in the number of nests each year and this trend was correlated with the proportion of recruited mothers the conservation project could be judged successful; and (ii) that there was a significant correlation between annual nest abundance and SST from shallow waters of the SCS and annual average anomalies registered by the ONI in the equatorial Pacific Ocean. We hypothesized that the number of nests would be smaller for nesting seasons following El Niño events, since warming of the West Pacific waters during this period would decrease the quantity and quality of feeding resources for future migrating females. By testing these hypotheses, we hoped to elucidate the effectiveness of conservation efforts in the CHTS; to gain a better understanding of the population dynamics of green turtles in the SCS, especially concerning the effects of climate variability in an annual time scale; and to provide a background for predicting nest abundance in the CHTS in the following seasons, permitting more assertive conservation plans.
Material and methods
Study site
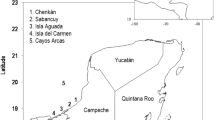
The CHTS is located at the northern-most part of Redang Island (5°46′30''N, 103°0′54″ E), off the northwest coast of Terengganu, Malaysia (Fig. 1). Chagar Hutang is one of the most important rookeries for green turtles along the coast of Peninsular Malaysia, recording between 700 and 1500 nests per year, in addition to a small number of hawksbill turtle nests (averaging 20 per year) (Pers Comm). Conservation efforts are managed by the Sea Turtle Research Unit (SEATRU) of Universiti Malaysia Terengganu. Although nesting may occur throughout the year, the peak season is from May to August. Green turtles nesting at Redang Island can migrate up to 1500 km to reach foraging grounds as far away as Bugsuk Island (south of Palawan) to the East and Bangka Island (east of Sumatra) to the South (Luschi et al. 1996; Fig. 1).
Location of Chagar Hutang Turtle Sanctuary (CHTS), in Redang Island, Malaysia, and the five 2° × 2° cells from ERSST.v4 data containing known feeding grounds of green turtles nesting at the CHTS, namely Palawan Island (A); Brunei Bay (B); Natuna Island (C); Tamberlan Island (D); and Bangka Island (E); Dark grey area denotes the 50 2° × 2° cells of ERSST.v4 covering shallow and coastal waters of the SCS. Dashed line demarks the 200 m bathymetry contour
Databases
Nesting data collection began at CHTS in 1993, when resources allowed for the monitoring of clutches and tagging of turtles during the peak months of the nesting season (June to September). Chagar Hutang Beach is 320 m long, which allowed complete overnight inspections and recording of all nesting females and nests placed on the beach during the survey periods. As financial and structural resources increased, longer time spans during the nesting season were covered. From 2008 onwards, nests were recorded and protected all year round. To standardise comparisons, annual abundances were calculated considering only nests recorded between June and September from 1993 to 2016. The modal re-migrant interval for the green turtles nesting at CHTS was 3 years (Chew 2015). Thus, the proportion of recruits to re-migrants was calculated from 1996 onwards, to avoid overestimation of recruits in the first 3 years of the project due to newly marked mothers that could have actually returned to their nesting grounds before the beginning of the tagging effort.
A time series was created with annual means of 50 2° × 2° resolution cells of the National Oceanic and Atmospheric Administration (NOAA) Extended Reconstructed Sea Surface Temperature version 4 (ERSST.v4) dataset from 1990 to 2016 covering all the shallow waters of the SCS, from Hainan Island, China, to Belitung Island, Indonesia, and from the Gulf of Thailand to Palawan Island, Philippines (Fig. 1), so to consider SST from all the known and potential feeding grounds of the green turtles nesting at the CHTS (Papi et al. 1995; Luschi et al. 1996; Van de Merwe et al. 2009). A time series was created from the ONI average monthly anomalies from July to June from 1990 to 2016. El Niño/La Niña peak in December, but ONI anomalies associated with these events can be identified a couple of months before and after (Trenberth 1997); therefore, the averaging from July to June of the following year was chosen to obtain annual means clearly representing ENSO oscillations. ONI was chosen over more-holistic ENSO proxies (i.e., Multivariate ENSO Index.) for being derived from the ERSST, which allowed direct comparison to the SST series obtained from SCS.
Data analysis
Long-term trend and annual oscillation were inferred from the log-transformed annual nest abundance data by Seasonal and Linear Trend decomposition using Loess, or simply STL (Cleveland et al. 1990). This specific type of generalised additive modelling uses Loess, a type of nonparametric regression smoothing, to decompose a time series into additive frequency components of variation: namely a general trend; seasonal or quasi-periodic oscillation; and a remainder component. STL was previously used to infer long-term trend sand seasonal oscillations in turtles nesting in Malaysia (Chaloupka 2001), Hawaii (Balazs and Chaloupka 2004), and the southwest Indian Ocean (Lauret-Stepler et al. 2007). STL was also used in this study to extract any effects of the Pacific Decadal Oscillation (PDO) and anthropogenic climate change from the ERSST and ONI anomalies series, leaving only the fluctuations caused by the quasi-periodic climatic oscillations to be used in the statistical tests. For the decomposition, a seasonal window of 3 years was chosen for the high-frequency component to reflect the 2–3 years quasi-periodicity of ENSO, while a trend window of 9 years was assumed for the low-frequency component to represent the PDO at the turn of the millennium, based on Krishnamurthy and Krishnamurthy (2013) modelling.
Linear regression was then used to test the correlation between the STL long-term trend smoother of nest abundance and the annual proportion of recruited mothers, and between the STL quasi-periodic smoothers of nest abundance and ERSST at shallow waters of the SCS and ONI anomalies. For the ERSST databases measured locally at the SCS, correlation was tested over time series with lags of 3, 3.5, 2.5, 2.0, 1.5, 1.0, and 0.5 years (i.e., nest abundance in 1993 was correlated with averaged ERSST from 1990, 1991, 1992, and 1993 and the averages from June to July in between these years, and so forth for all the other years in the series). The same approach was used for the ONI series; however, for the reasons explained above, the lags were 3.5, 2.5, 1.5, and 0.5 years (i.e., nest abundance in 1993 was compared to ONI average anomalies from the 12-month periods between July and June for 1989–1990, 1990–1991, 1991–1992, 1992–1993, and so forth for the following years of the series).
Positive ONI anomalies are frequently followed by negative ones the next year, comprising the El Niño–La Niña oscillation. Similarly, peaks of nest abundance in a given year may be caused by (or lead to) drops in the previous (or the next) year, since green turtles rarely nest in two consecutive years (Broderick et al. 2001). To test whether this autocorrelation was present, auto and cross-correlation functions were applied to the STL short-frequency smoother of the ONI and nests’ abundance data series. Residual-fits and qq-plots were produced to evidence the model fit. Statistical tests were performed using R 3.2.5 through the car (Fox and Weisberg 2019) and vegan (Oksanen et al. 2018) packages, and significance was assumed at the 95% confidence level.
Results
The monthly nest abundance in CHTS is presented in Fig. 2. There was a concentration of nest abundance between April and September, and a 3 years quasi-periodic oscillation after 2005. Peaks normally occurred in June or July; however, the highest abundance was recorded in May 2016. Anomalous peaks were also observed in August 2003 and May 2014.
The STL decomposition of annual nest abundance is summarised in Fig. 3. The STL long-term smoother (with low-frequency variation of 9 years; Fig. 3, trend) fitted well with the 24 years’ series of nest abundance (r2 = 0.5502; P < 0.0001). There was a general decline in nest abundance during the first half of the period, followed by a general increase in the second half. The STL seasonal smoother (with high-frequency variation of 3 years; Fig. 3, seasonal) also showed a good fit to the oscillation during the same period (r2 = 0.4581; P < 0.0005). The remainder accounted for 7.2% of the variation in the data series and was much higher in the first 3 years of the conservation project than during the rest of the period (Fig. 3, remainder).
STL decomposition plot of the number of green turtles’ clutches at CHTS, Redang Island, Malaysia (1993–2016). Data show the log-transformed annual nesting series corresponding to the 4 months during peak season (Jun–Sep); Seasonal shows the fitted 3 years quasi-periodic trend or high-frequency variation in nest abundance (bandwidth of trend filter = 3 years); Trend shows the fitted long-term trend or low-frequency variation in estimated annual number of clutches (bandwidth of trend filter = 9 years); Remainder shows the residual component after seasonal quasi-periodicity and long-term trend components have been fitted to the series. The three components, seasonal, trend, and remainder, sum to the series shown in the data. Grey vertical bars on the right of each panel indicate relative variation in scaling among the components and original data series
Annual Oscillation and proportion of recruits
The annual number of re-migrant turtles visiting the CHTS did not vary greatly, and remained below 100 during the study period. Recruits however, presented a bigger fluctuation and there was a notable increase after 2005, following the same general increment in nest abundance (Fig. 4a). The proportion of recruits over re-migrants showed a declining trend during the first 10 years of the conservation project, from 2005; however, the pattern was then inverted and the proportion started to increase until reaching stability in 2009, oscillating between 70 and 80% (Fig. 4a). The STL long-term smoother showed a significant correlation with the proportion of recruits over the years (β = 1.681; r2 = 0.5158; P < 0.0005; Fig. 4b and Appendix: Fig. 7).
a Temporal variation in the proportion of recruits over re-migrants (dashed red line, right axis), and the STL long-term trend smoother (continuous black line, left axis; corresponding to the trend component in Fig. 3). b Linear regression of STL values on the proportion of recruits over re-migrants. Green solid line shows the best fit and dashed lines show the 95% confidence interval
Annual Oscillation and ENSO
The analysis of ONI data revealed positive anomalies corresponding to the 1997–1998 El Niño (the strongest in the study period) as well as the peaks of the 2009–2010 and 2015–2016 events, and the three small El Niños of the 2000s. The longest (and strongest) La Niña of 1998–2001 was clearly evident, as well as the 1995–1996, 2007–2008, and 2010–2012 episodes. A complete analysis of the time series is provided in Appendix: Fig. 8.
For the ERSST series of SCS, the STL’s seasonal component matched the El Niño peaks of 1997–1998 and 2009–2010 (Appendix: Fig. 8b). La Niña peaks in 1995–1996 and 2007–2008 are clearly evident in the seasonal decomposition, while local conditions seemed to mask the effects of the other less-strong oscillations of ENSO. A significant correlation was found for the STL quasi-periodic smoother of nest abundance and both ONI anomalies and ERSST data series. For ONI anomalies, the best fit occurred for data that lagged 2.5 years (β = – 0.221; r2 = 0.2799; P = 0.0079), but a significant correlation was also found for a lag of 1.5 years (β = 0.206; r2 = 0.2425; P = 0.0145). Notably, the correlation to nest abundance was negative (or positive) for ONI series delayed by 2.5 (or 1.5) years respectively (Fig. 5).
Correlation between the STL seasonal component of nests series (solid line in a and c, seasonal component in Fig. 3) and ONI average anomalies (red dashed lines in a and c, seasonal component in Fig. 8a) lagged 2.5 years (a) and 1.5 years (c), followed by their respective scatterplots with regression lines and 95% confidence interval (solid and dashed green lines in b and d). Values of ONI anomalies are inverted in a to highlight the negative correlation
Figure 6a illustrates the significant autocorrelation in Nest abundance series with 1 year lag while Fig. 6b, although not showing significant autocorrelation in the ONI series, shows the 3 years’ quasi-periodicity of ENSO, with positive anomalies corresponding to El Niño always followed by biennial negative anomalies corresponding to La Niña. These factors combined result in the significant cross-correlation between these two series with 1 and 2 years of delay (Fig. 6c). Note that, since ONI anomalies series are averaged over a 12-month period from July to June of the following year, it is in 6 months of dissonance to nest abundance series, therefore the 1- and 2-year lag of Fig. 6c correspond to the 2.5- and 1.5-year lag of Fig. 5a,c.
Autocorrelation functions of seasonal component of STL for a Nests’ abundance and b ONI anomalies. c Cross-correlation function of both series, for a given lag t, cross-correlation is computed for ONI series delayed t years. Observations outside the limit of dashed lines denote significant auto or cross-correlation
Linear regression also showed a considerable fit between the STL seasonal components of nest abundance and ERSST from shallow waters of SCS lagged in 2 years (β = – 0.3596; r2 = 0.2059; P = 0.0260). This result reflected the delayed response of the SCS’s SST to ENSO, with the local SST reflecting the ENSO around 6 months later (Klein et al. 1999; Wu et al. 2014). Thus, there was a negative correlation between nest abundance in the CHTS and the ONI series 2.5 years before and the ERSST from the SCS 2 years before.
Discussion
Green turtle recruitment
The trend smoother in Fig. 3 illustrated that the number of clutches laid on the CHTS were in continuous decline between 1993 and 2005, then progressively increasing between 2005 and 2016. Normally, green turtle populations from the west pacific reach maturity between 25 and 50 years of age (Limpus and Chaloupka 1997; Chaloupka et al. 2004). This late-maturing nature of green turtles requires decades before conservation efforts are reflected in increased reproductive success (Mazaris et al. 2017), therefore, it is plausible that the trend in number of nests continued to decline even after 12 years of the establishment of the CHTS. The same argument applies to the larger remainders in the first 3 years of the conservation project (Fig. 3, remainder). Since the reproductive cycle of green turtles in the CHTS normally takes 3 years, one could expect that recovery from an adverse environment would not be observed until the fourth year of the conservation project, when the initial turtles that had laid their eggs in 1993 started to return for their second reproductive cycle under SEATRU supervision, which was roughly around 1996. Only then could a stable oscillation of the reproductive cycle occur, as it can be observed in the seasonal and remainder components of Fig. 3.
We have found a correlation between green turtle recruitment and long-term trend in nest abundance (Fig. 4). Given the late-maturing nature of green turtles and the 24 years long time series available for this study, it is too soon to conclude that this is a consequence of conservation efforts alone. Nevertheless, it has been shown that individuals from Hawaii, in the north Pacific Ocean, can start reproducing within 17–19 years of age (Houtan et al. 2014) or as early as 12–20 years of age in the Caribbean Sea (Bell et al. 2005; Zurita et al. 2011). In the SCS, the only study that assessed recruitment interval of green turtles used data from the Turtle Island Park (TIP), in Sabah, northerner Borneo. The analysis of the long-term nesting data (1979–2016) had shown that the age-to-maturity for female green turtles in the TIP was 19 years (Joseph 2017). Since green turtles reproducing in the CHTS belong to the same population as the ones from TIP they could have an equally shorter age-to-maturity in comparison to other green turtles elsewhere. Therefore, it is possible that individuals born in the CHTS in 1993 could already have returned for their first reproductive cycle as early as the 2010´s, leading to the stable proportion of recruits observed by the end of the time series (Fig. 4). However, more evidence is needed to prove that green turtles in the SCS can reach sexual maturity at an age earlier than 20 years. In any case, most of the individuals born under SEATRU supervision were still immature, a proportion that is due to be reversed in the coming years, when it will be possible to access the consequences of the conservation programme over the recruitment of mature females with more clarity and potentially corroborate the correlation between recruitment and long-term trend in nest abundance at that rookery.
Although an increase of conservation efforts normally takes three decades or more to result in green turtle population recovery (Mazaris et al. 2017), some studies observed positive effects after 27 years in the Mediterranean Sea (Omeyer et al. 2021), after 25 years in the West Pacific (Chaloupka et al. 2008), and as early as after 22 years in the North Atlantic Ocean (Blumenthal et al. 2021). In the present study, there was a notable continuous increase in the proportion of recruit mothers and a positive trend of nest abundance in the second half of the series (Fig. 4); this may be an indication of the start of a population recovery during the analysed period, proving the decisive role of monitoring and protection of rookeries in promoting population recovery over the decades (Godley et al. 2020). Nevertheless, what is seen here is just a partial glimpse of the total population of green turtles connected to the CHTS. The abundance of nests estimates the number of reproducing females, but information about juveniles and adult males is still lacking and can be accessed only by surveying turtle populations in their foraging grounds (Hawkes et al. 2005). In the South Atlantic populations, where long-term data are available for foraging grounds, changes in population size and structure are more readily accessible and demonstrate the success of conservation efforts in connected nesting sites (Silva et al. 2017). Moreover, it has been demonstrated that guaranteeing the food supply is necessary for the species to show resilience to climate change (Patrício et al. 2019). The conservation of rookeries must therefore be coupled to fishing regulations and the establishment of marine parks, to promote the full protection of marine turtles throughout their life cycle and to allow effective population growth.
ENSO and nest abundance
The STL seasonal components of nest abundance and averaged ONI anomalies showed the highest correlation with a 2.5-year lag (Fig. 5a, b); however, a significant correlation was also present with a 1.5-year lag (Fig. 5c, d). More precisely, positive (or negative) ONI anomalies at the turn of the year had a direct correlation (or negative correlation) to the abundance of nests after roughly 18 months and a negative (or positive) correlation after roughly 30 months. As seen in Fig. 5a, negative ONI anomalies, corresponding to cold La Niña events, seemed to match all the peaks in nest abundance 2.5 years later, with the exception of that in 2004. Also, positive ONI anomalies, corresponding to warm El Niño events, seemed to match, to a lesser extent, the years with nest shortages (Fig. 5a). Correlation with ONI anomalies 1.5 years prior seemed to match nest abundance relatively well until 2009, after which the peaks observed in 2010, 2013, and 2016 were more closely related to negative ONI anomalies 2.5 years before (Fig. 5a, b). These results suggest that La Niña episodes with extremely low anomalies are responsible for peaks of nest abundance 2.5 years later in the CHTS, and not the El Niño episodes causing the opposite effect, as previously proposed for other green turtle rookeries (Limpus and Nichols 1988, 2000; Chaloupka 2001; Santidrián Tomillo et al. 2020). Since years with peaking nest abundance are normally preceded and followed by years with nest shortages, and ENSO warm phases are frequently followed by cold ones the next year and vice versa, a negative cross-correlation was found with 1 year of difference (Fig. 6c); thus, the positive correlation between nest abundance and ONI anomalies lagged by 1.5 years (Fig. 5c, d).
The green turtles’ nesting season in SCS peaked within 6 months of dissonance to the GBR; thus, non-integer values of 1.5 or 2.5 years were found here, as opposed to the integer interval of 2 years found for Australia (Limpus and Nicholls 1988, 2000). In the same way that negative phases of SO implied El Niño conditions and consequently cooler waters in the GBR, promoting turtle nutrition and re-migration after 2 years (Limpus and Nichols 2000), negative ONI anomalies, associated with La Niña events, decreased the SST in the SCS 6 months later and promoted an increase in the number of nests after 2.5 years. In summary, the ENSO, by cooling down the SCS’s waters around 6 months after La Niña peaks, will have positive effects on turtles re-migrating to the CHTS for their next nesting cycle within 2 years. These findings help to explain the findings of Santidrián Tomillo et al. (2020) in Costa Rica, where there was no strong correlation between El-Niño peaks and green turtle’s reproductive success, although these events have significantly reduced the hatching success and nest abundance of other sea turtle species.
Correlation between ENSO and nest abundance in the SCS was investigated by Chaloupka (2001), who found a strong association between the Southern Oscillation Index (SOI) and nesting at two rookeries in the eastern SCS 1.5-years prior. Chaloupka (2001) advocated that El Niño events lead to nesting peaks around 18 months later. However, the present findings indicate that La Niña episodes are more likely to cause these peaks with 30 months of delay, and spurious correlations resulting from autocorrelation in the data series, as explained above, are the most reasonable explanation for the 18-month-gap correlation found by Chaloupka (2001) and the present study. The author refers to the Lough (1994) study to support the argument that El Niño episodes decrease the SST in the west Pacific Ocean, and consequently provide better nutrition for turtles reproducing during the next year. It is true that positive ENSO phases are correlated to low SST in the GBR (Lough 1994); however, this cannot be generalised to the whole western edge of the Pacific Ocean. ENSO’s atmospheric bridge has induced the opposite effect in the SCS, where positive phases of ENSO have led to an increase of SST in the region during the following months (Klein et al. 1999; Wang et al. 2006; Wu et al. 2014). Hence, the negative correlations of nest abundance to ONI and ERSST series 2.5 and 2 years prior, respectively.
Vitellogenesis in green turtles takes 9 months, and fat accumulation must occur before it starts (Kwan 1994); it therefore requires more than 1 year for the whole process to be finished before a turtle can re-migrate to its rockery. Thus, if considering travel time between foraging and reproduction grounds, 2 years is the minimum period necessary between the completion of a reproductive cycle and the beginning of the next one (Broderick et al. 2001). Taking this into consideration, alongside the fact that a higher SST affects sea-grass growth (Marbà and Duarte 2010), a higher water temperature at the green turtles’ feeding grounds will deplete their accumulation of fat and delay the process of vitellogenesis, consequently reducing the number of individuals returning to their rookeries after 2 years.
Beyond influencing green turtle’s caloric uptake, ENSO can change their diet by shifting the availability of food sources. In the southern Atlantic Ocean, ENSO was observed to cause diet shifts in juvenile green turtles, where strong El Niño/La Niña events were responsible for shifting the preference of consumption from seagrasses of the species Halodule wrightii to the green algae Ulva sp. (Gama et al. 2016). In this case, the authors advocated that both strong positive and strong negative peaks of ENSO caused a reduction of sea-grass biomass, the former by increasing rainfall in the region leading to greater turbidity, and the latter by cooling down the SST, in both cases reducing the growth capacity of H. wrightii and allowing more resilient Ulva sp. to increase in dominance (Gama et al. 2016).
Warm SST during El Niño episodes, besides facilitating migration of green turtles to foraging grounds on the coast of Peru, also produces a bloom of the jellyfish Chrysaora plocamia, providing them with an alternative food source that they can opportunistically feed upon when juvenile and mature (Quiñones et al. 2010). These conditions favour an anomalous increase in the abundance of green turtles on the coast of Peru during positive ENSO phases (Quiñones et al. 2010; Castro et al. 2012). Moreover, in GBR, higher levels of triglycerides in green turtles’ blood samples were found to peak during the 1997–1998 El Niño, indicating that they were experiencing a higher nutritional uptake during this period (Hamann et al. 2005). These findings support the hypothesis that this modulation of green turtles’ nesting by ENSO has a nutritional basis.
Limitations and future perspectives
To date, five feeding grounds have been identified for green turtles nesting at CHTS: Natuna Island, identified by Papi et al. (1995); and Bangka, Tamberlan, the Palawan Islands and Brunei Bay, all identified by Luschi et al. (1996) (Fig. 1). However, these might not be the only feeding grounds of the green turtles visiting Redang Island. Indeed, the green turtles nesting in Ma’Daerah Turtle Sanctuary in mainland Terangganu were found to travel to foraging grounds at Ly Son Island in Central Vietnam, the Sabah coast in Malaysian Borneo, the Thousand Islands in Java, and Pemanggil Island in Peninsular Malaysia (Van de Merwe et al. 2009). Since Ma’Daerah beach is located just 150 km south of Redang Island and most of its turtles’ migration routes overlap those identified by Papi et al. (1995) and Luschi et al. (1996), it is likely that turtles nesting in the CHTS can also access these feeding grounds. Therefore, expanding the knowledge of the migration patterns of green turtles reproducing in this rookery, via satellite tracking for example (Papi et al. 1995; Luschi et al. 1996), will be essential for understanding how climate conditions throughout the SCS influence their life cycle.
There was an apparent association between an increase of immature female growth rates in southern GBR and the 1982–1983 El Niño (Limpus and Chaloupka 1997). The inversed picture is found for the turtles in the Atlantic Ocean, where the 1997–1998 El Niño led to an ecological regime shift that is very likely to be responsible for the decrease in growth rate of green turtles, loggerheads and hawksbills (Bjorndal et al. 1993). Thus, recruitment itself is likely to be subject to ENSO and future studies should take this into consideration when investigating the increment in nest abundance in a long-term.
Conclusions
In summary, the proportion of recruited mothers had a positive effect on nest abundance in the CHTS, leading to a positive trend since 2005. Annual oscillation in nest abundance was significantly linked to ENSO. La Niña peaks promoted a general decrease of SST in the SCS in the following months, which was reflected in the increased nest abundance in the CHTS 2 years later. These findings highlight the importance of implementing long-term conservation projects, such as the one put in practice in the CHTS, coupled to transnational efforts to safeguard green turtles during their regular migrations and at their foraging grounds.
Availability of data and material
Green turtle nesting data is managed by SEATRU and available upon request (https://seatru.umt.edu.my). SST data series are publicly available at NOAA website (https://www.ncdc.noaa.gov/data-access/marineocean-data/extended-reconstructed-sea-surface-temperature-ersst-v4).
Code availability
Not applicable.
References
Balazs GH, Chaloupka M (2004) Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol Cons 117:491–498. https://doi.org/10.1016/j.biocon.2003.08.008
Bell CDL, Parsons J, Austin TJ, Broderick AC, Ebanks-petrie G, Godley BJ (2005) Some of them came home: the Cayman Turtle Farm headstarting project for the green turtle Chelonia mydas. Oryx 39(2):137–148. https://doi.org/10.1017/S0030605305000372
Bjorndal K, Bolten A, Lagueux C (1993) Decline of the Nesting Population of Hawksbill Turtles at Tortuguero, Costa Rica. Conservation Biology 7(4):925–927. http://www.jstor.org/stable/2386826
Blumenthal JM, Hardwick JL, Austin TJ, Broderick AC, Chin P, Collyer L, Ebanks-Petrie G, Grant L, Lamb LD, Olynik J, Omeyer LCM, Prat-Varela A, Godley BJ (2021) Cayman Islands Sea Turtle Nesting Population Increases Over 22 Years of Monitoring. Front Mar Sci 8:1–12. https://doi.org/10.3389/fmars.2021.663856
Broderick AC, Godley BJ, Hays GC (2001) Trophic status drives interannual variability in nesting numbers of marine turtles. Proc Biol Sci London B 268(1475):1481–1487. https://doi.org/10.1098/rspb.2001.1695
Castro J, de la Cruz J, Ramirez P, Quinones J (2012) Captura incidental de tortugas marinas durante El Nino 1997 1998, en el norte del Peru. Lat Am J Aquat Res 40(4):970–979. https://doi.org/10.3856/vol40-issue4-fulltext-13
Chaloupka M (2001) Historical trends, seasonality and spatial synchrony in green sea turtle egg production. Biol Cons 101:263–279. https://doi.org/10.1016/S0006-3207(00)00199-3
Chaloupka M, Limpus C, Miller J (2004) Green turtle somatic growth dynamics in a spatially disjunct Great Barrier Reef metapopulation. Coral Reefs 23:325–335. https://doi.org/10.1007/s00338-004-0387-9
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17(2):297–304. https://doi.org/10.1111/j.1466-8238.2007.00367.x
Chan E-H (2006) Marine turtles in Malaysia: On the verge of extinction? Aquatic Ecosyst Health Management 9(2):175–184. https://doi.org/10.1080/14634980600701559
Chew VYC (2015) Tagging, Nesting and Photo-Identification of Green Turtles (Chelonia mydas) at Chagar Hutang, Redang Island (Master’s Thesis).
Cleveland RB, Cleveland WS, McRae JE, Terpenning P (1990) STL: a seasonal-trend decomposition procedure based on Loess. J off Stat 6:3–73
Fox J, Weisberg S (2019) An R Companion to Applied Regression, Third edition. Sage, Thousand Oaks CA. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Gama LR, Domit C, Broadhurst MK, Fuentes MMPB, Millar RB (2016) Green turtle Chelonia mydas foraging ecology at 25° S in the western Atlantic: evidence to support a feeding model driven by intrinsic and extrinsic variability. Mar Ecol Prog Ser 542:209–219. https://doi.org/10.3354/meps11576
Godley B, Broderick A, Colman L, Formia A, Godfrey M, Hamann M, Nuno N, Omeyer LCM, Patricio AR, Phillott AD, Rees AF, Shanker K (2020) Reflections on sea turtle conservation. Oryx 54(3):287–289. https://doi.org/10.1017/S0030605320000162
Hamann M, Jessop TS, Limpus CJ, Whittier JM (2005) Regional and annual variation in plasma steroids and metabolic indicators in female green turtles, Chelonia mydas. Mar Biol 148:427–433. https://doi.org/10.1007/s00227-005-0082-6
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ (2005) Status of nesting loggerhead turtles Caretta caretta at Bald Head Island (North Carolina, USA) after 24 years of intensive monitoring and conservation. Oryx 39(1):65–72. https://doi.org/10.1017/S0030605305000116
Heppell SS, Snover ML, Crowder LB (2003) Sea Turtle Population Ecology. Lutz PL, Musick JA, Wyneken J [eds] The Biology of Sea Turtles, vol II. CRC Press, New York, pp 277–306
HoutanVan HKS, Hargrove SK, Balazs GH (2014) Modeling sea turtle maturity age from partial life history records. Pac Sci 68(4):465–477. https://doi.org/10.2984/68.4.2
Joseph J (2017) Marine Turtle Landing, Hatching, and Predation in Turtle Island Park (TIP), Sabah. Technical Report for the Coastal and Marine Resources Management in the Coral Triangle-Southeast Asia (TA 7813-REG). 64 pp.
Kittinger JN, Houtan KSV, Mcclenachan LE, Lawrence AL (2013) Using historical data to assess the biogeography of population recovery. Ecography 36(8):868–872. https://doi.org/10.1111/j.1600-0587.2013.00245.x
Klein SA, Soden BJ, Lau NC (1999) Remote sea surface temperature variations during ENSO: Evidence for a tropical atmospheric bridge. J Clim 12(4):917–932. https://doi.org/10.1175/1520-0442(1999)012%3c0917:RSSTVD%3e2.0.CO;2
Krishnamurthy L, Krishnamurthy V (2013) Influence of PDO on South Asian monsoon and monsoon-ENSO relation. Clim Dyn 42:9–10. https://doi.org/10.1007/s00382-013-1856-z
Kwan D (1994) Fat reserves and reproduction in the green turtle, Chelonia mydas. Wildl Res 21:257–265. https://doi.org/10.1071/WR9940257
Lauret-Stepler M, Bourjea J, Roos D, Dominique P, Ryan P, Ciccione S, Grizel H (2007) Reproductive seasonality and trend of Chelonia mydas in the SW Indian Ocean: a 20 yr study based on track counts. Endangered Species Res 3:217–227. https://doi.org/10.3354/esr003217
Limpus CJ, Chaloupka M (1997) Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 149:23–34. https://doi.org/10.3354/meps149023
Limpus CJ, Nicholls N (1988) The Southern Oscillation Regulates the Annual Numbers of Green Turtles (Chelonia mydas) Breeding around Northern Australia. Aust J Wildl Res 15:157–161. https://doi.org/10.1071/WR9880157
Limpus CJ, Nicholls N (2000) ENSO regulation of Indo-Pacific green turtle populations. Hammer GL, Nicholls N, Mitchell C [eds] The Australian Experience. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 399–408
Lough JM (1994) Climate variation and El Niño-Southern Oscillation events on the Great Barrier Reef: 1958 to 1987. Coral Reefs 13:181–195. https://doi.org/10.1007/BF00301197
Luschi P, Papi F, Liew HC, Chan EH, Bonadonna F (1996) Long-distance migration and homing after displacement in the green turtle (Chelonia mydas): a satellite tracking study. J Comp Physiol A 178(4):447–452. https://doi.org/10.1007/BF00190175
Marbà N, Duarte CM (2010) Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob Change Biol 16(8):2366–2375. https://doi.org/10.1111/j.1365-2486.2009.02130.x
Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC (2017) Global sea turtle conservation successes. Sci Adv 3:9. https://doi.org/10.1126/sciadv.1600730
Van de Merwe J, Ibrahim K, Lee S, Whittier J (2009) Habitat use by green turtles (Chelonia mydas) nesting in Peninsular Malaysia: local and regional conservation implications. Wildlife Res 36:637–645. https://doi.org/10.1071/WR09099
Mortimer JA (2012) Seasonality of green turtle (Chelonia mydas) reproduction at Aldabra Atoll, Seychelles (1980–2011) in the regional context of the Western Indian ocean. Chelonian Conserv Biol 11(2):170–181. https://doi.org/10.2744/CCB-0941.1
Mortimer JA, Esteban N, Guzman AN, Hays GC (2020) Estimates of marine turtle nesting populations in the south-west Indian Ocean indicate the importance of the Chagos Archipelago. Oryx 54(3):332–343. https://doi.org/10.1017/S0030605319001108
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D et al. (2018) Vegan: community ecology package. R package vegan, vers. 2.5–2. https://cran.r-project.org/web/packages/vegan/index.html
Omeyer LCM, Stokes KL, Beton D, Çiçek BA, Davey S, Fuller WJ, Godley BJ, Sherley RB, Snape RTE, Broderick AC (2021) Investigating differences in population recovery rates of two sympatrically nesting sea turtle species. Anim Conserv. https://doi.org/10.1111/acv.12689
Papi F, Liew HC, Luschi P, Chan E (1995) Long-range migratory travel of a green turtle tracked by satellite: Evidence for navigational ability in the open sea. Mar Biol 122:171–175. https://doi.org/10.1007/BF00348929
Patrício AR, Varela MR, Barbosa C, Broderick AC, Catry P, Hawkes LA, Regalla A, Godley BJ (2019) Climate change resilience of a globally important sea turtle nesting population. Glob Change Biol 25(2):522–535. https://doi.org/10.1111/gcb.14520
Quiñones J, González Carman V, Zeballos J, Purca S, Mianzan H (2010) Effects of El Niño-driven environmental variability on black turtle migration to Peruvian foraging grounds. Hydrobiologia 645(1):69–79. https://doi.org/10.1007/s10750-010-0225-8
Santidrián Tomillo P, Fonseca LG, Ward M, Tankersley N, Robinson NJ, Orrego CM, Paladino FV, Saba VS (2020) The impacts of extreme El Niño events on sea turtle nesting populations. Clim Change 159(2):163–176. https://doi.org/10.1007/s10584-020-02658-w
Seminoff JA (Southwest Fisheries Science Center, U.S.) (2004) Chelonia mydas. The IUCN Red List of Threatened Species; 2004. https://doi.org/10.2305/IUCN.UK.2004.RLTS.T4615A11037468.en
Seminoff JA, Jeffrey A, Allen CD, Balazs GH, Dutton PH, Peter H, Eguchi T, Haas H, Hargrove SA, Jensen M, Klemm DL, Lauritsen AM, MacPherson SL, Opay P, Possardt EE, Pultz S, Seney EE, Van Houtan KS, Waples RS (2015) Status review of the green turtle (Chelonia mydas) under the Endangered Species Act. NOAA Tech Memo. NOAA-TM-NMFS-SWFSC-539. California, United States of America. https://repository.library.noaa.gov/view/noaa/4922
Silva BMG, Bugoni L, Almeida BADL, Giffoni BB, Alvarenga FS, Brondizio LS, Becker JH (2017) Long-term trends in abundance of green sea turtles (Chelonia mydas) assessed by non-lethal capture rates in a coastal fishery. Ecol Ind 79:254–264. https://doi.org/10.1016/j.ecolind.2017.04.008
Solow AR, Bjorndal KA, Bolten AB (2002) Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol Lett 5:742–746
Trenberth KE (1997) The Definition of El Niño. Bull Am Meteor Soc 78(12):2771–2777. https://doi.org/10.1175/1520-0477(1997)078%3c2771:TDOENO%3e2.0.CO;2
Zurita JC, Herrera R, Arenas A, Negrete AC, Gómez L, Prezas B, and Sasso CR (2011) Age at first nesting of green turtles in the Mexican Caribbean. In: Jones TT, Wallace BP (eds) Proceedings of the 31st Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum (NOAA NMFS-SEFSC-631), p. 89
Wang C, Wang W, Wang D, Wang Q, Nin E (2006) Interannual variability of the South China Sea associated with El Niño. J Geophys Res 111:1–19. https://doi.org/10.1029/2005JC003333
Weber SB, Weber N, Ellick J, Avery A, Frauenstein R, Godley BJ, Sim J, Williams N, Broderick AC (2014) Recovery of the South Atlantic’s largest green turtle nesting population. Biodivers Conserv 23(12):3005–3018. https://doi.org/10.1007/s10531-014-0759-6
Wu R, Chen W, Wang G, Hu K (2014) Relative contribution of ENSO and East Asian winter monsoon to the South China Sea SST anomalies during ENSO decaying years. J Geophys Res Atmos 119:5046–5064. https://doi.org/10.1002/2013JD021095
Acknowledgements
The authors would like to thank the anonymous reviewers for their useful comments; Aimi, Mann, Jangga and Pak Uda for logistical help in the CHTS and in mainland Terengganu as well as the hundreds of volunteers who helped collecting the data; and Nicholas Tolen for proof-reading the first version of this manuscript.
Funding
José Francisco Carminatti Wenceslau was supported by the European Commission as part of the Erasmus + scholarship. Field work were supported by the SEATRU-Universiti Malaysia Terengganu Sea Turtle Fund (Vot No. 63130) and by the Institute of Oceanography and Environment, Universiti Malaysia Terengganu, Higher Institution Center of Excellence, HICoE Phase I (Vot. No. 66928).
Author information
Authors and Affiliations
Contributions
JFCW and JJ conceived the ideas and collected the data; JFCW and GS performed the statistical analysis; MUR and MFA helped interpreting the results; and JFCW led the writing. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
There was no animal manipulation nor testing involved in this study. Permission to use nesting activity data was granted by the Sea Turtle Research Unit (SEATRU) of Universiti Malaysia Terengganu as the owner of the data.
No approval of research ethics committees was required given there were no animal manipulations nor testing involved throughout this study.
Additional information
Responsible Editor: P. Casale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
STL decomposition plots of a ONI average anomalies and b ERSST averages from SCS for the period 1990–2016. Top rows in both images show raw data; Seasonal row corresponds to the fitted 2–3 years quasi-periodic trend or high-frequency variation (bandwidth of trend filter = 3 years); Trend row shows the fitted long-term trend or low-frequency variation (bandwidth of trend filter = 9 years). Bottom row shows the remainder component after quasi-periodicity and long-term trend components (middle rows) have been fitted to the series (upper row). Remainder, Trend and Seasonal components sum to the series shown in data row. Grey vertical bar at right of each panel indicates relative variation in scaling amongst the components and original data series
Rights and permissions
About this article
Cite this article
Wenceslau, J.F.C., Rusli, M.U., Akhir, M.F. et al. Recruitment and El Niño-Southern Oscillation long-term effects on green turtle (Chelonia mydas) nest abundance. Mar Biol 168, 180 (2021). https://doi.org/10.1007/s00227-021-03989-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03989-7