Abstract
Mulloway (Argyrosomus japonicus) are an iconic recreational, indigenous, and commercial fishery species with declining numbers across some parts of their range, with relatively little known about their movements. During the Austral summers and autumns from 2011 to 2014, we deployed 19 pop-up satellite archival tags (PSATs) on mature mulloway at an aggregation site within the Great Australian Bight Marine Park (GABMP), to examine their movement patterns. Twelve tags provided data from deployments ranging from 8 to 110 days including five tags that gathered data over autumn and seven over summer. Five of the seven mulloway tagged during summer likely remained in the vicinity of the tagging location and hence within or in close proximity to marine-protected areas (MPAs) over summer; however, relatively large horizontal movements were observed over autumn for most fish, including a maximum net displacement of ~ 550 km. The median pop-up distance from deployment was 51 and 212 km for summer-and autumn-tagged fish, respectively. Depths encountered by the tagged mulloway ranged from the surface to 56.5 m deep. Our study provides new information on the dispersal of a poorly understood fish species which could aid their conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predatory demersal fish form important commercial, recreational, and traditional fisheries but stocks are depleted on a global scale (Myers and Worm 2003). As such, it is imperative to understand their broad scale movements to help manage and conserve stocks under extractive pressure and subject to habitat loss from anthropogenic developments and climate change. For large bodied demersal fish that form high value fisheries, population movement information is historically acquired from fisheries data and targeted research fishing (e.g., stratified trawl), whereas limited scale mark–recapture studies have provided some information on individual movements (Griffiths 1996). Traditionally, natural tags (genetic and otolith techniques) and artificial tags (mark/recapture and acoustic tags) have also been utilised on demersal fish to obtain some limited information on movements (Palumbi 2004). Whilst recent research combining otolith and archival tags have validated the utility of otoliths to provide location (e.g., Darnaude and Hunter 2018), there is substantial investment required to obtain data from natural and artificial tags from the same individuals. Furthermore, there is a species-specific aspect to otolith chemical signatures and a reliance on encountering heterogenic environments (Gillanders 2005).
Pop-up satellite archival tags (PSATs) have been utilised to gain movement information on aquatic animals for the last decade. For example, unprecedented information has been acquired on the movement of pelagic white sharks (Carcharodon carcharias), tuna (e.g., Thunnus orientalis) (Block et al. 2011), benthic stingrays (Dasyatis brevicaudata) (Le Port et al. 2008), and flatfish (Hippoglossus stenolepis) (Loher 2008). The pop-up feature means the movement information is fishery independent and hence useful for movement studies of marine animals in very remote areas.
Mulloway (Argyrosomus japonicus, Sciaenidae) are a demersal species that form important fisheries across their Australian and South African range. Unfortunately, in some parts of the range, mulloway abundance is declining (Silberschneider and Gray 2008; Ferguson et al. 2014) and stocks have been reported as collapsed in South Africa (Griffiths 1997; Taylor et al. 2006). Like many Sciaenids, mulloway are particularly vulnerable to habitat destruction and poorly managed recreational and commercial fishing, mainly due to reliance on critical habitats such as low energy nurseries (e.g., estuaries) and also because they mature relatively late (4–6 years and ~ 90 cm) (Griffiths 1996). Mulloway nursery habitat and exact size at first sexual maturity depends on the regional stock, location, and other biological factors, including sex (Ferguson 2010, Barnes unpublished data). To enhance fishing opportunities, mulloway are being restocked in some parts of their Australian (Taylor et al. 2006) and South African (Palmer and Snowball 2009) ranges. In other parts of their range, there is potential for marine-protected areas (MPAs) to provide some protection and allow numbers to rebuild; however, there is little information on the spatial scale of mulloway movement in the marine environment in Australia (but see Hall 1986). Hence, it is difficult to ascertain that the level of protection MPAs may provide due to the lack of spatial information and the likelihood of ontogenetic shifts, around the onset of sexual maturity and beyond (Griffiths 1996).
Most mulloway movement studies have been undertaken in estuaries (Taylor et al. 2006; Næsje et al. 2012). This is despite mulloway having either a marine life stage for estuary-associated populations, or being exclusively marine in others (Lenanton 1982; Ferguson et al. 2011; Barnes et al. 2016). Næsje et al. (2012) found that the acoustically tagged mulloway in their study showed both mobile and resident periods within an estuary. It is, however, difficult to ascertain the degree to which migratory and resident contingents comprise mulloway populations from the tagging literature. In South Africa, adult fish were found to be migratory based on mark–recapture and commercial catch records, although precise distances moved were unclear (Griffiths 1996). Mulloway have been subjected to mark and recapture using conventional tags in South Australia (SA), but recapture rates were very low (4.6%) (Hall 1986). Nonetheless, some fish moved distances of approximately 200 km (Hall 1986). Therefore, more reliable information on the species range is required and will be beneficial for fisheries and conservation managers.
In the eastern section of the Great Australian Bight (GAB), on the far west coast (FWC) of SA, there is a unique population of mulloway (Barnes et al. 2016). Here, particularly, along the surf beaches of the Yalata Indigenous Protected Area (Fig. 1), large adult mulloway are thought to spawn in relatively shallow water just behind the surf- line in late spring and early summer, and the area is a year round nursery for early life stages (Rogers et al. 2014). The population is genetically differentiated from others (Barnes et al. 2016), exhibits life-history strategies distinct from those of surrounding populations (e.g., lack of estuary association), and is faster growing than some neighbouring populations (Ferguson 2010). This population has cultural significance and also supports a large, but isolated recreational fishery (Rogers et al. 2014), as well as commercial catches via various gear types and licenses both at the Commonwealth and state agency level (Ferguson and Ward 2003). Recent anecdotal concerns from the local indigenous and fishing communities suggest that the GAB mulloway population is declining. However, there are two relatively new SA (state agency level) MPAs [Far West Coast Marine Park (FWCMP) and Nuyts Archipelago Marine Park (NAMP)] in the eastern GAB (Fig. 1) that when combined with existing Commonwealth, MPAs may benefit mulloway due to range overlap.
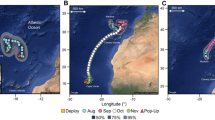
Study area: Inset 1 sets the broad geographical context, and identifies the area, where mulloway tagging work was focussed (dashed line rectangle) as depicted in the main map. The main map incorporates the area of the Great Australian Bight (GAB), where tagged mulloway were tracked. Shown in main map is the GAB marine park network and Western Australian (WA) and South Australian (SA) state borders. Fine dashed and blue zones are commonwealth parks, whereas nearshore brown, burgundy, and green zones are the state-level park networks. Collectively, these zones form the GAB Marine Park (GABMP). Zones that are managed to protect mulloway and other biota are sanctuary (green) and national park (blue); the restricted access zone may also offer protection (very small zone at the head of the Bight whale sanctuary—not shown). Inset 2 (solid line rectangle) highlights the Yalata section of the Far West Coast Marine Park (YFWCMP) and this inset is shown in full in Fig. 4
Here, we aimed to determine the spatial scale of movements associated with mulloway tagged with PSATs at the GAB during summer and autumn spanning three seasons. Using the results generated, we test the hypothesis that GAB mulloway is non-migratory. Finally, we assess the potential level of protection afforded by MPAs in the GAB.
Materials and methods
Study area
The GAB is a pristine region of the south coast of Australia encompassing the southwestern section of SA and south-eastern Western Australia (WA) (Fig. 1). The area is very isolated, abundant in marine life and has a variety of oceanographic influences. Within the GAB are high-energy sandy beach and reef lagoons in the Yalata section of the FWCMP (herein YFWCMP); this zone forms critical fish habitat for many species (Rogers et al. 2014).
The marine park network in the study region is comprised of protected areas from two levels of government (state and Commonwealth). The state-level protected areas are the Far West Coast Marine Park (FWCMP) and Nuyts Archipelago Marine Park (NAMP) and the Commonwealth protected areas are the Commonwealth Marine Reserves collectively referred to as the Great Australian Bight Marine Park (GABMP). Zones where mulloway cannot be extracted are sanctuary and national park (green and blue, respectively—see Fig. 1). Mulloway are regularly captured in and around the GABMP; by indigenous, recreational, and commercial sectors. The indigenous and recreational sectors particularly target the YFWCMP.
Mulloway capture
Adult mulloway (Table 1) were caught by individual hook (Gamakatsu® Octopus Pattern 10/0) and line from the beach. This configuration was chosen, because large fish could be landed relatively quickly and handling effects minimised. All work on fish was carried out on the beach and included: hook removal, measuring, and inspection of hooking site for excessive bleeding and tagging. Beach operations were carried out just above the surf line, to minimise time out of water and facilitate release of the fish in the best possible condition. Restraints or recovery tanks were not used as the fish are very docile, meaning that they could be tagged and released in under 4 min. As the fish could not be externally sexed, differences in movement and behaviour between male and female fish were not investigated. Mulloway were targeted for tagging in the Austral spring and summer between 2011/12 and 2013/14 (Table 1). Spring and summer provided the most likely time to capture mulloway from the beach, as the fish are inshore, particularly in the YFWCMP. Late summer captures facilitated the autumn deployments.
PSAT specifications
MiniPAT [mini-pop-up archival tag, Wildlife Computers (Redmond, WA, USA)] were used due to their relatively small size (12 cm long and ~ 53 g total mass), so as to minimise impact on the animal. The PSATs recorded depth, temperature, and light levels. All sensors sampled every 3 s; however, data were internally summarised for transmission. If a tag was physically retrieved, the total, non-summarised data could be downloaded on a personal computer. The depth sensor range was 0–1700 m with a resolution of 0.5 m. The temperature sensor range was − 40 to 60 °C with a sensor resolution of 0.05 °C. The time series (detailed sample data) depth information was summarised at 5 min intervals, whilst the time series temperature function was turned off to avoid problems with data transmission. Histogram messages or time at depth and time at temperature data were internally binned (12 bins each) at 6 h intervals, programming the tag to split the day into four periods, which were summarised in 24 h packages. The tags were also programmed to transmit 9 light-level samples for each light transition (dusk and dawn) to enable the estimation of the geographic position of mulloway (details below) via the light sensor with a range of 5 × 10−12 to 5 × 10−2 W cm−2. The deployment period was programmed at 100 days to be a trade-off between a reasonable duration to record seasonal changes, minimise risk of biofouling of sensors, and removal by other animals. Premature release was programmed to occur if the depth was constant (within 2 m ± 0.5 m) for 5 days. After the tags released (pop-up) from the fish, the summarised data and pop-up position were transmitted via the Argos satellite network.
PSAT attachment operations (tagging)
Extensive testing of various anchoring systems (the device that penetrates the animal and attaches it for deployment) was performed on mulloway carcasses at South Australian Research and Development Institute—Aquatic Sciences (West Beach, Adelaide, South Australia). The best practice assembly (i.e., least invasive but adequate holding power) consisted of a 30 mm titanium or surgical stainless-steel blade attached to the tag (Wildlife Computers—miniPAT) by 400 lb monofilament. This assembly was attached to the animal in the field using a stainless-steel applicator needle. All surgical and anchoring equipments were disinfected using 100% ethanol and betadine before applying to the individual fish. The needle and anchor were inserted between the bony pterygiophores on the left-hand side under the dorsal fin as this position produced the maximum holding strength of the anchor whilst keeping the trailing tag from interfering with the animal during swimming.
Horizontal movements
Mulloway position at the time of pop-up was calculated via the Doppler-shift service (precision < 1 km) from Argos data collection and location. The straight line or net displacement distance (km) between the tag deployment location and the pop-up location was calculated using R (Version 3.3.2, R Core Team 2013) software and functions within the package “geosphere”. Distance estimates were rounded to the nearest kilometre. For interpretation of straight-line movements, fish were classed by the migration direction [east (E), west (W)] or fish that did not appear to leave the YFWCMP which were termed non-migrant.
Daily geographical positions were estimated using the Wildlife Computers Global Position Estimator (GPE3) software. The details of GPE3 have been previously reported (see Stewart et al. 2016). Briefly, the Hidden Markov model (time series) at a 0.25° grid resolution incorporates environmental and habitat variables, such as temperature, daylight and barriers to movement, and the maximum swimming speed of the study animal (Pedersen et al. 2011). For mulloway, swimming speed was estimated to be 1 m s−1, which was calculated from acoustic telemetry data in Taylor et al. (2006). This model was used to create probability distribution surfaces (polygons) of tagged fish and the “most likely” daily location points determined using a spline interpolation (Pedersen et al. 2011). In one case, no pop-up Argos position reported for the tag, so the final day geolocational “most likely” estimate was used. Polygons and positions were visualised using “Raster” geospatial package in R.
Statistical data analysis
Statistical tests were conducted to explore relationships between mulloway behaviour and environmental variables, such as temperature, depth, season, and migration direction and distance. Data were checked for normality and homogeneity of variance using boxplots and quantile–quantile plots. Variables were tested for their influence on the net displacement via a generalised linear model (GLM). The covariates included deployment duration (days the fish were at liberty), size of fish [total length (TL)], and deployment season (summer or autumn). The net displacement response variable was regressed against the predictor variables with a Gaussian distribution family; predictor variables were removed, where not significant and the model rerun. The effect of deployment season and size of the fish on the net displacement direction (either west, east or no movement outside of the YFWCMP) were also tested using a multinomial logistic regression (MLR). The displacement direction response variable was regressed against the predictor covariates (TL and season). The influence of season on the time fish spent within temperature ranges (the binned temperature summary data) and the time fish spent in depth ranges (the binned depth summary data) was tested using x2 and Fisher’s exact test, respectively. These environmental variables were parameterized by determining the greatest percentage of time fish spent in certain bins or ranges and allocating the fish to that bin. All statistical analyses were performed using R software, with the package “nnet” used for the multinomial test.
Results
Tag deployments and data analysis
A total of 19 tags were deployed on mulloway that were presumed to be sexually mature (based on size) ranging from 94 to 151 cm total length. All fish were captured in a relatively small section of the GAB (30 km stretch) in western SA within the YFWCMP (Fig. 1).
Twelve tags provided data for analysis (Table 1). Eleven tags transmitted data to satellite but with variation in data days and data quality (53–110 data days and 28–89% Argos messages decoded) and one non-transmitting tag (tag 3) was recovered (see below). Satellite transmission days varied from 1 to 9 full days. There were missing time series depth data points in all of the Argos downloads, totalling between 288 and 19,968 data points, depending on the tag (Supplementary Fig. 1 and Table 2). The missing data were due to incomplete transmission packets creating gaps in the time series rather than a problem with the tag (Table 1).
The 12 reporting tags provided information on the pop-up location (except tag 3), daily position estimates via dusk and dawn light levels, time series of depth, and time at depth and temperature histograms (except tag 9 (see below)—Tables 1 and 2). Four tags were retrieved by members of the public, who found them on beaches (Table 1): these tags provided a full archival record. The data suggest that 2 tags (2 and 3) were snagged on an underwater structure(s) after 8 and 31 days (respectively) attached to the host fish. The snagging was inferred from a rapid transition from activity indicated by vertical movement in the water column to relative uniformity in depth profiles (see Supplementary Fig. 1). The period these tags appeared snagged was beyond the premature release period of 5 days; it appears that the tidal fluctuation, recorded by the static tags, prevented premature release implementation. As such, data were not analysed beyond the possible snagging date, except for the pop-up position of tag 2. Depth and temperature data from tag 9 fluctuated erratically due to an undetermined cause (see Supplementary Fig. 1); hence, only light data were used to determine the geolocation of this fish.
Horizontal movements
The minimum straight line or net displacement distance travelled by tagged mulloway ranged from 10 to 594 km, with a mean distance of 139.29 (SD 171.03); there was no significant influence of deployment duration (8–110 days) on distance travelled (p > 0.05, GLM, Supplementary Fig. 2). Most tags popped up within 3 km of the shore except tags 1 and 10 which were ~ 30 and 15 km offshore, respectively (Fig. 4). The greatest net displacements (straight-line distance between tagging and pop-up) occurred during autumn deployments (e.g., tags 1 and 10, Figs. 3 and 4). The influence of season was statistically significant (β = − 294.43, standard error (SE) = 104.49, t value = − 2.82, p < 0.05, GLM), despite the two small autumn net displacements (tags 11 and 12). The deployment season (autumn or summer) did not significantly affect the displacement direction (east, west, or non-migrant, p > 0.05, MLR) (Fig. 3). Similarly, the size of the tagged fish (TL) did not significantly affect the direction (p > 0.05, MLR) or distance of the displacements (p > 0.05, GLM, Supplementary Fig. 2).
Polygons of probability density surfaces for tagged mulloway with light to dark shades representing 95%, 75%, and 50% contours (respectively), for autumn, summer, and tag 9 (summer) deployments (first, second, and third multi-plot panels, respectively); the second plot on the third panel is the Yalata inset (Fig. 1) with summer pop-ups. Solid circles depict tagging location, upside down triangles are Argos pop-up location and upright triangles estimated pop-ups (from the relevant most likely position estimate), positions. Coloured areas are marine park zones with green representing sanctuary zones, orange (YFWCMP) and burgundy the Nuyts sections of the state-level Far West Coast Marine Park, whilst blue is Commonwealth Marine National Parks. Depth contours are also shown
Probability distribution polygons support approximately linear migrations of those tagged mulloway that moved relatively large distances (tags 1 and 10) (Fig. 4). The distribution contours vary along the linear migrations and suggest rapid linear movement (95% contour) and slower movement or residential behaviour (50% contour). In cases of limited movement (tags 2, 6, 7, 8, and 9), the polygons occupy an area of ~ 1 degree in diameter or about 110 km. In the case of tag 11 and to a lesser degree tag 9, the polygons suggest offshore migrations (at right angles to the tagging location), of hundreds of kilometres, before returning to very near the place of capture and tagging (Fig. 4).
Depth and temperature
The mean depth recorded by tags ranged from 2.5 (± 2.73 SD) to 29.4 (± 14.37 SD) m with overall minimum and maximum values of 0.0 and 56.5 m (Table 2). The average mean depth for all 11 fish (tag 9 was omitted due to unreliable data) was 9.72 (± 6.19 SD) m (Table 2). Five tagged fish (1, 3, 5, 8, and 10) showed relatively rapid (< 24 h) and large depth changes (> 20 m) from relatively shallow (< 12 m) to deeper waters and then returned to shallow water (Supplementary Fig. 1). However, there was no obvious pattern to these large depth changes. There was a seasonal aspect to depth habitat preference (Fig. 2). Over autumn fish spent most of their time in deeper parts of their depth range (~ 41% at 20.1–50 m, Supplementary Table 1) with the exception of two fish, tags 11 and 12 (Fig. 5). Conversely, over summer fish spent most of their time at relatively shallow depths (~ 50% at 2.1–10.0 m, Supplementary Table 1), with one individual at depths of 0.1–2.0 m (tag 7, Fig. 5). Two autumn fish (tags 11 and 12) had shallower depth ranges, similar to those displayed by fish tagged in the summer. Fish tagged over summer spent significantly more time in shallower waters than fish tagged over autumn (X2 = 6.97, df 2, p = 0.03, n = 11, Supplementary Fig. 3), despite tags 11 and 12 not conforming to the deeper autumn trend. Temperature distributions suggest that a greater range of temperatures were experienced by mulloway over summer (Fig. 5). All tagged mulloway inhabited waters between 15.1 and 27.0 °C (Fig. 5). Despite their shallow depth range, tags 11 and 12 experienced the coolest water of the autumn deployments (~ 15% of time at 15.1–18.0 °C). Nearly, all autumn-tagged fish (except tag 5) inhabited waters ranging from 18.1 to 21.0 °C for the majority of their deployment (~ 80% of time for all autumn fish), whilst summer tagged fish spent most time in 18.1–21.0 and 21.1–24.0 °C water (~ 52 and 37% of time, respectively—Supplementary Table 1). However, the influence of season on the time spent at certain water temperatures was not statistically significant (Fisher’s exact test p = 0.45).
Pop-up positions
Five of the seven fish tagged in summer popped up (released from fish) within the YFWCMP (the zone of capture and tagging). The fish were tags 2, 6, 7, 8, and 9 (Fig. 4). All autumn fish and two summer fish popped up outside of the YFWCMP. Tag 3 did not transmit to Argos; however, the last maximum likely daily location of this individual suggests that it was outside of the YFWCMP at the end of the deployment (Fig. 4).
Discussion
We were able to determine the spatial scale of mulloway movement, rejecting our original hypothesis that mulloway are non-migratory. A population genetics study suggests that the range of movements is hundreds of kilometres on the southwest coast of Australia, supporting the present study (Barnes et al. 2016). However, genetics suggest potentially longer movements occur in the south east of Australia, where there is evidence of ‘isolation by distance’ (Barnes et al. 2016). Hence, it is possible that different genetic populations in varying environments exhibit different life-history traits including movements (Ferguson 2010). The scale of the observed movements is also supported by the limited mark–recapture work done by Hall (1986) on the same genetic population. Mulloway home ranges, and therefore, genetically similar populations were found to be constrained by biogeographic migration barriers (e.g., fronts at the mouth of gulfs) (Barnes et al. 2016). It is likely that the study population’s home range is governed by barriers in the form of unsuitable habitat on lower Eyre Peninsula (Hall 1986) and the mouth of Spencer Gulf (e.g., salinity and temperature fronts) to the east and to the west by oceanographic influences and unsuitable habitat (Farmer 2008).
It has previously been suggested that mulloway likely spawn in the surf zone in late spring and summer in SA (Ferguson et al. 2014). This timing is similar to other areas (e.g., southeast and south Western Australia, Gray and McDonall 1993; Parsons et al. 2009), although mulloway have also been observed to spawn year round in sub-tropical regions (with seasonal peaks) (Farmer 2008). Prior research suggests that adult mulloway are aggregating at YFWCMP to spawn (Hall 1986; Rogers et al. 2014). Fish have been observed in spawning condition during evisceration by recreational anglers (Hall 1986; Barnes pers. obs.) and groups of fish observed tailing with an oily slick around them (Barnes, pers. obs.). Tailing is a behaviour, where a fish’s tail leaves the water, whilst the fish is near vertical with its head down and is anecdotal evidence of fish spawning. In addition, it is unusual to catch large mature mulloway (TL > 1 m) in autumn, winter, and early spring at YFWCMP, but sub-adults and juveniles are caught year round (Barnes, pers. obs.). The movement data from the present study support the seasonal spawning aggregation at the YFWCMP hypothesis. For example, there was a seasonal aspect to tagged mulloway behaviour. The seasonal difference in movements and environments experienced by the tagged mulloway suggests a shallow water summer residency (i.e., in or near the surf zone) in late spring and summer.
No information is available on overwintering of mulloway in SA, but seasonal movements have been observed in southeast SA, also in autumn (Hall 1986), South Africa (Griffiths 1996), and in other Sciaenids. Weinstein et al. (2009) found that weakfish (Cynoscion regalis) move to warmer waters to overwinter in autumn. The seasonal movement strategy is likely philopatric, a strategy common in other Sciaenids (Thorrold et al. 2001; Gold and Turner 2002; Hutchings and Griffiths 2010; Potts et al. 2010) and other mulloway populations have their own regularly visited regions (Griffiths 1996; Hall 1986). It is widely accepted that philopatry may also drive population differentiation (e.g., Svedäng et al. 2007) and makes Sciaenid populations vulnerable to fishing activity (Thorrold et al. 2001; Erisman et al. 2017) and habitat destruction (Ferguson et al. 2008).
Tags 11 and 12 were outliers and did not move west in autumn. Different size-dependent migratory strategies within populations have been reported for mulloway in other regions (Griffiths 1996) and other large bodied predatory fish including Sciaenids (Potts et al. 2010; Semmens et al. 2010). Differences in mitochondrial and nuclear genetic data from South African mulloway (dusky kob) have been attributed to separate female migration behaviour (Mirimin et al. 2016). However, different movement strategies, at the population level, may not be the case for mulloway at Yalata which is supported by the seasonality of size structure in catches. Hence, the westerly movement is likely size dependent (although not statistically significant but with relatively low replication), as the two autumn outliers were only 105 and 110 cm TL compared to tags 5 and 10 which were 128 and 150 cm TL, respectively. Determining size-dependent movement is complicated in this case and may be influenced by environmental and other biological factors (e.g., water temperature and sex). More replication or other targeted research would be required to definitively address the migration complexities of the study population.
Information on mulloway movement and the habitat data (e.g., depth) provided valuable insight into the ecology of a unique and isolated demersal fish population, despite inherent problems with the technique. The return rate of tags was reasonable (63%) and compared favourably with other demersal tagging applications such as data storage tags (DST), which can be as low as 6% (Miller and Able 2002), but which can be up to 50% (e.g., see Hunter et al. (2006)). However, the rate was slightly lower than some studies on fish with different habitat preferences, such as flatfish (Hippoglossus stenolepis) (e.g., Armsworthy et al. 2014) and tuna (e.g., Thunnus orientalis) (e.g., Block et al. 2011). These fish live in habitats that are often relatively free of solid obstructions (e.g., soft sediment and water column), whereas mulloway frequent a range of habitat types including reefs and shipwrecks; hence, they may interact with more structure at times which may snag the trailing tag. We lost approximately 160 days of data due to snagging of two tags which would be unlikely in pelagic species. The variety of habitat encountered by demersal species should be a consideration in future PSAT tagging projects. In addition, light based geolocation is more suited to pelagic rather than sea-bed dwelling organisms, due to water column attenuation (Liu et al. 2017); however, mulloway are a coastal species and, as such, are found in relatively shallow water. The depth habitat occurrence of the target population of the present study was only previously supported by anecdotal evidence. The depth data show that tagged fish inhabited the photic zone (upper 80 m) and hence were recording light and dark regularly.
The pop-up position of some of the migratory-tagged mulloway was outside of the YFWCMP by reasonably substantial distances. Therefore, mulloway only receive partial protection from the YFWCMP. Importantly, the YFWCMP provides protection to spawning and juvenile fish and the associated habitat. Spawning aggregation protection is an important first step in managing data poor fisheries (Erisman et al. 2017) and nursery protection has also been suggested as critical for another exploited large bodied Sciaenid (Potts et al. 2010). In addition, the other MPAs in the area (Commonwealth and state) may help slow the harvest and protect habitat (e.g., on the migration route), although our data cannot quantify the level of protection. However, different or new technology in the future may be able to more precisely assess mulloway interactions with MPAs during migration.
Fishery-independent data obtained from PSATs shed new light on mulloway movement ecology. It is now clear that some mulloway migrate from western SA to WA. These data highlight the important movement information that can be obtained through PSATs on otherwise difficult to track species, potentially aiding management decisions on fish sustainability and conservation.
References
Armsworthy SL, Trzcinski MK, Campana SE (2014) Movements, environmental associations, and presumed spawning locations of Atlantic halibut (Hippoglossus hippoglossus) in the northwest Atlantic determined using archival satellite pop-up tags. Mar Biol 161:645–656
Barnes TC, Junge C, Myers SA, Taylor MD, Rogers PJ, Ferguson GJ, Lieschke JA, Donnellan SC, Gillanders BM (2016) Population structure in a wide-ranging coastal teleost (Argyrosomus japonicus, Sciaenidae) reflects marine biogeography across southern Australia. Mar Freshw Res 67:1103–1113
Block BA, Jonsen I, Jorgensen S, Winship A, Shaffer SA, Bograd S, Hazen E, Foley D, Breed G, Harrison A-L (2011) Tracking apex marine predator movements in a dynamic ocean. Nature 475:86–90
Darnaude AM, Hunter E (2018) Validation of otolith δ18O values as effective natural tags for shelf-scale geolocation of migrating fish. Mar Ecol Prog Ser 598:167–185
Erisman E, Heyman W, Kobara S, Ezer T, Pittman S, Aburto-Oropeza O, Nemeth RS (2017) Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish 18:128–144
Farmer BM (2008) Comparisons of the biological and genetic characteristics of the Mulloway Argyosomus japonicus (Sciaenidae) in different regions of Western Australia. Dissertation, Murdoch University, Perth
Ferguson GJ (2010) Impacts of river regulation, drought and exploitation on the fish in a degraded Australian estuary, with particular reference to the life-history of the Sciaenid, Argyrosomus japonicus. Dissertation, University of Adelaide, Adelaide
Ferguson G, Ward T (2003) Mulloway (Argyrosomus japonicus) fishery. Fishery Stock Status Report for PIRSA Fisheries. SARDI Aquatic Sciences RD03/0040, pp 1–55
Ferguson GJ, Ward TM, Geddes MC (2008) Do recent age structures and historical catches of mulloway, Argyrosomus japonicus (Sciaenidae), reflect freshwater inflows in the remnant estuary of the Murray River, South Australia? Aquat Living Resour 21:145–152
Ferguson GJ, Ward TM, Gillanders BM (2011) Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish Res 110:75–83
Ferguson GJ, Ward TM, Ivey A, Barnes T (2014) Life history of Argyrosomus japonicus, a large sciaenid at the southern part of its global distribution: implications for fisheries management. Fish Res 151:148–157
Gillanders BM (2005) Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuar Coast Shelf Sci 64:47–57
Gold J, Turner T (2002) Population structure of red drum (Sciaenops ocellatus) in the northern Gulf of Mexico, as inferred from variation in nuclear-encoded microsatellites. Mar Biol 140:249–265
Gray C, McDonall V (1993) Distribution and growth of juvenile mulloway, Argyrosomus hololepidotus (Pisces: Sciaenidae), in the Hawkesbury River, south-eastern Australia. Mar Freshw Res 44:401–409
Griffiths M (1996) Life history of the dusky kob Argyrosomus japonicus (Sciaenidae) off the east coast of South Africa. S Afr J Mar Sci 17:135–154
Griffiths M (1997) Management of South African dusky kob Argyrosomus japonicus (Sciaenidae) based on per-recruit models. S Afr J Mar Sci 18:213–228
Hall D (1986) An assessment of the mulloway (Argyrosomus hololepidotus) fishery in South Australia with particular reference to the Coorong Lagoon: discussion paper. Department of Fisheries, South Australia
Hunter E, Berry F, Buckley AA, Stewart C, Metcalfe JD (2006) Seasonal migration of thornback rays and implications for closure management. J Appl Ecol 43:710–720
Hutchings K, Griffiths M (2010) Life-history strategies of Umbrina robinsoni (Sciaenidae) in warm-temperate and subtropical South African marine reserves. Afr J Mar Sci 32(1):37–53
Le Port A, Sippel T, Montgomery JC (2008) Observations of mesoscale movements in the short-tailed stingray, Dasyatis brevicaudata from New Zealand using a novel PSAT tag attachment method. J Exp Mar Biol Ecol 359:110–117
Lenanton R (1982) Alternative non-estuarine nursery habitats for some commercially and recreationally important fish species of south-western Australia. Mar Freshw Res 33:881–900
Liu C, Cowles GW, Zemeckis DR, Cadrin SX, Dean MJ (2017) Validation of a hidden Markov model for the geolocation of Atlantic cod. Can J Fish Aquat Sci 74:1862–1877
Loher T (2008) Homing and summer feeding site fidelity of Pacific halibut (Hippoglossus stenolepis) in the Gulf of Alaska, established using satellite-transmitting archival tags. Fish Res 92:63–69
Miller M, Able K (2002) Movements and growth of tagged young-of-the-year Atlantic croaker (Micropogonias undulatus L.) in restored and reference marsh creeks in Delaware Bay, USA. J Exp Mar Biol Ecol 267:15–33
Mirimin L, Macey B, Kerwath S, Laberth S, Bester-van der Merwe A, Cowley P, Bloomer P, Roodt-Wilding R (2016) Genetic analyses reveal declining trends and low effective population size in an overfished South African sciaenid species, the dusky kob (Argyrosomus japonicus). Mar Freshw Res 67:266–276
Myers RA, Worm B (2003) Rapid worldwide depletion of predatory fish communities. Nature 423:280–283
Næsje TF, Cowley PD, Diserud OH, Childs A-R, Kerwath SE, Thorstad EB (2012) Riding the tide: estuarine movements of a sciaenid fish, Argyrosomus japonicus. Mar Ecol Prog Ser 460:221–232
Palmer RM, Snowball JD (2009) The willingness to pay for dusky kob (Argyrosomus japonicus) restocking: using recreational linefishing licence fees to fund stock enhancement in South Africa. ICES J Mar Sci 66:839–843
Palumbi SR (2004) Marine reserves and ocean neighborhoods: the spatial scale of marine populations and their management. Annu Rev Environ Resour 29:31–68
Parsons MJ, McCauley RD, Mackie MC, Siwabessy P, Duncan AJ (2009) Localization of individual mulloway (Argyrosomus japonicus) within a spawning aggregation and their behaviour throughout a diel spawning period. ICES J Mar Sci 66:1007–1014
Pedersen MW, Patterson TA, Thygesen UH, Madsen H (2011) Estimating animal behavior and residency from movement data. Oikos 120:1281–1290
Potts WM, Sauer WHH, Henriques R, Sequesseque S, Santos CV, Shaw PW (2010) The biology, life history and management needs of a large sciaenid fish, Argyrosomus coronus, in Angola. Afr J Mar Sci 32:247–258
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rogers P, Barnes T, Wolf Y, Gregory P, Williams N, Madonna A, Loisier A (2014) On-site recreational fishery survey and research of mulloway (Argyrosomus japonicus) in the Yalata Indigenous Protected Area and Far West Coast Marine Park between 2009 and 2013. SARDI Research Report Series-South Australian Research and Development Institute, South Australia, p 759
Semmens JM, Buxton C, Forbes E, Phelan M (2010) Spatial and temporal use of spawning aggregation sites by the tropical sciaenid Protonibea diacanthus. Mar Ecol Prog Ser 403:193–203
Silberschneider V, Gray CA (2008) Synopsis of biological, fisheries and aquaculture-related information on mulloway Argyrosomus japonicus (Pisces: Sciaenidae), with particular reference to Australia. J Appl Ichthyol 24:7–17
Stewart JD, Beale CS, Fernando D, Sianipar AB, Burton RS, Semmens BX, Aburto-Oropeza O (2016) Spatial ecology and conservation of Manta birostris in the Indo-Pacific. Biol Cons 200:178–183
Svedäng H, Righton D, Jonsson P (2007) Migratory behaviour of Atlantic cod Gadus morhua: natal homing is the prime stock-separating mechanism. Mar Ecol Prog Ser 345:1–12
Taylor M, Laffan S, Fielder D, Suthers I (2006) Key habitat and home range of mulloway Argyrosomus japonicus in a south-east Australian estuary: finding the estuarine niche to optimise stocking. Mar Ecol Prog Ser 328:237–247
Thorrold SR, Latkoczy C, Swart PK, Jones CM (2001) Natal homing in a marine fish metapopulation. Science 291:297–299
Weinstein M, Litvin S, Guida V, Chambers R (2009) Is global climate change influencing the overwintering distribution of weakfish Cynoscion regalis? J Fish Biol 75:693–698
Acknowledgements
We thank the following for their support during this project: Teddy Edwards, Brian Quema, Bubbles and other members of Yalata Land Management and the Yalata community, Blair Middlemiss, Wayne Ragless, Amanda Woods, Geoff Rogers, Andrew Brooks, Cindy Strachan, Darren Hoad, Kris Ellis, Danielle Manetti, Harley Donnithorne, Paul Lehmann, Colin Bailey, Hall Print and Wildlife Computers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study has no potential conflict of interest.
Ethical approval
Animal ethics approval was via: Primary Industries and Regions South Australia Animal Ethics Application 19/11 and the University of Adelaide S-2009-129. This project was funded by Yalata Land Management, Department for Environment, Water and Natural Resources—Alinytjara Wilurara, the University of Adelaide, the Australian Research Council (FT100100767) (awarded to BMG) and the Nature Foundation.
Additional information
Responsible Editor: E. Hunter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barnes, T.C., Rogers, P.J., Wolf, Y. et al. Dispersal of an exploited demersal fish species (Argyrosomus japonicus, Sciaenidae) inferred from satellite telemetry. Mar Biol 166, 125 (2019). https://doi.org/10.1007/s00227-019-3575-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3575-4