Abstract
In host–parasite coevolution, parasite innovations including the acquisition of new habitats and novel traits can trigger evolutionary breakthroughs and enhance parasite diversification via accumulation of new hosts. All species of the family Cymothoidae are obligate fish parasites, attaching to exterior body surfaces of fish, the buccal or opercular cavities, or burrowing into abdominal muscle tissue. In the present study, we constructed a molecular phylogeny of 27 cymothoid species that parasitise 38 fish species, combined with 2 prior cymothoid datasets, based on the sequences of mitochondrial 16S rRNA and COI genes. We explored the evolution of the host attachment mode, and the habitat shift from saline water to freshwater. Our evolutionary trees include two freshwater clades, an abdominal burrower clade, and cymothoid clades that are closer to the base of Cymothoidae than those initially analysed. We found that the basal clade of Cymothoidae was Elthusa sacciger, which is parasitic in the opercular cavity of synaphobranchid eels. This result suggests that cymothoids may have originated in deep seas, subsequently expanded to shallow seas, and then to brackish and/or freshwater, by shifting host species. Invasion of freshwater habitats has occurred at least twice; freshwater abdominal muscle burrowers living on armoured catfish constitute a clade allied to E. sacciger. The ancestral host attachment site, based on our dataset, was the opercular cavity, followed (sequentially) by buccal colonisation and attachment to the external body.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During host–parasite coevolution, parasite innovation (acquisition of new traits and colonisation of new habitats) can enhance parasite diversification via accumulation of new host species (Zietara and Lumme 2002; Ricklefs et al. 2014). The family Cymothoidae is a diverse group of Isopoda containing 366 species of 42 genera to the best of our knowledge (Table S1), all of which are obligate parasites on fish, feeding on blood, mucus, and/or tissue (Adlard and Lester 1995; Horton and Okamura 2003; Bruce and Schotte 2008). Cymothoids have been identified in marine actinopterygian fish (notably Perciformes, Clupeiformes, Beloniformes, and Tetraodontiformes) and certain elasmobranchs (Brusca 1981; Smit et al. 2014). Cymothoids have expanded their distribution to include freshwater fish, being currently parasitic on freshwater actinopterygians including Characiformes, Siluriformes, and Cypriniformes. 310 species (85%) of 32 genera (76%) are marine, 33 species (9%) of 9 genera (21%) are fresh water inhabitants. At least 27 species in nine genera (including Riggia, Braga, Asotana, and Paracymothoa) inhabit Amazonia in South America. Several species of the genus Ichthyoxenos live in freshwater habitats of eastern and south-eastern Asia, and central Africa (Brusca 1981; Tsai and Dai 1999).
Cymothoids parasitise the exterior bodies of fish (including the fins), or the buccal or opercular cavities, or burrow into abdominal muscular tissue to create capsules (with small openings) in the abdominal cavity (Brusca 1981; Bruce 1990; Smit et al. 2014). Each cymothoid species exhibits a specific attachment mode to its host fish, and is morphologically specialised in this context (Bruce 1986, 1987, 1990). Seven genera (e.g. Anilocra, Nerocila, and Renocila), 16 genera (e.g. Ceratothoa and Cymothoa), 17 genera (e.g. Elthusa and Ryukyua), four genera (e.g. Ichthyoxenos and Artystone) are known as parasites on fish body surface, in the buccal cavity, opercular cavity, burrowing into abdominal cavity, respectively (Table S1). Host specificity and host range vary among cymothoid species. In general, however, cymothoids that attach to external surfaces of fish exhibit wider host ranges than other species, sometimes straddling fish orders. On the other hand, cymothoids that burrow into the abdominal muscles of fish are highly species-specific, being parasitic on only one or several species within a single family (Tsai and Dai 1999; Thatcher 2006; Yamano et al. 2011). Cymothoids that are parasitic in the buccal and opercular cavities exhibit intermediate width of host range (Bruce 1986; Hadfield et al. 2015).
We used a molecular phylogenetic approach to explore cymothoid evolution in terms of the acquisition of attachment modes, shifts in such modes, habitat expansion (particularly to freshwater), and acquisition of new host fish. Brusca (1981) suggested that external surface attachment was an ancestral trait, citing morphological, zoogeographic, and ecological data. Jones et al. (2008) suggested that buccal- or opercular cavity-dwelling were ancestral in nature, and that external attachment had evolved on several occasions; mitochondrial 16S rRNA sequence data were used to support these conclusions. However, molecular methods have never been used to analyse cymothoids that burrow under the host epidermis to occupy the abdominal cavity, and few data are available on freshwater cymothoids. Thus, it remains unclear whether several cymothoids independently acquired the ability to live in freshwater, or whether a single group acquired that competence and has subsequently diversified. Although there have been several molecular phylogenetic studies on the family Cymothoidae, the reference frameworks are unclear; ambiguous sequences were included in previous phylogenies based on 16S rRNA and COI sequences (Ketmaier et al. 2008), and were stored in GenBank as below. The 16S rRNA gene sequences differ by a maximum of 5 of the 317 bases among four species in three genera (Ceratothoa italica [EF455804–6], C. collaris [EF455807], Nerocila bivittata [EF455810], and Anilocra physodes [EF455808–9]). Such extreme similarity differs from the findings of another study (Jones et al. 2008), which also sequenced 16S rRNA gene, and we found that the sequences in question were those of some members of the genus Ceratothoa. It may be that Ketmaier et al. (2008) included these inappropriate sequences in their analyses.
In the present study, we newly collected two freshwater cymothoid species; these were a burrowing parasite (Artystone sp.) from an armoured catfish (Epactionotus yasi) living in the Iguazu river, South America, and a buccal parasite (Ichthyoxenos tanganyikae) from a cichlid (Simochromis diagramma) of Lake Tanganyika. We also collected samples of ten genera of marine cymothoids from the buccal and opercular cavities, and the body surfaces, of their various host fish (ranging from anguilliformes to perciformes) living in various depths (See Table 1). We sought to describe the evolution within the family Cymothoidae. In particular, we explored how the organisms acquired novel host attachment modes, shifted such modes, and expanded into freshwater habitats. We also sought to resolve taxonomic confusion within the family. We constructed phylogenetic trees based on mitochondrial 16S rRNA and COI sequences; we used the families Aegidae, Bopyridae, Cirolanidae, Corallanidae, and Sphaeromatidae as outgroups.
Materials and methods
Sample collection
We collected 29 cymothoid species from 59 species of host fish, as well as 2 species of Aegidae, 1 species of Corallanidae, and 1 species of Sphaeromatidae (Table 1). 27 species of 12 genera cymothoids were newly collected for molecular phylogeny. In total, 15 genera of all the 43 genera of Cymothoidae are included in this study. Host fish were identified by reference to Nakabo (2013). We distinguished four cymothoid attachment modes (Brusca 1981; Smit et al. 2014): (1) buccal-dwelling, where both males and females live inside the buccal cavities of host fish; (2) opercular cavity-dwelling, where females attach to a specific site within the opercular cavity and their body form is distinctly asymmetrical, reflecting the shape of the host operculum; (3) external body-attaching, where the parasites attach to the external body surface (including the fins) of the hosts, being cryptically coloured in contrast to the pale colour of parasites exhibiting other attachment modes; and (4) abdominal-burrowing, where parasites become buried under the host epidermis, forming a capsule with a small opening within myomeric tissue to occupy the abdominal cavity.
DNA extraction
Samples of dorsal musculature (ca. 2 × 2 mm3) were excised from ethanol-preserved specimens. Total genomic DNA was extracted with Genomic DNA Purification Kit (Promega, Wisconsin, USA) following the manufacturer’s protocol.
PCR and sequencing
The 16S rRNA gene region of mitochondrial DNA (mtDNA) was amplified using the forward primer (16Sar) 5′–CGCCTGTTTAACAAAAACAT–3′ and the reverse primer (16Sbr) 5′–CCGGTCTGAACTCAGATCATGT–3′ (Simon et al. 1994) via polymerase chain reaction (PCR). Each PCR was performed in a 10 μL reaction volume containing 3.35 μL sterile distilled H20, 5.0 μL Ampdirect Plus (Shimadzu, Kyoto, Japan), 0.3 μL each primer (10 μM solutions), 0.05 μL Taq DNA polymerase (BIOTAQ HS DNA Polymerase, Bioline, London, UK), and 1 μL template. The thermal cycle profile was as follows: initial denaturation at 94 °C for 10 min; 30 cycles of 94 °C for 30 s, 48 °C for 1 min, and 72 °C for 1 min; and a final extension at 72 °C for 10 min. The COI regions of mtDNA were amplified using the forward primer LCO1490 (5′–GGTCAACAAATCATAAAGATATTGG–3′) and the reverse primer HCO2198 (5′–TAAACTTCAGGGTGACCAAAAAATCA–3′) (Folmer et al. 1994) using the volumes described above. The thermal cycle profile was as follows: initial denaturation at 94 °C for 10 min; 35 cycles of 94 °C for 30 s, 40 °C for 1 min, and 72 °C for 1.5 min; and a final extension at 72 °C for 10 min. PCR products were purified using polyethylene glycol following a published protocol (Rosenthal et al. 1993) and subjected to direct cycle sequencing employing BigDye Terminator version 3.1 technology (Applied Biosystems [ABI], Foster City, CA, USA) using the PCR primers. The sequencing protocol used was that recommended by ABI. Labelled fragments were sequenced on an ABI 3130 platform. All pseudogenes (nuclear copies of mtDNA) evident on electropherograms were excluded by checking for double peaks and mismatches in overlapping sequences of any given taxon (Mindell et al. 1999); we used Geneious version 7.1.7 software to this end (Kearse et al. 2012).
Sequence analysis
Forward and reverse sequences were assembled using Geneious version 7.1.7. For the 16S rRNA genes, assembled sequences were aligned using MAFFT 7.273 software running the Q-INS-i option that aligns sequences by considering RNA secondary structure (Katoh and Standley 2013). Next, ambiguities were removed by trimAl version 1.2 software (Capella-Gutiérrez et al. 2009). For the COI genes, the assembled sequences were translated into proteins and aligned to identify pseudogenes containing unexpected insertions, deletions, frameshifts, and stop codons (Mindell et al. 1999). The protein sequences were then reverse-translated prior to phylogenetic analysis using MEGA 6.06 software (Tamura et al. 2013).
We ran χ2 homogeneity tests to explore the compositional homogeneity of nucleotides within 16S rRNA genes, and those of all codons of COI genes. These tests showed that the third codon positions of the COI genes exhibited significant heterogeneity in terms of base frequency. Most phylogenetic reconstruction algorithms assume homogeneity in nucleotide base compositions and can give rise to phylogenetic inaccuracies when this assumption is violated (e.g. Tarrío et al. 2001; Hassanin 2006). Therefore, the first, second, and third codon positions of the COI genes were separately analysed. The third codon position was subjected to RY coding. Thus, the pyrimidines C and T formed a single character, as did the purines A and G (Phillips and Penny 2003).
Phylogenetic analysis
Phylogenetic trees were constructed based on the individual 16S rRNA and COI datasets, and their combination. We used both the maximum likelihood (ML) and Bayesian inference (BI) methods. Individual substitution models were used to analyse the 16S rRNA data, and the first, second, and third codon positions of COI. We calculated Akaike Information Criteria and selected the best substitution model using Kakusan 4 software (Tanabe 2011). Following this model selections, we used the GTR + G model when applying both the ML and BI methods to the 16S rRNA data. For COI analysis, we employed the GTR + G model when evaluating all three codon positions by the ML method, and the SYM + G, GTR + G, and F81 + G models to evaluate the first, second, and third codon positions, respectively, when applying the BI method. For the combined dataset, we used the GTR + G model to evaluate all markers by the ML method, and the GTR + G, SYM + G, GTR + G, and F81 + G models to evaluate the 16S rRNA data, and the first, second, and third codon positions of COI, respectively, when applying the BI method. ML and BI analyses were performed using RAxML version 8 (Stamatakis 2014) and MrBayes version 3.2.6 (Ronquist et al. 2012) software, respectively. In the BI method, two independent Markov-chain Monte Carlo runs, each of 1,000,000 generations, were performed; the trees were sampled every 100 generations. The first 4000 trees were discarded as burn-in. A majority-rule consensus tree was constructed from the remaining 6000 trees. We confirmed that all analyses attained the stationary condition well before the end of the burn-in period. To this end, we plotted the natural logarithms of the likelihoods of all sampled trees against the generation time, and confirmed that average standard deviation of split frequencies and the potential scale reduction factor for all parameters reached 0.006 and 1.00, respectively. We also confirmed that evaluating effective sample size values for all parameters were more than 200.
Evolution of the attachment mode
To explore how attachment modes were acquired by, and shifted within, Cymothoidae, we identified the ancestral mode using the Trace Character History function of Mesquite 3.04 (Maddison and Maddison 2015) software running an ML reconstruction algorithm.
Results and discussion
Phylogeny of the Cymothoidae
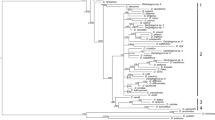
Three phylogenetic trees were constructed based on the 16S rRNA, COI, and combined datasets. These trees included two freshwater clades, an abdominal burrower, and the clade closer to the base of Cymothoidae (which were analysed for the first time). The topologies were nearly consistent among the trees, although the bootstrap values/Bayesian posterior probabilities were low in both the 16S rRNA and COI trees (Figs. 1, 2, 3, S1). We base most of our results on the tree constructed using the combined dataset (Figs. 1, S1).
A maximum likelihood tree of the family Cymothoidae based on combined data on mitochondrial 16S rRNA and COI genes. The numbers adjacent to branches are the maximum likelihood bootstrap values followed by the Bayesian posterior probabilities. Values >50 and 0.5 (bootstrap values and probabilities, respectively) are shown. The host fish families and the numbers of families (informative in terms of phylogenetic position; 1 to 536 indicate Myxinidae to Diodontidae, respectively; Nelson et al. 2016) are shown in parentheses or following silhouettes of the families. Dark- and light-grey fish inhabit deep (>200 m) and shallow water, respectively; ‘FW’ identifies freshwater fish. See Table 1 for details of host fish species. The photograph of Artystone sp. is reproduced with the kind permission of I. Seidel
Maximum likelihood tree of the family Cymothoidae based on mitochondrial 16S rRNA sequence data. The numbers adjacent to the branches are the maximum likelihood bootstrap values followed by the Bayesian posterior probabilities. Values >50 and 0.5 (bootstrap values and probabilities, respectively) are shown. The host fish species are shown in parentheses
Maximum likelihood tree of the family Cymothoidae based on mitochondrial COI sequence data. The numbers adjacent to the branches are the maximum likelihood bootstrap values followed by the Bayesian posterior probabilities. Values >50 and 0.5 (bootstrap values and probabilities, respectively) are shown. The host fish species are shown in parentheses
Phylogenetic analysis revealed that the basal branches within the family Cymothoidae were Elthusa sacciger; parasitic in the opercular cavity of synaphobranchid eels living in the deep sea (between 400 and 3000 m) of the Western Pacific, and the freshwater abdomen-burrowing Artystone sp., parasitic on an armoured catfish of the Iguassu River, South America. The opercular cavity parasite clade, including the genera Elthusa and Cterissa, arose next (Figs. 1, 2, 3, and S2). These parasites colonise both deep-water fish (Ereuniidae, Hemitripteridae, Hexagrammidae, and Macrouridae) and shallow water coastal fish belonging to Holocentridae. The mouth-dwelling Ceratothoa and Glossobius are next; most of their host fish species are coastal in nature. The next clade contains only Ichthyoxenos tanganyikae, which parasitises the mouth of a Tanganyikan cichlid (Simochromis diagramma); this species seems to form a monophyletic group with the Mothocya species parasitic in the opercular cavities of marine Belonidae and Hemiramphidae. Subsequently, two clades of parasites living in the buccal and opercular cavities arose; one clade includes Elthusa vulgaris and the other includes Lobothorax and Ryukyua. Finally, Cymothoa (parasitic in the mouth) and Anilocra and Nerocila (parasitic on the external body surfaces of marine fish) arose and became diversified.
The abdominal muscle burrower Artystone sp. parasitises a freshwater loricariid fish, Epactionotus yasi, and forms a highly supported clade with Alitropus typus, a species of Aegidae that is parasitic in the opercular cavities or on the body surfaces of fish that live in brackish or freshwater of southern Asia to eastern Australia (Pillai 1967; Bruce 1983; Nair and Nair 1983; Bruce 2009). This was apparent in the phylogenetic tree based on COI sequences (Fig. 3).
Habitat expansion and shift to a new host fish
One of the basal taxa of Cymothoidae is Elthusa sacciger, which is parasitic on deep-sea Synaphobranchidae. Smit et al. (2014) indicated that Cymothoidae may have originated in the Jurassic era (199–145 Ma) because fossils of bopyrid isopods, a family closely related to Cymothoidae (Boyko et al. 2013), have been dated to that time. In addition, the fossil record suggests that cirolanid isopods scavenged a marine fish, Pachyrhizodus marathonensis, of the extinct order Crossognathiformes, in the Albian, Early Cretaceous (113–100 Ma) (Wilson et al. 2011). In terms of marine fish, most existing orders that marine cymothoids parasitise were absent in the Jurassic era (Inoue et al. 2015), the exception being the order Anguilliformes. Deep-sea Synaphobranchidae originated in the Triassic era (Inoue et al. 2010; Johnson et al. 2012), and it is thus possible that the isopods parasitic in the opercular cavity of these deep-sea eels constitute the origin of Cymothoidae, which later diversified and expanded their habitat to include shallow seas and freshwater by evolving to live with newly derived fish. Intensive collection of deep-sea cymothoids is required to rigorously define the phylogenetic position of parasites of synaphobranchid eels and to evaluate the hypothesis we advance above. On the other hand, phylogeny of cymothoids and that of host fishes are not concordant because of dynamic host shift by cymothoids (Fig. 1). Especially, cymothoids living in the buccal cavity and on external body surfaces of fishes have wide ranges of host fish species (Fig. 1; Table 1) and suggesting frequent host shift in the coevolution between cymothoids and host fishes.
In the present study, we analysed two freshwater species, one of which was a flesh-burrowing Artystone sp. parasitic on an armoured catfish, Epactionotus yasi, of the Iguassu River basin, South America; the other was the freshwater buccal parasite, Ichthyoxenos tanganyikae, which is parasitic on a Tanganyikan cichlid, Simochromis diagramma. Artystone sp. forms a highly supported clade with Alitropus typus, a species of Aegidae that is parasitic in the opercular cavity or body surface of brackish or freshwater fish of southern Asia to eastern Australia (Pillai 1967; Bruce 1983; Nair and Nair 1983; Bruce 2009). This suggests that Alitropus (which contains only A. typus) may belong to Cymothoidae rather than Aegidae. Alitropus typus has seven pairs of prehensile legs and short coxae on pereopods 5–7, and is unique among aegid species (Chilton 1926; Bruce 1983; note that Rocinela simplex of Chilton [1926] is a junior synonym of A. typus). However, this pereopod morphology is common in Cymothoidae (Brusca 1981). Further, this clade diverges near the base of the cymothoid phylogenetic tree, suggesting that the lineage may have immigrated to freshwater in Gondwanaland, and diversified following the break-up of that continent. Alitropus typus and Artystone sp. colonise siluriform fish that also originated in Gondwanaland (106.1 Ma; Near et al. 2012), partly supporting this hypothesis.
In contrast, Ichthyoxenos tanganyikae is closely related to the opercular cavity-parasitic genus Mothocya that lives in shallow coastal regions, suggesting that this species also arose by invasion of the lacustrine habitat, perhaps via the Congo River where a close relative, I. expansus, has been described from the opercular cavity of the characiform fish Eugnathichthys eetveldii (Fryer 1968; Lincoln 1972). Ichthyoxenos tanganyikae is apparently asymmetrical, as are cymothoids that parasitise the opercular cavity, suggesting that this mouth-parasitic group originated from a parasite of the opercular cavity (Fig. 4). These two freshwater clades arose independently from different marine ancestors; the Artystone clade evolved much earlier than did the I. tanganyikae clade.
Reconstruction of the Cymothoidae attachment mode using a maximum likelihood tree based on combined data on mitochondrial 16S rRNA and COI sequences. The pie charts indicate the relative likelihoods of the attachment mode for each clade. Asterisks indicate nodes where only one state was significant upon comparison of log-likelihoods. The host fish species are shown in parentheses. The photographs of Artystone sp. and Elthusa sacciger are reproduced with the kind permission of I. Seidel and H. Hirasaka, respectively
Evolution of the attachment mode to host fish
Our results suggest that the ancestor of the family Cymothoidae was most likely an opercular parasite (Fig. 4), although more taxon sampling and molecular analyses are necessary to resolve the basal part of cymothoid phylogeny. Subsequently, at a very early stage, certain lineages seem to have invaded freshwater habitats and some acquired the ability to burrow into the abdominal cavity. All abdominal cavity-burrowing species (the genera Ichthyoxenos, Artystone, and Riggia) are strictly confined to brackish or freshwater, with the exception of only one species, Ourozeuktes bopyroides, that is marine and parasitic on a leatherjacket (Acanthaluteres spilomelanurus) of one of the most derived fish orders, Tetraodontiformes (Brusca 1981; Saunders 2012; Trilles and Hipeau-Jacquotte 2012). Further analyses on these species are required to define the timing and frequency of evolution of abdominal cavity burrowers. Mouth-dwelling capacity evolved only once and then became diversified. Three clades of mouth parasites have reverted to opercular cavity parasitism. The final evolutionary step was attachment to the external surface of fish. Based on the 16S rRNA data, parasites of the buccal or opercular cavity may be the ancestors of organisms exhibiting external attachment today (Jones et al. 2008). Our 16S rRNA and COI dataset supports this suggestion. Furthermore, we have defined three clades near the base of the tree, suggesting that parasites of the opercular cavity are the most ancient. For the first time, we used molecular data to reveal the ancient origin of freshwater abdominal burrowers, and the independent expansion into a freshwater habitat by a Tanganyikan mouth-dwelling cymothoid.
Phylogenetic relationships among species of Cymothoidae and fish parasites of Aegidae
Taxonomic studies of the family Cymothoidae are challenging when only morphological data are available; intra-specific variation is sufficiently great to sometimes overwhelm inter-specific variation (Smit et al. 2014). This is attributable to the morphological convergence of cymothoid species that share morphologically similar host fish, and divergence among cymothoid populations that utilise different species of host fish but nonetheless belong to a single species. In such cases, molecular markers are important in taxonomic terms, as has also been found for bivalves parasitic on various host species (Goto et al. 2012). Taxonomy and phylogeny based on both morphology and molecular markers have been conducted on Cymothoidae (Hadfield 2012; Martin 2015), and most genera are well established. Our results also confirmed the monophyly of most genera. However, the genus Elthusa is not a monophyletic group, but rather contains at least three distinct clades: Elthusa sacciger; Elthusa spp. parasitic in the opercular cavity of Ereuniidae, Hemitripteridae, Hexagrammidae, Macrouridae; and E. vulgaris. In addition, our data suggest that Alitropus typus belongs to Cymothoidae, not Aegidae; both molecular phylogeny and morphology support this suggestion.
Conclusions
We constructed a molecular phylogeny of the family Cymothoidae based on mitochondrial 16S rRNA and COI sequences. The phylogenetic tree suggests that cymothoid isopods may have originated in the deep sea, and then expanded their habitats to include shallow seas and brackish or freshwater by shifting their host species. Invasion of freshwater has occurred (independently) at least twice. The ancestral attachment mode is most likely to be the opercular cavity-dwelling. The ability to live in the buccal cavity and on external body surfaces evolved subsequently.
References
Adlard RD, Lester RJG (1995) The life-cycle and biology of Anilocra pomacentri (Isopoda, Cymothoidae), an ectoparasitic isopod of the coral-reef fish, Chromis nitida (Perciformes, Pomacentridae). Aust J Zool 43:271–281. doi:10.1071/ZO9950271
Boyko CB, Moss J, Williams JD, Shields JD (2013) A molecular phylogeny of Bopyroidea and Cryptoniscoidea (Crustacea: Isopoda). Syst Biodivers 11:495–506. doi:10.1080/14772000.2013.865679
Bruce NL (1983) Aegidae (Isopoda: Crustacea) from Australia with descriptions of three new species. J Nat Hist 17:757–788. doi:10.1080/00222938300770591
Bruce NL (1986) Revision of the isopod crustacean genus Mothocya Costa, in Hope, 1851 (Cymothoidae: Flabellifera), parasitic on marine fishes. J Nat Hist 20:1089–1189
Bruce NL (1987) Australian Pleopodias Richardson, 1910, and Anilocra Leach, 1818 (Isopoda: Cymothoidae), crustacean parasites of marine fishes. Rec Aust Mus 39:85–130
Bruce NL (1990) The genera Catoessa, Elthusa, Enispa, Ichthyoxenus, Idusa, Livoneca and Norileca n.gen. (Isopoda, Cymothoidae), crustacean parasites of marine fishes, with descriptions of eastern Australian species. Rec Aust Mus 42:247–300. doi:10.3853/j.0067-1975.42.1990.118
Bruce NL (2009) The marine fauna of New Zealand: Isopoda, Aegidae (Crustacea). NIWA Biodivers Mem 122:1–252
Bruce NL, Schotte M (2008) Cymothoidae. In: Boyko CB, Bruce NL, Merrin KL, Ota Y, Poore GCB, Taiti S, Schotte M, Wilson GDF (eds) World Marine, Freshwater and Terrestrial Isopod Crustaceans database. http://www.marinespecies.org/aphia.php?p=taxdetails&id=118274. Accessed 13 Dec 2016. (2008 onwards)
Brusca RC (1981) A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool J Linn Soc 73:117–199
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi:10.1093/bioinformatics/btp348
Chilton C (1926) Zoological results of a tour in the far East. The Tanaidacea and Isopoda of Tale Sap. Rec Indian Mus 28:173–185
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fryer G (1968) A new parasitic isopod of the family Cymothoidae from clupeid fishes of Lake Tanganyika: a further Lake Tanganyika enigma. J Zool 156:35–43
Goto R, Kawakita A, Ishikawa H, Hamamura Y, Kato M (2012) Molecular phylogeny of the bivalve superfamily Galeommatoidea (Heterodonta, Veneroida) reveals dynamic evolution of symbiotic lifestyle and interphylum host switching. BMC Evol Biol 12:1–13. doi:10.1186/1471-2148-12-172
Hadfield KA (2012) The biodiversity and systematics of marine fish parasitic isopods of the family Cymothoidae from Southern Africa. Dissertation, University of Johanesburg
Hadfield KA, Bruce NL, Smit NJ (2015) Review of Mothocya Costa in Hope 1851 Crustacea Isopoda Cymothoidae from southern Africa, with the description of a new species. Afr Zool 50:147–163. doi:10.1080/15627020.2015.1043943
Hassanin A (2006) Phylogeny of Arthropoda inferred from mitochondrial sequences: strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Mol Phylogenet Evol 38:100–116. doi:10.1016/j.ympev.2005.09.012
Horton T, Okamura B (2003) Post-haemorrhagic anaemia in sea bass, Dicentrarchus labrax (L.), caused by blood feeding of Ceratothoa oestroides (Isopoda: Cymothoidae). J Fish Dis 26:401–406. doi:10.1046/j.1365-2761.2003.00476.x
Inoue JG, Miya M, Miller MJ, Sado T, Hanel R, Hatooka K, Aoyama J, Minegishi Y, Nishida M, Tsukamoto K (2010) Deep-ocean origin of the freshwater eels. Biol Lett 6:363–366. doi:10.1098/rsbl.2009.0989
Inoue J, Sato Y, Sinclair R, Tsukamoto K, Nishida M (2015) Rapid genome reshaping by multiple-gene loss after whole-genome duplication in teleost fish suggested by mathematical modeling. Proc Natl Acad Sci USA 112:14918–14923. doi:10.1073/pnas.1507669112
Johnson GD, Ida H, Sakaue J, Sado T, Asahida T, Miya M (2012) A ‘living fossil’ eel (Anguilliformes: Protoanguillidae, fam. nov.) from an undersea cave in Palau. Proc R Soc B 279:934–943. doi:10.1098/rspb.2011.1289
Jones CM, Miller TL, Grutter AS, Cribb TH (2008) Natatory-stage cymothoid isopods: description, molecular identification and evolution of attachment. Int J Parasitol 38:477–491. doi:10.1016/j.ijpara.2007.07.013
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi:10.1093/molbev/mst010
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi:10.1093/bioinformatics/bts199
Ketmaier V, Joyce DA, Horton T, Mariani S (2008) A molecular phylogenetic framework for the evolution of parasitic strategies in cymothoid isopods (Crustacea). J Zool Syst Evol Res 46:19–23. doi:10.1111/j.1439-0469.2007.00423.x
Lincoln R (1972) A new species of Lironeca (Isopoda; Cymothoidae) parasitic on cichlid fishes in Lake Tanganyika. Bull Br Mus Nat Hist Zool 21:329–337
Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis version 3.04. http://mesquiteproject.org. Accessed 13 Dec 2016
Martin MB (2015) Taxonomy and phylogeny of the buccal-attaching Cymothoidae (Crustacea: Isopoda) of Australia. Dissertation, University of Tasmania
Mindell D, Sorenson M, Dimcheff D, Hasegawa M, Ast J, Yuri T (1999) Interordinal relationships of birds and other reptiles based on whole mitochondrial genomes. Syst Biol 48:138–152
Nair GA, Nair NB (1983) Effect of infestation with the isopod, Alitropus typus M. Edwards (Crustacea: Flabellifera: Aegidae) on the haematological parameters of the host fish, Channa striatus (Bloch). Aquaculture 30:11–19. doi:10.1016/0044-8486(83)90147-3
Nakabo T (2013) Fishes of Japan with pictorial keys to the species. Tokai University Press, Kanagawa
Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL (2012) Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci USA 109:13698–13703. doi:10.1073/pnas.1206625109
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world. Wiley, Hoboken
Phillips MJ, Penny D (2003) The root of the mammalian tree inferred from whole mitochondrial genomes. Mol Phylogenet Evol 28:171–185. doi:10.1016/S1055-7903(03)00057-5
Pillai NK (1967) Littoral and parasitic isopods from Kerala: families Eurydicidae, Corallanidae and Aegidae-2. J Bombay Nat Hist Soc 64:267–283
Ricklefs RE, Outlaw DC, Svensson-Coelho M, Medeiros MCI, Ellis VA, Latta S (2014) Species formation by host shifting in avian malaria parasites. Proc Natl Acad Sci USA 111:14816–14821. doi:10.1073/pnas.1416356111
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys029
Rosenthal A, Coutelle O, Craxton M (1993) Large-scale production of DNA sequencing templates by microtitre format PCR. Nucl Acids Res 21:173–174. doi:10.1093/nar/21.1.173
Saunders B (2012) Discovery of Australia’s fishes: a history of Australian ichthyology to 1930. CSIRO Publishing Collingwood, VIC
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Floors P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Smit NJ, Bruce NL, Hadfield KA (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasit Wildl 3:188–197. doi:10.1016/j.ijppaw.2014.03.004
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi:10.1093/bioinformatics/btu033
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tanabe AS (2011) Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol Ecol Resour 11:914–921. doi:10.1111/j.1755-0998.2011.03021.x
Tarrío R, Rodríguez-Trelles F, Ayala FJ (2001) Shared nucleotide composition biases among species and their impact on phylogenetic reconstructions of the Drosophilidae. Mol Biol Evol 18:1464–1473. doi:10.1093/oxfordjournals.molbev.a003932
Thatcher VE (2006) Isopoda. In: Thatcher VE (ed) Amazon fish parasites. Pensoft Pub, Sofia, pp 416–453
Trilles J-P, Hipeau-Jacquotte R (2012) Symbiosis and parasitism in the Crustacea. In: Forest J, von Vaupel Klein JC (eds) Treatise on zoology—anatomy, taxonomy, biology. The Crustacea. Brill, Leiden, pp 239–317. doi:10.1163/9789004188259_006
Tsai M-L, Dai C-F (1999) Ichthyoxenus fushanensis, new species (Isopoda: Cymothoidae), parasite of the fresh-water fish Varicorhinus barbatulus from northern Taiwan. J Crustacean Biol 19:917–923. doi:10.1163/193724099X00600
Wilson GDF, Paterson JR, Kear BP (2011) Fossil isopods associated with a fish skeleton from the Lower Cretaceous of Queensland, Australia—direct evidence of a scavenging lifestyle in Mesozoic Cymothoida. Palaeontology 54:1053–1068. doi:10.1111/j.1475-4983.2011.01095.x
Yamano H, Yamauchi T, Hosoya K (2011) A new host record of Ichthyoxenus amurensis (Crustacea: Isopoda: Cymothoidae) from the Amur bitterling Rhodeus sericeus (Cypriniformes: Cyprinidae). Limnology 12:103–106. doi:10.1007/s10201-010-0325-1
Zietara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (monogenea, gyrodactylidae). Evolution 56:2445–2458. doi:10.1111/j.0014-3820.2002.tb00170.x
Acknowledgements
We sincerely thank J. Akahane, G. Akinaga, S. N. Chiba, R. Dohi, A. Fujii, K. Goto, H. Hamaoka, T. Hattori, T. Hayakawa, N. Hayashida, I. Hirabayashi, H. Hirasaka, S. Isozaki, G. Itani, M. Ito, M. Ito, H. Matsuba, Kanagawa Prefectural Museum of Natural History, H. Katahira, E. Katayama, Y. Kimura, K. Koeda, T. Kunishima, H. Mishima, K. Miyamoto, Y. Miyazaki, T. Moritaki, F. Nakao, N. Nakayama, M. Nishiguchi, M. Ogishi, J. Ohtomi, T. Oka, T. Okada, I. Oura, K. Sakai, T. Sakai, T. Sakumoto, M. Sakurai, T. Sato, T. P. Satoh, Seikai National Fisheries Research Institute, H. Senou, T. Shigeta, S. Sodeyama, R. Shimomura, T. Shinonome, K. Tachihara, R. Tagashira, M. Takami, T. Takaya, F. Tashiro, Toba Aquarium, Tohoku National Fisheries Research Institute, T. Tsukayama, A. Umemoto, M. Utsunomiya, Y. Yagi, A. Yasui, and S. Yoshimi for providing specimens and/or assisting sample collection. Photos in Figs. 1, 4 courtesy of I. Seidel and H. Hirasaka. We are also grateful to members of the Maneno Team (Tanganyika Research Project Team) and staffs of Lake Tanganyika Research Unit, Mpulungu, Zambia, for their support. Research in Lake Tanganyika was conducted under permission from the Zambian Ministry of Agriculture, Food, and Fisheries. This study was supported by Showa Seitoku Memorial Foundation (Grant No. H26) and the Mikimoto Fund for marine Ecology (Grant No. H24).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical standard
All experiments were conducted in accordance with the Ethical Guidelines for Animal Experiments of Ehime University.
Additional information
Responsible Editor: T. Reusch.
Reviewed by C. Boyko and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hata, H., Sogabe, A., Tada, S. et al. Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Mar Biol 164, 105 (2017). https://doi.org/10.1007/s00227-017-3138-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3138-5