Abstract
Softer rocks in the intertidal zones of southern Japan are occasionally excavated by the rock-boring sea urchin, Echinostrephus molaris, and the pits are often succeeded by non-boring sea urchins, Anthocidaris crassispina and Echinometra tsumajiro after the death of Ec. molaris. Although the rock-boring sea urchin can fold their thin spines and retreat deeply into the pit bottoms, non-boring sea urchins with stouter spines cannot retreat deeply, thus, leaving spaces between their spines and the pit wall. To evaluate the uniqueness of these pits as microhabitats, we conducted an extensive census of biota both inside and outside of the pits occupied by rock-boring and non-boring sea urchins in tidal pools at Shirahama in southern Japan (33°69′51″N, 135°33′58″E). Macrophytes were only observed outside the pits, whereas sessile filter feeders and detritus feeders were found at similar frequencies in all of the microhabitats. The abundance and species richness of algal grazers and carnivores, however, were significantly higher in outside and inside the pits occupied by non-boring sea urchins compared to pits occupied by rock-boring sea urchins. The pit occupied by a non-boring sea urchin was specifically inhabited by a limpet-like trochid snail, Broderipia iridescens, the biology of which is almost completely unknown. Our data suggest that this trochid species is the first example of obligate inquiline with a non-boring pit-inhabiting sea urchin, adapted to the life in the pits, where the limpet benefit by sneaking in the gap between the pit wall and sea urchin spines, escaping from contact with the spines and being protected from attack by predaceous muricid snails.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystem diversity increases when ecosystem engineers colonize, as they create novel microhabitats for other organisms in different trophic levels (Jones et al. 1994, 1997). Rock-borers are important physical ecosystem engineers in the intertidal areas of marine rocky coasts because their empty pits protect various organisms (Davidson et al. 2010; Davidson and Grupe 2014). The hard substrata in rocky intertidal areas are unique habitats occupied by diverse macroalgae, microalgae, algal grazers, sessile filter feeders and carnivores. Softer substrata are often eroded physically by strong waves, or biologically by boring organisms such as isopods (Davidson et al. 2010), pholadid bivalves (Valentich-Scott and Dinesen 2004), rock-boring annelids including peanut worms (Hylleberg 1994) and sea urchins with strong solid spines and teeth (Solovjev and Markov 2013). The pits and trenches indented on the substrata yield unique microhabitats and refugia for various organisms.
Among these boring organisms, sea urchins are the most abundant borers of rocky substrata on temperate and tropical coasts. For example, Paracentrotus lividus excavates rock from Britain to Africa including the Mediterranean Sea, and Strongylocentrotus purpuratus bores rock along the coast of California (Solovjev and Markov 2013). In the western Pacific to Indian Oceans, Echinostrephus molaris bores softer mudstone and limestone, and infests algal fragments transported to the pits (Kobayashi and Tokioka 1976; Campbell et al. 1973). These rock-boring sea urchins can excavate rocky substrata with pentaradially symmetrical teeth formed from mosaic calcite crystals which are precisely shaped into plates and fibers (Killian et al. 2011). Tropical sea urchin species such as Echinothrix diadema, Diadema setosum, Diadema savignyi and Echinometra mathaei use these teeth to erode dead coral and feed on surficial and endolithic algae associated with the coral skeleton (Bak 1994; Dumont et al. 2013; Carreiro-Silva and McClanahan 2001).
Sea urchins living in the intertidal zone have evolved to defend against echinoid-feeding predators such as crabs, gastropods and fishes by having numerous long stout spines and by retreating into the pits excavated in the rocks. Therefore, the surface of a sea urchin is inhabited by various organisms such as eulimid snails (Delongueville and Scaillet 2009), gammarid amphipods (Parker 1936) and alpheid shrimps (Gherardi 1991; Nakashima 1987). Furthermore, the pits are also refugia for various small slimsy organisms. For example, the pits of the Atlantic sea urchin, Echinometra lucunter are inhabited by the porcellanid crab Clastotoechus vanderhorsti, the brittlestar Ophiothrix synoecina, and the clingfish Acyrtus rubiginosus, the former two of which are obligate commensal organisms of sea urchins (Schoppe and Werding 1996). Thus, the pits occupied by sea urchins are unique microhabitats protected from the strong waves and predators, and are open to inquilines. However, the pits are not always safe for the inquilines because the teeth and spines of the sea urchins may crush or scrape the organisms living on the pit walls. The adaptation that enables organisms to inhabit the pits occupied by sea urchins is intriguing.
Many pits are excavated by sea urchins on rocky substrata in tidal pools of Shirahama on southern coasts of Japan. Two types of rock beds are found, such as solid sandstone and softer mudstone, around Seto Marine Biological Laboratory of Kyoto University (Kishu Shimanto Research Group 1972). No pit or no rock-boring sea urchins have been found in the intertidal areas of the solid sandstone rockbeds. In contrast, many pits are excavated by rock-boring sea urchins in the intertidal areas of the softer rockbeds, and some of the pits are occupied by non-boring sea urchins, Anthocidaris crassispina and Echinometra tsumajiro (Kobayashi and Tokioka 1976). The rock-boring sea urchin Ec. molaris and these two non-boring sea urchins are, respectively, abbreviated as Es. molaris, Em. tsumajiro and A. crassispina afterward. A rare limpet-shaped trochid snail, Broderipia iridescens has been found in the pits occupied by the sea urchin species A. crassispina (Ohgaki et al. 2011). While most trochid snails are algal grazers with coiled shells that live in intertidal or subtidal areas, the biology of limpet-shaped trochids in the genus Broderipia are poorly understood, except for the brief report mentioned above.
Rock-boring sea urchins are physical ecosystem engineers in the intertidal areas of the Pacific coast, and their excavated pits are protected microhabitats. Moreover, the pits succeeded by the two non-boring sea urchins are another type of microhabitat. What microhabitats are created by the rock-boring and non-boring sea urchins are intriguing. To determine how these rock-boring and non-boring sea urchins affect the abundance and diversity of littoral organisms through utilization of their pits, we conducted an extensive survey on the biota outside and inside the pits of the rock-boring sea urchin, Es. molaris and the non-boring sea urchins, A. crassispina and Em. tsumajiro. Our data also revealed the remarkable biology of the limpet-like trochid snail, which is closely associated with non-boring sea urchins.
Materials and methods
Study sites
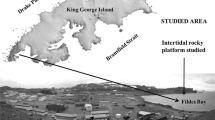
This study was conducted in the rocky intertidal zones around Seto Marine Biological Laboratory of Kyoto University at Shirahama, Wakayama prefecture (33°69′51″N, 135°33′58″E) (site B in Fig. 1), where the rock bed is formed from soft mudstone (Kishu Shimanto Research Group 1972) and bears many holes excavated by sea urchins (Fig. 2). The mean tidal level difference of spring tide at the study site is around 200 cm. Three tide pools (P1–P3) on the rocky shore facing Tanabe Bay with many sea urchin pits were chosen as study sites. The tidal pools P1 and P2 appear from the sea at a tide level of 60 cm, and P3 appeared at 40 cm. The area of P1 is about 20.4 m2, that of tide pool P2 is about 1.79 m2 and that of tide pool P3 is about 2.00 m2. After conducting preliminary surveys on the distribution of macrobenthos and macrophytes during May to December 2015, extensive surveys were conducted at spring tide during April to May 2016.
Schema of a rock bed with sea urchin pits in a tidal pool near the Seto Marine Biological Laboratory of Kyoto University at Shirahama. The depth of tidal pools varied from 30 to 150 cm. The tide level difference between mean low water (MLW) and mean high water (MHW) is about 200 cm during spring tide. The burrowing sea urchin Echinostrephus molaris and the non-burrowing sea urchin Anthocidaris crassispina and Echinometra tsumajiro live in pits at various depth in a soft mudstone wall
Internal structure of pit
First, to investigate the internal structure of a pit, five sea urchins of each species were removed, and the pit was filled with oil-based clay to make a mold. The sea urchins were soaked in an undiluted hypochlorite solution for about 3 h until all of the spines, tube feet, and pedicellaria detached from the shell. The pit mold and the sea urchin shell skeleton were photographed laterally for cross-sectional views. Next, each shell was vertically split into five pieces, and one of the pieces was horizontally split into upper and lower parts and soaked in hypochlorite. The size distributions of the detached spines in the upper and lower parts of the shell were measured for each sea urchin species (10/species).
Occupation of the pits by sea urchins
More than 700 pits were discovered in the three surveyed tidal pools (Fig. 1b), which were, respectively, occupied by one of the three sea urchin species; rock-boring Ec. molaris, and non-boring Ec. tsumajiro and A. crassispina. Although Em. tsumajiro and other congeneric species excavate trenches in dead corals and coral limestone in the Ryukyu Archipelago (e.g. Kyan Cape at Okinawa, site G in Fig. 1a), they are treated as non-boring sea urchins in this study because they scarcely bore into mudstone. All of these pits were marked and mapped, and the occupants of all of the pits were identified during low tide.
Survey of biota inside/outside the pits
All of the macrobenthos and macroalgae were collected and counted inside and outside of 20 pits of each sea urchin species and 20 quadrats (10 × 10 cm, nearly equal to the internal area of a pit) set on rocks outside the sea urchin pits to compare the biota. The sea urchins inside the pits were removed using a hooked steel spatula, and all of the organisms in the pits were collected carefully and identified. Macrobenthos found outside (in the quadrats) and inside the pits of each sea urchin species were classified into the following functional guilds: algal grazer, commensal, drilling carnivore, sessile filter feeder, scavenger, parasite, detritus feeder, omnivore and general carnivore (i.e. fish). B. iridescens was treated as a separate category to discriminate it from algal grazers.
Distribution and behavior of Broderipia iridescens
In addition to Shirahama, extensive search for Broderipia snails was made at Izu (34°77′55″N, 138°76′68″E, Shizuoka Prefecture), Ryunohama in Yokonami Peninsula (33°43′39″N, 133°44′90″E), Kashiwajima Island (32°76′72″N, 132°62′51″E), Uguru-shima Island (32°79′93″N, 132°49′56″E), Okinoshima Island (32°73′27″N, 132°54′32″E, Kochi Prefecture) and Kyan cape in Okinawa Island (26°07′89″N, 127°66′79″E, Okinawa Prefecture) (Fig. 1). The relative position of the snails with their host sea urchin was recorded, and the lengths of the major and minor axes, as well as the height were recorded for 20 Shirahama specimens. Further observations on the interactions between the snail and its host sea urchins were made in aquaria, where glasses were cast as artificial pits.
Results
Posture of sea urchin in pits
Sea urchin pits were only found in tidal pools consistently filled with water on rock beds in the low intertidal zone (Fig. 3a, b), and the pits were occupied by the three sea urchin species Es. molaris, A. crassispina, and Em. tsumajiro (Fig. 3c–e). Although the internal structure was similar among the pits occupied by the sea urchin species, the space remaining between the pit bottom and the ventral side of the sea urchin varied among species (Fig. 4). The rock-boring sea urchin Es. molaris has a shell whose upper and lower halves are flattened and domed, respectively, and the latter fits exactly with the pit bottom (Fig. 4a). Es. molaris retreated deep into the bottom of the pit for protection by folding up their thin spines and not leaving any space below (Fig. 4b). On the other hand, the non-boring sea urchins, A. crassispina and Em. tsumajiro, have shells whose upper and lower halves are domed and flattened, respectively, and the latter does not fit the pit bottom (Fig. 4c). Thus, the non-boring sea urchins could not fold their spines and retreat deeply into the pit bottom. Therefore, the pit bottoms usually had spaces in which the spines of pit-occupying sea urchins could not reach.
Internal structure of pits and posture of a pit-inhabiting sea urchin. a Normal posture of the boring sea urchin, Echinostrephus molaris. b Defending posture of Es. molaris retreating deep into the bottom of a pit by folding their lateral spines flat against the shell (indicated by arrowhead). c Posture of the non-boring sea urchins, Anthocidaris crassispina and Echinometra tsumajiro which unable to retreat deeply into bottom of a pit
To confirm these patterns, the total numbers and size distributions of spines were compared between the upper and lower halves of shells and among the three sea urchin species (Fig. 5). The total number of spines in one-fifth of the shell was greater in Es. molaris (mean, 482) than in A. crassispina (mean, 441) or Em. tsumajiro (mean, 392), and the interspecific difference was due to the limited number of spines on the upper shell halves of the latter two species (Fig. 5). The variance in spine length was high in all of the three species, and sea urchins of each species generally had few long stout primary spines and dense short thin secondary spines. The mean length of the spines on the upper half was greater than that on the lower half of the Es. molaris shell (3.2. vs. 2.0 mm), but the relationship was inverted in Em. tsumajiro (2.6 vs. 2.8 mm) and A. crassispina (1.8 vs. 2.1 mm). Another unique aspect of the Es. molaris spine size distribution was the marked predominance of short spines on the lower half shells, which corresponded to the abovementioned exact fit of the shells to the pit bottoms.
Pit occupants
A total of 784 pits were found in the three tidal pools, and all of the pits were occupied by one of the abovementioned three sea urchin species (Table 1). The most common species was the rock-boring sea urchin, Es. molaris (479 pits) followed by Em. tsumajiro (253 pits) and A. crassispina (52 pits).
Biota inside and outside of sea urchin pits
In total, 7 macroalgal and 30 macrobenthic species were recorded outside and inside the Shirahama sea urchin pits. Macroalgae were only recorded outside the pits. A total of 21 and 24 macrobenthic species were recorded from outside and inside the pits, respectively (Table 2). Mean macrophyte species richness and density of macrobenthos of a pit are shown in Fig. 6, the numbers of pits/quadrats in which macrobenthos (sorted by guild) were recorded are shown in Fig. 7, and the mean numbers of macrobenthos in a pits/quadrats are shown in Fig. 8. Species with only one individual were excluded from these analyses.
Comparison of species richness of marcophytes (a) and macrobenthos (b), and a comparison of macrobenthos density (c) among four microhabitats, i.e. outside of pits and pits occupied by three sea urchin species. The numbers of species and total number of macrobenthos individuals found in pits of Echinostrephus molaris were significantly lower than those of found outside the pits and the pits of non-boring sea urchins
Frequency of pits/quadrats in which selected organisms were recorded. Algal grazers, drilling carnivores, sessile filter feeders, detritus feeders, omnivores and general carnivores appeared outside and inside pits, and pits of burrowing and non-boring sea urchins. Drilling carnivores appeared significantly more frequently outside pits (Fisher’s exact test; P < 0.05). Scavangers and macroalgae appeared only outside the pits, and Broderipia iridescens and commensal shrimp appeared only inside the pits of non-boring sea urchins. Parasites appeared only on the surface of Anthocidaris crassispina
Numbers of organisms recorded in a pit/quadrat. Broderipia iridescens and commensal shrimp were found only inside the pits of non-burrowing sea urchins. Significantly more drilling carnivores were found outside the pits than those found inside the pits (Fisher’s exact test; P < 0.05). Scavengers were found only outside the pits, whereas a parasitic snail was found only on the shell surface of Anthocidaris crassispina
Outside of the pits, seven macroalgal species were found: a green alga species (Monostroma nitidum), three red alga species (Pterocladiella tenuis, Amphiroa echigoensis, Palisada intermedia) and three brown alga species (Sargassum patens, Dictyota dichotoma, Padina arborescens). In clear contrast to outside of pits, no macrophytes were found inside the pits. The species richness and density of macrobenthos were significantly low in the pits of Es. molaris (Fisher’s exact test; P < 0.05), while those were almost the same level in outside of pits and the pits of Em. tsumajiro and A. crassispina (Fig. 6).
Three gastropod algal grazer species (two chitons Rhyssoplax kurodai and Acanthochitona achates, and the trochid B. iridescens) were recorded. The chitons were found outside and inside the pits of non-boring sea urchins, but never in the pits of rock-boring sea urchins. B. iridescens (1–3/pit) was only recorded from inside the pits of non-boring sea urchins. In contrast, no algal grazer was found inside the pit of a rock-boring sea urchin. Because the distribution of B. iridescens is unique, it is shown separately from the other algal grazers (Figs. 7, 8).
From two to eight Vitreolina aurata snails were found on sea urchin shells, with their proboscises inserted into the sea urchin epidermis (Fig. 9a).
Organisms found inside sea urchin pits. a, b The commensal shrimp Arete dorsalis on Anthocidaris crassispina. c The parasitic gastropod Vitreolina aurata on A. crassispina. d Broderipia iridescens snails (arrowheads) in a pit of the non-boring sea urchin, Echinometra tsumajiro. e–g Dorsal, ventral and side views of a living B. iridescens snail. h The sea urchin A. crassispina in a glass in an aquarium, with a B. iridescens snail (indicated by arrowhead) positioning itself in the space between sea urchin spines. Scale bar a–d, h 1 cm, e–g 5 mm
The alpheid shrimp Arete dorsalis was recorded as commensal species only from the pits of the non-boring sea urchins, A. crassispina and Em. tsumajiro (Fig. 9b, c). The shrimp was usually found in a pair, and their color was similar to their host sea urchins (i.e. purple with no line on A. crassispina and brown with a vertical white line on Em. tsumajiro).
Four muricid gastropod species (Thais clavigera, Cronia fusca, Cronia margariticola, Tenguella musiva) were recorded as drilling carnivores. These molluscan drilling carnivores were recorded significantly more frequently outside than inside the pits (Fig. 7, Fisher’s exact test; P < 0.05), and the recorded number of gastropods was also greater outside than inside the pits (Fig. 8, Fisher’s exact test; P < 0.05).
Two fish species (Conidens laticephalus and Rhabdoblennius nitidus) were recorded as general carnivores. The clingfish (Gobiesocidae) C. laticephalus was found only inside the sea urchin pits, suggesting an intimate association with the sea urchins. Another fish R. nitidus (Blennidae) was recorded only outside the pits (Table 2), particularly in dead shells of the sessile vermetid gastropod Serpulorbis imbricatus.
Two gastropod species (the hipponicid Antisabia foliacea and the calyptraeid Crepidula gravispinosus), four bivalve species (three arcids, such as Barbatia foliata, Barbatia obtusoides, Arca boucardi, mytilid Septifer bilocularis) and a polychaete species (the serpulid Hydroides elegans) were recorded as sessile filter feeders. Although these sessile bivalves were found outside and inside the pits, the sessile gastropod A. foliacea was only found deep inside the pit. This gastropod species has a flattened shell and was even found in Es. molaris pits.
The gastropod species Euplica versicolor was recorded as scavenger outside the pits. The eulimid snail species V. aurata was recorded only on the shells of A. crassispina, suggesting that it is a specific parasite of the sea urchin.
Five polychaetes (Armandia amakusaensis, Nereiphylla castanea, Typosyllis okadai, Lepidonotus tenuisetosus, Nonparahalosydna pleiolepis) and an ophiuroid (Ophiactis savignyi) were recorded as detritus feeders without specific preference for outside or inside the pits or the pit host (Fisher’s exact test; P > 0.05).
Five arthropod species (two amphipod species Aoroides rubellus, Maera serratipalma, and three hermit crab species Clibanarius virescens, Pagurus nigrivittatus, Pagurus filholi) were recorded as omnivores without specific preference to outside or inside the pits (Fisher’s exact test; P > 0.05), but no omnivore was found in Es. molaris pits.
Obligate inquilinism of Broderipia iridescens
After an extensive search for Broderipia snails in the three Shirahama tidal pools, 136 Broderipia snails were found exclusively in pits of non-boring sea urchins, but never in pits of rock-boring sea urchins or outside the pits. We also searched for Broderipia snails in southern Japan and found them exclusively in pits or crevices occupied by A. crassispina at Izu, Ryunohama beach, Kasiwajima Island, Uguru-shima Island and Okino-shima Island (Fig. 1a). Broderipia snails were also found in coral reef trenches at Kyan Cape in Okinawa Island (Fig. 1a), which were excavated in coral limestone by Echinometra spp. sea urchins.
Broderipia snails attached to the pit walls near the aperture of the pit (Fig. 9c) or on the pit bottom without leaving home scars. The number of snails found near pit apertures was significantly greater than that on the pit bottom (Fisher’s exact test; P < 0.05) (Table 1), suggesting a preference for the pit aperture over the pit bottom. Broderipia snails immediately escaped deep into the pit when touched with thin tweezers. This snail has a thin shell with an inner iridescent pearl-tint surface and external surface normally covered with crustose coralline algae, creeps swiftly using three pairs of long lateral extended tentacles (Fig. 9d–f). The shells of Broderipia snails are small and flattened, the mean lengths of major and minor axes, and height were 6.1, 4.1 and 1.6 mm, respectively (ranges 5.0–7.5, 3.1–5.0 and 1.2–2.1 mm, respectively n = 20). Anthocidaris sea urchins were kept in glasses in laboratory aquaria, and the Broderipia snails were introduced. The Broderipia snails always crept toward the sea urchins, and settled on the wall just below the apical parts of long spines (Fig. 9g). In the condition where there is no pit in aquaria, sea urchins continued to walk around searching for pits, and the Broderipia snails followed after the walking sea urchins.
Discussions
Rock pits excavated by rock-boring sea urchins are unique microhabitat in rocky intertidals in southern Japan. The pits are narrower toward the inside and that the pit bottom fit the domed shell bottom of the rock-boring sea urchin, Es. molaris (Fig. 2). Sea urchins usually stay near the aperture of their pits to ingest drifting algal fragments (Kobayashi and Tokioka 1976; Campbel et al. 1973), and retreated deep into the bottom of the pit by folding up their spines when they were poked with a steel spatula. Because sea urchins heavily graze the pit substrata, macrobenthic and macroalgal abundance and species richness were higher outside than inside the pits. This tendency was similar to the result reported in the northwest coast of the US (Davidson and Grupe 2014). In contrast to the northwest coast of the US where only one species of sea urchin inhabits in pits, there are three species of pit-inhabiting sea urchins in southern Japan. The pits occupied by the two non-boring sea urchins are another unique microhabitat, which are obligately inhabited by the limpet-like trochid snails.
The non-boring sea urchins, A. crassispina and Em. tsumajiro, have flattened shell bottoms and long spines even on the ventral side of their shells, and were unable to withdraw deep into the pits. In addition, the spines of these non-boring sea urchins were broader than those of Es. molaris, and they are unable to fold their spines flat against their shells. Therefore, unoccupied spaces were detected inside the pits of non-boring sea urchins, particularly between the shell and pit bottoms, and between the upper reach of their spines and the sidewall of the pit near the pit aperture.
The size distribution of spines and their arrangement also differed among sea urchin species (Fig. 4), which contributed to create diverse microhabitats around their bodies. The wider space between the sparse spines of the non-boring sea urchins functions as refugia for small commensal organisms, such as the gammarid amphipod Amphilocus neapolitanu (Parker 1936), the alpheid shrimp Athanas indicus (Gherardi 1991) and A. dorsalis (synonym for A. kominatoensis) (Nakashima 1987).
The limited number of spines and the flat ventral side of the non-boring sea urchins contributed to creating a space between the sea urchin and the pit wall. Our data show that the space between the pit wall and the non-boring sea urchins was inhabited by unique macrobenthos (Table 2). Although chitons were also found outside of the pits, Broderipia snails were found only inside the pits of non-boring sea urchins. Because pressure by these molluscan algal grazers did not seem to differ substantially between outside and inside the non-boring sea urchin pits, the noticeable absence of macroalgae in the sea urchin pits suggests that grazing pressure by these sea urchins is high.
Other organisms found exclusively in the pits of non-boring sea urchins were the alpheid shrimp A. dorsalis and the gobiesocid fish C. laticephalus. This alpheid shrimp was commensal with two species of non-boring sea urchins. The body color of this shrimp mimics the sea urchin spines (i.e. purple on purple A. crassispina shells, and brown on browmn E. tsumajiro shells). The absence of the shrimp on the rock-boring sea urchins suggests that the narrow space between dense spines was insufficient for the commensal life of the shrimp. In contrast to the commensal species, the parasitic snail was specific to the non-boring sea urchin A. crassispina.
Muricid snails were frequently found drilling carnivores outside the sea urchin pits, but were not often found in the pits. The lower frequency of these coiled gastropods in the pits suggests that the sea urchin spines functioned as an effective guard against these predacious organisms. Sessile filter feeders, such as gastropods, bivalves, and polychaetes, were found inside and outside the sea urchin pits. Because these filter feeders are frequently attacked by muricid snails (Gordillo 1998; Harper and Morton 1997), sea urchin pits free from muricid snails should be safe refugia for sessile filter feeders once they successfully colonize the safe space in the pits.
Among organisms found in the sea urchin pits, B. iridescens was the only organism that lived on the pit wall near the sea urchin but never on the sea urchin itself. Our data show that B. iridescens is an obligate inquiline of the non-boring sea urchins, A. crassispina and Em. tsumajiro. All Broderipia snails were found in rocky substrate in the immediate vicinity of the sea urchins (Fig. 9c), suggesting that Broderipia snails are behaviorally adapted to symbiotic life. Our behavioral observations demonstrate that Broderipia snails with a pair of cephalic and three pairs of lateral tentacles extended (Fig. 9d–f) followed the walking sea urchins, and that the snails settled on substrate just below the apical portion of long spines (Fig. 9g). These long lateral tentacles are unique to Broderipia snails and related species (e.g. Synaptocochlea, Fossarina, and Roya) (Williams et al. 2010), but have never been observed on patellogastropod limpets, which have similar shell morphology and alga-grazing habits.
Sea urchins living in intertidal areas generally have sparse long stout spines, particularly on the dorsal side, and they have dense short spines, particularly on the ventral side. The former spines are used for defense, and the latter spines are used for excavating the substrate or grazing algae, but both types of spines may have harmful effects on Broderipia snails. Because rock-boring sea urchins covered with dense short spines vigorously excavate and graze the substrate, very few organisms can inhabit their pits. In contrast, the space between the sparse long spines in pits of non-boring sea urchins is the safest microhabitat for Broderipia snails. Our data show that Broderipia snails exclusively inhabited the pit wall near the aperture of the pits (Table 3). We found neither crack nor erosion on the external surface of Broderipia snails, which suggests that the limpet-like shape of the Broderipia snail decreases contact with the spines of the pit-occupying sea urchin. By minimizing direct contact with spines and teeth, Broderipia snails are thought to mitigate damage caused by the sea urchin. Broderipia snails retreated immediately and deeply into the pit when touched with tweezers, suggesting that they can escape to a safer site when encountered by predators.
Rock-boring sea urchins are important ecosystem engineers in tidal pools because they create protected microhabitats on hard substrata. Empty pits of the rock-boring sea urchin, S. purpuratus on the northwest Pacific Coast of the United States harbor more species of general carnivores than outside and inside the pits (Davidson and Grupe 2014). Furthermore, the pits of the rock-boring sea urchin Em. lucunter on the Atlantic coast of Central America enhance microbenthic diversity in the intertidal areas because three macrobenthic species (porcellanid crab, brittlestar and clingfish) are obligatorily associated with the pits occupied by the boring sea urchins (Schoppe and Werding 1996). This result is unexpected because the pits of rock-boring sea urchins are subject to high sea urchin grazing pressure and are usually deficient of inquilines. In contrast, rock-boring sea urchins on the southern coast of Japan excavate pits on intertidal rock beds, and the succession of the pits by non-boring sea urchins creates a novel microhabitat, where a unique inquiline has evolved. Thus, B. iridescens is the first example of an obligate inquiline in pits occupied by non-boring sea urchins. Although the biology of other species in the genus Broderipia is almost unknown, the unique limpet-like shell morphology of Trochidae might be an adaptation to the tight association with sea urchins. Although no fossils of this species have been reported, the appearance of intertidal sea urchins during a geological era would be tracked by the future discovery of Broderipia fossils. Additional studies on the microalgal flora outside and inside sea urchin pits and on the settlement of Broderipia larval snails will contribute to our understanding of their inquiline life.
References
Bak RPM (1994) Sea urchin bioerosion on coral reefs: place in the carbonate budget and relevant variables. Coral Reefs 13:99–103
Campbell AC, Dart JK, Head SM, Ormond RF (1973) The feeding activity of Echinostrephus molaris (de Blainville) in the central Red Sea. Mar Freshwater Behav Physiol 2:155–169.
Carreiro-Silva M, McClanahan TR (2001) Echinoid bioerosion and herbivory on Kenyan coral reefs: the role of protection from fishing. J Exper Mar Biol Ecol 262:133–153
Davidson TM, Grupe BM (2014) Habitat modification in tidepools by bioeroding sea urchins and implications for fine-scale community structure. Mar Ecol 36:185–194
Davidson TM, Shanks AL, Rumrill SS (2010) The composition and density of fauna utilizing burrow microhabitats created by a non-native burrowing crustacean (Sphaeroma quoianum). Biol Invasion 12:1403–1413
Delongueville C, Scaillet R (2009) Illustration de Vitreolina philippi (Ponzi, de Rayneval & Van den Hecke, 1854) sur Paracentrotus lividus (Lamarck, 1816) à Chypre Nord. NOVAPEX/Société 10:100
Dumont CP, Lau DC, Astudillo JC, Fong KF, Chak ST, Qiu JW (2013) Coral bioerosion by the sea urchin Diadema setosum in Hong Kong: susceptibility of different coral species. J Exper Mar Biol Ecol 441:71–79
Gherardi F (1991) Eco-ethological aspects of the symbiosis between the shrimp Athanas indicus (Coutière 1903) and the sea urchin Echinometra mathaei (de Blainville 1825). Trop Zool 4:107–128
Harper E, Morton B (1997) Muricid predation upon an under-boulder community of epibyssate bivalves in the Cape D’Aguilar Marine Reserve, Hong Kong. The Marine Flora and Fauna of Hong Kong and Southern China IV. Hong Kong University Press, Hong Kong, pp 263–284
Hylleberg J (1994) Phylum Sipuncula. Part 2. Cryptic fauna with emphasis on sipunculans in hump coral Porites lutea, the Andaman Sea, Thailand. Phuket Mar Biol Cent Res Bull 59:33–41
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78:1946–1957
Killian CE, Metzler RA, Gong Y, Churchill TH, Olson IC, Trubetskoy V, Christensen MB, Fournelle JH, De Carlo F, Cohen S, Mahamid J, Scholl A, Young A, Doran A, Wilt FH, Coppersmith SN, Gilbert PUPA (2011) Self-sharpening mechanism of the sea urchin tooth. Adv Funct Mater 21:682–690
Kishu Shimanto Research Group (1972) The Muro Group in the Upperreaches of Koza River in Wakayama Prefecture: The study of the Shimanto Terrain in the Kii Peninsula, Southwest Japan (Part 5). Chikyukagaku (in Japanese) 26:195–204
Kobayashi N, Tokioka T (1976) Preliminary observatoin on the maturation of the burrowing sea urchin, Echinostrephus aciculatus (A. Agassiz), in the vicinity of Seto. Publ Seto Mar Biol Lab 23:57–62
Nakashima Y (1987) Reproductive strategies in a partially protandrous shrimp, Athanas kominatoensis (Decapoda: Alpheidae): sex change as the best of a bad situation for subordinates. J Ethol 5:145–159
Ohgaki SI, Komemoto KI, Funayama N (2011) 3. Comments on selected species. Publ Seto Mar Biol Lab. Spec Publ Ser 11:15–23
Parker GH (1936) An inquiline gammarid on the sea-urchin Lytechinus. Ecology 17:185–186
Schoppe S, Werding B (1996) The boreholes of the sea urchin genus Echinometra (Echinodermata: Echinoidea: Echinometridae) as a microhabitat in tropical South America. Mar Ecol 17:181–186
Solovjev AN, Markov AV (2013) The role of echinoids in shaping environments. Paleontol J 47:480–484
Valentich-Scott P, Dinesen GE (2004) Rock and coral boring Bivalvia (Mollusca) of the middle Florida Keys, USA. Malacologia 46:339–354
Williams ST, Donald KM, Spencer HG, Nakano T (2010) Molecular systematics of the marine gastropod families Trochidae and Calliostomatidae (Mollusca: Superfamily Trochoidea). Mol Phylogen Evol 54:783–809
Gordillo S (1998) Trophonid gastropod predation on recent bivalves from the Magellanic Region. Bivalves: an eon of evolution. Paleobiological Studies Honoring Norman D. Newell :251–254.
Acknowledgements
We thank all the staffs of the Seto Marine Biological Laboratory for supporting our field survey, Ryo Nakayama and Youhei Yamauchi of the Laboratory for technical support, and Kasumi Kondo of Kyoto University for helping with the sampling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Grant-in-Aid for Scientific Research 2015–2019 (15H02420) to MK.
Conflict of interest
We have no conflict of interest.
Additional information
Responsible Editor: S. Connell.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Yamamori, L., Kato, M. The macrobenthic community in intertidal sea urchin pits and an obligate inquilinism of a limpet-shaped trochid gastropod in the pits. Mar Biol 164, 61 (2017). https://doi.org/10.1007/s00227-017-3091-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3091-3