Abstract
Intraspecific variation in coral colony growth forms is common and often attributed to phenotypic plasticity. The ability of other organisms to induce variation in coral colony growth forms has received less attention, but has implications for both taxonomy and the fates of corals and associated species (e.g. fishes and invertebrates). Variation in growth forms and photochemical efficiency of massive Porites spp. in lagoons of Moorea, French Polynesia (17.48°S, 149.85°W), were quantified in 2012. The presence of a vermetid gastropod (Ceraesignum maximum) was correlated with (1) reduced rugosity of coral colonies and (2) reduced photochemical efficiency (Fv/Fm) on terminal “hummocks” (coral tissue in contact with vermetid mucus nets) relative to adjacent “interstitial” locations (tissue not in contact vermetid mucus nets). A manipulative field experiment confirmed that the relative growth rate of coral tissue was greater in interstices than hummocks when vermetids were present and similar (but with a trend for faster growth on hummocks) when vermetids were absent. Collectively, these results indicate that vermetid gastropods interact (presumably via their mucus nets) with coral colony architecture to impair photochemical efficiency, reduce growth rates of specific portions of a coral tissue, and induce a smoothed colony morphology. Given that structural complexity of coral colonies is an important determinant of “habitat quality” for many other species (fishes and invertebrates), these results suggest that the vermetid gastropod, C. maximum (with a widespread distribution and reported increases in density in some portions of its range), may have important indirect effects on many coral-associated organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reef-building corals exhibit a diversity of growth forms that vary markedly among and within species (Chappell 1980; Veron 2000; Todd 2008). Particular growth forms may be used to classify putative species and can be a source of taxonomic confusion (Fukami et al. 2004; Forsman et al. 2009). More importantly, structurally complex growth forms may be used by coral-dwelling organisms for shelter and/or refuges from predators (Rogers 1990; Holbrook and Schmitt 2002; Bergsma 2012).
Intraspecific variation in coral growth forms is often attributed to phenotypic plasticity that may be adaptive (i.e. enabling long-lived colonies to maximise their fitness under changeable environmental conditions; Bruno and Edmunds 1997; Muko et al. 2000; Todd 2008; Padilla-Gamino et al. 2012). Morphological variation may result from interactions with other corals (Lang 1973; Connell 1976; Rinkevich and Loya 1985), putative disease agents (Aeby et al. 2011; Stimson 2011), or other coral-associated organisms (e.g. Abelson et al. 1991; Wielgus et al. 2002; Bergsma 2009), though the latter has received comparatively little attention (perhaps because it is perceived as rare or anomalous).
Previous experiments (Colgan 1985; Shima et al. 2010, 2013) indicate that the vermetid gastropod, Ceraesignum maximum (formerly Dendropoma maximum; see Golding et al. 2014 for recent taxonomic revision), reduces the growth and survival of several species of corals and may have the ability to dramatically alter coral growth forms (Colgan 1985; Zvuloni et al. 2008; Shima et al. 2010). C. maximum produces pelagic larvae (Phillips and Shima 2010) that settle and permanently attach to bare substrate (Phillips et al. 2014). Settled individuals grow by adding to uncoiled tubular shells and often become encased in a matrix of live and dead coral. They capture food using a mucus net (Fig. 1a), which is secreted and retracted at intervals (Kappner et al. 2000). The mucus nets of vermetids often come into direct contact with the terminal branches or “hummocks” of corals including colonies of massive Porites spp. that are prevalent within the lagoons of Moorea, French Polynesia (Fig. 1a, b; a species complex determined by Edmunds (2009) to be a mix of P. lobata and P. lutea, though phylogenetic analysis by Forsman et al. (2009) indicates taxonomic uncertainty within this group; hence, we refer to the complex collectively as “massive Porites spp.”). Our preliminary observations suggest that tissue of massive Porites spp. (on both P. lutea-like colonies and P. lobata-like colonies) that is in direct contact with vermetid mucus nets (e.g. on hummocks) appears lighter in colour, with a distinct corallite/coenosteum structure (Fig. 1a) relative to tissue of the same colony that is not in direct contact with vermetid mucus nets (i.e. recessed in the interstices between hummocks). In addition, hummocks on massive Porites spp. colonies with vermetids often appear locally flattened (Fig. 1b; see also Shima et al. 2010). In contrast, massive Porites spp. colonies (both P. lutea-like colonies and P. lobata-like colonies) without vermetid populations do not show the same degree of variation in tissue colour between hummocks and interstices, or locally flattened growth forms.
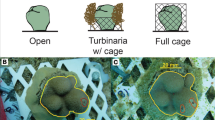
a Vermetid gastropod, Ceraesignum maximum (formerly listed in the genus Dendropoma), with a mucus net draped over a portion of a colony of massive Porites spp. Mucus nets used by vermetids for capture of food particles often come into direct contact with polyps on hummocks of massive Porites colonies but not with polyps in interstitial spaces. The portion of the Porites colony in background lacks vermetids and is noticeably more rugose. b Locally flattened appearance of hummocks on massive Porites colonies that are commonly associated with the presence of vermetids on Moorea. c Experimental unit (concrete block affixed to reef) used to evaluate the effects of vermetids on growth of massive Porites on hummocks (small coral attached to top of block) or in interstitial positions (small coral recessed within central portion of block). Vermetids embedded in rubble (treatment) or pieces of rubble without vermetids (control) were attached to periphery of block with cable ties
These observations led us to hypothesise that the vermetid gastropod, C. maximum, alters the growth form of massive Porites spp. when its mucus nets come into direct contact with exposed portions of the colony (i.e. on hummocks). In the absence of vermetids, we predicted that coral tissue in a hummock position would grow faster than coral tissue in interstices (because hummocks might be exposed to increased light and/or water flow; e.g. Dustan 1975) to yield colonies that become increasingly structurally complex with age. Conversely, we predicted that the presence of vermetids might reverse this pattern of differential growth between coral tissue in hummocks and interstitial positions (i.e. coral growth slows or ceases on hummocks where it is affected by vermetid mucus nets, but continues within the interstices until the gaps fill in) to yield colonies that become increasingly locally flattened with age. To test this hypothesis, we first quantified patterns and sources of spatial variation in the height of hummocks (to formally test our preliminary observations that hummock height appears greater on colonies without vermetids). In addition, we characterised aspects of the photobiology of coral tissue on hummocks or interstitial positions for colonies of massive Porites spp. that naturally had or lacked vermetids (to evaluate patterns and sources of variation in photophysiological performance). Finally, we conducted a field experiment to simulate hummocks and interstitial sites and quantified the growth rates of small coral colonies deployed to these positions in the presence and absence of transplanted vermetids.
Methods
Observational study
Structural morphology
We measured the heights of hummocks relative to adjacent interstices on massive Porites spp. colonies with and without the vermetid gastropod C. maximum. Specifically, we quantified the heights of 3 randomly selected hummocks on each of 10 randomly selected colonies (~1–2 m diameter) of each type (±vermetids), sampled from a site in Vaipahu Lagoon, Moorea, French Polynesia (17.48067°S, 149.84698°W). Hummock height was measured from the peak of a hummock to the bottom of the deepest adjacent interstice, with the depth probe of a dial calliper. We used a nested ANOVA (PROC GLM, SAS version 9.3) that accounted for the structure of our data (i.e. 3 measurements of hummock height nested within sampled colonies) to evaluate variation in hummock height as a function of the presence or absence of vermetids.
Photochemical efficiency
We measured maximum dark-adapted yield of PSII (Fv/Fm) of hummocks and interstices sampled from colonies (~1–2 m diameter) with and without vermetids using pulse amplitude modulated fluorometry (Diving PAM, Walz, Effeltrich, Germany). Diving PAM measurements were recorded from different colonies over 2 days just prior to sunrise (e.g. a dark-adaption period of >11 h) beginning at ~6:20 a.m. and ending when incident light (i.e. not shaded by surrounding mountains) began to affect the sampling locations (~7:00 a.m.). Diving PAM was configured with the 5.5-mm fibre-optic cable fitted with a clear plastic spacer that ensured a standardised placement of the sensor at 10 mm above the surface of the coral tissue. The instrument settings were adjusted to optimise detection of Fo, and the instrument was auto-zeroed prior to yield measurements (diving PAM settings, day 1: measuring light = 4, saturation intensity = 9, saturation width = 1.4, gain = 2 and damping = 3; day 2: measuring light = 8, saturation intensity = 8, saturation width = 0.8, gain = 1 and damping = 2). We quantified dark-adapted yield at ~10 randomly selected hummocks and ~10 nearby interstices per colony, sampling 13 randomly selected colonies without vermetids and 12 randomly selected colonies with vermetids. We evaluated variation in dark-adapted yield using a split-plot design (PROC MIXED, SAS version 9.3, with vermetid presence/absence as a whole-plot factor and hummock/interstice as a sub-plot factor).
Experimental study
We constructed a field experiment to quantify the effects of vermetids on growth of massive Porites spp. corals that were located in simulated hummock or interstitial positions. We collected 44 small colonies (≤5 cm diameter) of massive Porites spp from a site in the lagoon (2–3 m water depth) near to where we quantified structural morphology and photochemical efficiency. We transported corals to the flow-through sea water facility at the UC Berkeley Gump Research Station, attached each coral to a small (~8 × 8 cm) plastic base using A-788 splash zone epoxy (Z-spar), and estimated the initial skeletal mass of each focal coral and its base using a buoyant weight technique (Davies 1989). We used concrete blocks affixed with rebar to patch reefs in ~2 m water depth to provide attachment substrates for experimental corals (Fig. 1c). We deployed 22 concrete blocks and removed all naturally occurring vermetids on the reefs within ~1.5 m of each block; we randomly assigned half of the blocks (n = 11) to a “+vermetid” treatment and attached 2–3 clumps of coral rubble (each containing ~2–3 individual C. maximum vermetids, in tact in their tubes) to the block with cable ties. Clumps of rubble with vermetids were positioned with respect to current such that corals assigned to the top of blocks in this treatment would be attached downstream of vermetids and likely to encounter mucus nets. The remaining 11 blocks were assigned to a “−vermetid” treatment, and to these, we attached similar clumps of rubble without vermetids to control for unintended effects of added structure or other attributes of coral rubble (e.g. turf algae, microbial communities) on corals. We randomly assigned and then attached two corals to each concrete block with cable ties. One coral was attached to the top of each block, in a position intended to simulate a hummock. The second coral was attached within the diamond-shaped centre of the block (see Fig. 1c), 5–10 cm below the top of the block, to simulate an interstitial position, i.e. lower in elevation relative to the adjacent “hummock”, in a reduced flow and light environment, but also in a position that potentially afforded shelter from direct contact with vermetid mucus nets (for cases where vermetids were present). We surveyed the blocks after 127 d to confirm the effectiveness of our treatments (i.e. that transplanted vermetids remained alive and their mucus nets were associated with corals in “+vermetid” treatment and that corals in the “−vermetid” treatment were unaffected by vermetids). Of the 44 corals initially transplanted, 1 was lost altogether and 3 additional colonies were excluded from analyses (2 because they were assigned to the “−vermetid” treatment but were visibly in contact with mucus nets from naturally occurring vermetids; 1 because the attaching cable ties had broken and the colony was upside down and bleached). Following this survey, we recovered the corals and estimated the final skeletal mass (a combined buoyant weight of corals together with their bases). We quantified density of sea water associated with our initial and final estimates of buoyant weights and used a measure of aragonite density (2.93 g/cm3) to calculate skeletal growth (delta dry mass) of corals over the duration of the experiment. We evaluated variation in skeletal growth of corals using a split-plot design (PROC MIXED, SAS version 9.3, with vermetid presence/absence as a whole-plot factor and hummock/interstice as a sub-plot factor).
Results
Observational study
Structural morphology
Massive Porites spp. colonies lacking vermetids were more rugose relative to colonies with vermetids; hummock heights were ~3× greater when vermetids were locally absent (F1,18 = 55.88, P < 0.0001, Fig. 2a). Coral colonies exhibited significant variation in hummock heights irrespective of the presence/absence of vermetids (colonies nested within vermetid status: F18,40 = 3.33, P = 0.0008). However, most of the observed variation in hummock heights (~77.4 %) could be attributed to the presence/absence of vermetids, with only 9.9 and 12.7 % attributable to among-colony or within-colony variation, respectively (Fig. 2b).
a Disparity between hummocks and adjacent interstitial spaces on massive Porites spp. colonies where vermetids are naturally absent or present. Given are LS means ± 1 SE determined from a nested ANOVA; letters denote significant differences. b Per cent of variation in hummock heights attributable to I presence/absence of vermetids, II among colonies of a given vermetid status, or III within colonies (error term), from a nested ANOVA. c Variation in maximum dark-adapted yield of PSII (Fv/Fm) as a function of presence/absence of vermetids (whole-plot factor) and colony position (hummock vs. interstices; the sub-plot factor of a split-plot sampling design). Given are LS means ±1 SE determined from a mixed-effect ANOVA. Because multiple comparisons cannot be readily discerned from plots of LS means, we show results of a multiple comparisons test (using Tukey adjustment) as a forest plot. (d) Roman numerals in bars of c identify the pairwise comparisons shown in (d). Given in d are the estimated differences between each pairwise comparison, ±95 % CI (and where these do not overlap 0, the differences are statistically significant and indicated with solid error bars). P values for each post hoc test are also given
Photochemical efficiency
Interstitial positions had elevated dark-adapted yield (Fv/Fm) relative to hummocks regardless of the presence/absence of vermetids (main effect of colony position: F1,471 = 63.01, P < 0.0001, Fig. 2c, d). Dark-adapted yield was more strongly constrained on hummocks relative to interstitial spaces when vermetids were present (interaction term: F1,471 = 12.40, P = 0.0005, Fig. 2c, d). Because of the significant interaction term, the main effect of vermetid presence/absence was not significant (F1,23 = 1.51, P = 0.23; i.e. the effect of vermetids on dark-adapted yield was dependent upon colony position).
Field experiment
The effect of vermetids on coral growth was dependent upon colony position (F1,16 = 9.90, P = 0.0062). When vermetids were absent, corals showed a trend for faster growth on hummocks relative to interstices (Fig. 3a), although post hoc tests show this to be non-significant (Fig. 3b, I vs. II). In contrast, corals on hummocks were strongly disadvantaged in the presence of vermetids, growing significantly slower than corals in adjacent interstices (Fig. 3b, III vs. IV) and also slower than corals on hummocks without vermetids (Fig. 3b, I vs. III). Due to the strong interaction term, neither the main effect of vermetid treatment (F1,20 = 3.07, P = 0.095) nor the main effect of colony position (F1,16 = 0.98, P = 0.34) was significant.
a Variation in skeletal growth of massive Porites over 127 d, as a function of experimental manipulation of the presence/absence of vermetids (whole-plot factor) and colony position (hummock vs. interstices; the sub-plot factor of a split-plot experimental design). Given are LS means ±1 SE determined from a mixed-effect ANOVA. b Multiple comparisons for (a) (see Fig. 2d legend for detailed description)
Discussion
Our experimental results strongly support our hypothesis that the vermetid gastropod, C. maximum, alters within-colony variation in growth rates to reduce rugosity. We used small individual coral colonies as proxies for different portions of a single colony and found that protruding colonies (hummocks) that come into direct contact with vermetid mucus nets grew more slowly than those in adjacent interstitial spaces (separated by a distance of ≤10 cm). Where vermetids were absent, corals in the same spatial configuration did not vary significantly in their growth rate, although those on hummocks showed a trend for faster growth relative to interstices. Vermetids appear to have the ability to reverse the growth-form trajectory of massive Porites spp.: when vermetids are absent, coral colonies become increasingly rugose (with higher hummocks); when vermetids are present, gaps fill in and coral colonies become locally flattened.
Our experimental design relies upon individual small colonies (from which we can more easily quantify growth) to serve as proxies for differing portions of a single large colony. Thus, our experimental replicates differ from natural reefs in at least two important ways: (1) corals within replicates of our experiment likely vary in genotype, and (2) our design does not allow for the possible translocation of materials from the adjacent tissue positions (e.g. Stimson 2011), which could theoretically ameliorate the effects of vermetids on tissues in the hummock position. However, because our experimental design randomised the assignment of corals with respect to whole-plot and sub-plot factors, variation among genotypes is unlikely to generate the patterns that we observed (and any effect of among-colony variation in coral performance would simply make the detection of patterns more difficult). Additionally, the concordance between our experimental results and our observational study suggests to us that any translocation of materials that might be capable of mitigating the deleterious effects of vermetids on hummock tissue is unlikely. We also note that the relative height of hummocks to interstices is greater for our experiment than for most natural hummock/interstitial portions of single colonies, but the fact that vermetids ameliorated these more extreme differences serves primarily to strengthen our inferences.
Our experimental results are supported by our measurements of hummock heights on colonies where vermetids were naturally present or absent. We found hummocks to be ~3× higher on colonies without vermetids. Interestingly, most of the spatial variation in hummock height (>77 %) could be explained simply by the presence or absence of vermetids, with <10 % attributable to variation among colonies (which may include more than one putative species of Porites). Though we intentionally chose to sample discrete colonies with/without vermetids to evaluate the hypothesised vermetid effect (because we wanted to ensure statistical independence of observations at the level of this effect), our qualitative observations suggested that hummock heights varied in a similar magnitude within colonies (i.e. for a given colony with vermetids present, hummock heights were ~3× higher at locations furthest from the nearest vermetid).
The noticeable variation in coral tissue colour and corallite/coenosteum structure (Fig. 1a, b) that is often associated with (i.e. immediately beneath) the mucus nets of vermetids strongly indicates the mucus nets (as opposed to something else about vermetids; e.g. faecal pellets, which often accumulate in interstices) as the most likely cause of reduced coral performance. Mucus nets may be allelopathic (e.g. Pawlik et al. 2007), or they may alter local oxygen concentrations and/or microbial community composition (e.g. Smith et al. 2006) to induce within-colony heterogeneity in coral performance. However, we speculate that such mechanisms may not be likely to induce heterogeneity in growth responses at the scale of adjacent hummocks and interstices (i.e. often separated by only ~1 cm). Alternatively, physical contact with and/or abrasion by mucus nets may cause polyps on hummocks to remain retracted, reducing photosynthetic activity and growth (see River and Edmunds 2001 for a similar effect induced by contact with algae).
We quantified maximum dark-adapted yield and found that colonies with vermetids had reduced photosynthetic efficiency on hummocks (where direct contact with vermetid mucus was likely) but not in interstitial locations (where direct contact was unlikely). When taken together with our experimental results, these data may suggest that vermetid mucus induces within-colony heterogeneity in coral growth rates via direct or indirect effects on photochemical pathways.
Regardless of the precise mechanism, our observational and experimental components both support the hypothesis that vermetids reduce rugosity of massive Porites colonies. Morphological variation that is induced by such biological interactions may be perceived as rare or anomalous (but see Abelson et al. 1991; Wielgus et al. 2002; Bergsma 2009) and consequently has received less attention than environmentally driven variation, but this can have similarly important implications for the performance of the affected colony and/or other organisms that may depend resources (e.g. food, shelter) related to colony morphology. For example, flattening of coral colonies is often observed under reduced light levels, and this has been suggested as an adaptive response to reduce the quantity of tissue that must be sustained for a given light level and/or to minimise self-shading (Dustan 1975; Todd 2008 and further references therein). Coral morphology is also an important determinant of water flow (e.g. Wainwright and Koehl 1976), and consequently, effects of vermetids over a large expanse of reef may alter important ecosystem services. At a fine scale, the effect of vermetids may alter boundary layers that affect the diffusion of materials (e.g. nutrients, bicarbonate, gases; e.g. Lesser et al. 1994) and/or prey capture by polyps (e.g. Sebens and Johnson 1991). The induced reduction in rugosity of corals is likely to be correlated with a reduction in coral tissue surface area, which may limit food resources of corallivorous fish (e.g. butterflyfish: Graham et al. 2009) and invertebrates (e.g. crown of thorns seastars: Kayal et al. 2012). Many fishes and invertebrates use structurally complex coral colonies as sheltering sites and/or refuges from predators (Gladfelter and Gladfelter 1978; Holbrook and Schmitt 2002; Shima et al. 2008; Nakamura et al. 2009; Stier et al. 2010), and it follows that induced reductions in rugosity of coral colonies may decrease the quality of habitat for these coral-associated organisms. Bergsma (2009) found that tube-dwelling gammarid amphipods induce changes in the morphology of Montipora sp. (in this case, the epibiotic symbionts cause flattened colonies to become branched), and a subsequent paper showed that this induced growth form increased the abundance and diversity of fish through a “morphology-mediated facilitation cascade” (Bergsma 2012). Based upon Bergsma’s (2012) findings, we might expect the localised flattening induced by vermetids to reduce abundance and diversity of fish that use massive Porites spp. Previous work by members of our group suggests that massive Porites colonies on Moorea are used several common species of labrid fishes including the sixbar wrasse (e.g. Shima 1999, 2001a, b, 2002; Geange and Stier 2009). Variation in attributes of these reefs may be directly or indirectly mediated by vermetid-induced morphological variation to affect demographic rates and ecological interactions of resident fish (e.g. Shima and Osenberg 2003, Shima et al. 2008; Geange and Stier 2009).
Morphological variation in corals that is induced by organisms such as vermetids may be widespread. C. maximum has a widespread distribution throughout the Indo-Pacific, and its effects on the morphology of corals have been noted from the Red Sea (Zvuloni et al. 2008), Guam (Colgan 1985), and French Polynesia (Shima et al. 2010 and this study). C. maximum densities have increased by ~200× in the lagoons of Moorea from 1997 to 2008 (Y. Chancerelle and B. Salvat, personal communication), and this study suggests that most of the observable morphological variation in hummock height of massive Porites spp. can be explained by the presence or absence of these animals. These observations challenge the perspective that such growth forms may be “anomalies” (similar to the “growth anomalies” that are thought to be caused by a disease agent; e.g. Aeby et al. 2011; Stimson 2011), because this would suggest that they are rare occurrences or perhaps ecologically trivial. To the contrary, vermetids and other organisms (e.g. Bergsma 2012) are likely to play an important role in the structure of coral reef communities.
References
Abelson A, Galil BS, Loya Y (1991) Skeletal modifications in stony corals caused by indwelling crabs: hydrodynamical advantages for crab feeding. Symbiosis 10:233–248
Aeby GS, Williams GJ, Franklin EC, Haapkyla J, Harvell CD, Neale S, Page CA, Raymundo L, Vargas-Angel B, Willis BL, Work TM, Davy SK (2011) Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. Plos One 6:e16887
Bergsma GS (2009) Tube-dwelling coral symbionts induce significant morphological change in Montipora. Symbiosis 49:143–150
Bergsma GS (2012) Coral mutualists enhance fish abundance and diversity through a morphology-mediated facilitation cascade. Mar Ecol Prog Ser 451:151–161
Bruno JF, Edmunds PJ (1997) Clonal variation for phenotypic plasticity in the coral Madracis mirabilis. Ecology 78:2177–2190
Chappell J (1980) Coral morphology, diversity and reef growth. Nature 286:249–252
Colgan MW (1985) Growth rate reduction and modification of a coral colony by a vermetid mollusc, Dendropoma maxima. Proceedings of the 5th international coral reef symposium 6:205–210
Connell JH (1976) Competitive interactions and the species diversity of corals. In: Mackie GO (ed) Coelenterate ecology and behavior. Plenum Press, New York, pp 51–68
Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weight technique. Mar Biol 101:389–395
Dustan P (1975) Growth and form in the reef-building coral Montastrea annularis. Mar Biol 33:101–107
Edmunds PJ (2009) Effect of acclimatization to low temperature and reduced light on the response of reef corals to elevated temperature. Mar Biol 156:1797–1808
Forsman ZH, Barshis DJ, Hunger CL, Toonen RJ (2009) Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol Biol 9:45
Fukami H, Budd AF, Paulay G, Sole-Cava A, Chen CA, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427:832–835
Geange SW, Stier AC (2009) Order of arrival affects competition in two reef fishes. Ecology 90:2868–2878
Gladfelter WB, Gladfelter EH (1978) Fish community structure as a function of habitat structure on West Indian patch reefs. Rev Biol Trop 26:65–84
Golding RE, Bieler R, Rawlings TA, Collins TM (2014) Deconstructing Dendropoma: a systematic revision of a world-wide worm-snail roup, with descriptions of new genera (Caenogastropoda: Vermetidae). Malacologia 57:1–97
Graham NAJ, Wilson SK, Pratchett MS, Polunin NVC, Spalding MD (2009) Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodivers Conserv 18:3325–3336
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Kappner I, Al-Moghrabi SM, Richter C (2000) Mucus-net feeding by the vermetid gastropod Dendropoma maxima in coral reefs. Mar Ecol Prog Ser 204:309–313
Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart Céline, Michonneau F, Penin L, Planes S, Adjeroud M (2012) Predator Crown-of-Thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7(10):e47363
Lang JC (1973) Interspecific aggression by scleractinian corals. II. Why the race is not always to the swift. Bull Mar Sci 23:260–279
Lesser MP, Weis VM, Patterson MR, Jokiel PL (1994) Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J Exp Mar Biol Ecol 178:153–179
Muko S, Kawasaki K, Sakai K, Tasku F, Sigesada N (2000) Morphological plasticity in the coral Porites sillimani and its adaptive significance. Bull Mar Sci 66:225–239
Nakamura Y, Shibuno T, Lecchini D, Kawamura T, Watanabe Y (2009) Spatial variability in habitat associations of pre- and post-settlement stages of coral reef fishes at Ishigaki Island, Japan. Mar Biol 156:2413–2419
Padilla-Gamino JL, Hanson KM, Stat M, Gates RD (2012) Phenotypic plasticity of the coral Porites rus: acclimatization responses to a turbid environment. J Exp Mar Biol Ecol 434:71–80
Pawlik JR, Steindler L, Henkel TP, Beer S, Ilan M (2007) Chemical warfare on coral reefs: sponge metabolites differentially affect coral symbiosis in situ. Limnol Oceanogr 52:907–911
Phillips NE, Shima JS (2010) Reproduction of the vermetid gastropod Dendropoma maximum (Sowerby, 1825) in Moorea, French Polynesia. J Molluscan Stud 76:133–137
Phillips NE, Shima JS, Osenberg CW (2014) Live coral cover may provide resilience to damage from the vermetid gastropod Dendropoma maximum by preventing larval settlement. Coral Reefs. doi:10.1007/s00338-014-1198-2
Rinkevich B, Loya Y (1985) Intraspecific competition in a reef coral: effects on growth and reproduction. Oecologia 66:100–105
River GF, Edmunds PJ (2001) Mechanisms of interaction between macroalgae and scleractinians on a coral reef in Jamaica. J Exp Mar Biol Ecol 261:159–172
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202
Sebens KP, Johnson TJ (1991) Effect of water movement on prey capture and distribution of reef corals. Hydrobiologia 226:91–101
Shima JS (1999) Variability in relative importance of determinants of reef fish recruitment. Ecol Lett 2:304–310
Shima JS (2001a) Recruitment of a coral reef fish: roles of settlement, habitat, and postsettlement losses. Ecology 82:2190–2199
Shima JS (2001b) Regulation of local populations of a coral reef fish via joint effects of density- and number-dependent mortality. Oecologia 126:58–65
Shima JS (2002) Mechanisms of density- and number-dependent population regulation of a coral-reef fish. Mar Fresh Res 53:175–179
Shima JS, Osenberg CW (2003) Cryptic density dependence: effects of spatio-temporal covariation between density and site quality in reef fish. Ecology 84:46–52
Shima JS, Osenberg CW, St Mary CM (2008) Quantifying site quality in a heterogeneous landscape: recruitment of a reef fish. Ecology 89:86–94
Shima JS, Osenberg CW, Stier AC (2010) The vermetid gastropod Dendropoma maximum reduces coral growth and survival. Biol Lett 6:815–818
Shima JS, Phillips NE, Osenberg CW (2013) Consistent deleterious effects of vermetid gastropods on coral performance. J Exp Mar Biol Ecol 439:1–6
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett 9:835–845
Stier AC, McKeon CS, Osenberg CW, Shima JS (2010) Guard crabs alleviate deleterious effects of vermetid snails on a branching coral. Coral Reefs 29:1019–1022
Stimson J (2011) Ecological characterization of coral growth anomalies on Porites compressa in Hawai’i. Coral Reefs 30:133–142
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Wainwright SA, Koehl MAR (1976) The nature of flow and the reaction of benthic Cnidaria to it. In: Mackie GO (ed) Coelenterate ecology and behaviour. Plenum Press, New York, pp 5–21
Wielgus J, Glassom D, Ben-Shaprut O, Chadwick-Furman NE (2002) An aberrant growth form of Red Sea corals caused by polychaete infestations. Coral Reefs 21:315–316
Zvuloni A, Armoza-Zvuloni R, Loya Y (2008) Structural deformation of branching corals associated with the vermetid gastropod Dendropoma maxima. Mar Ecol Prog Ser 363:103–108
Acknowledgments
Funding was provided by grants from Victoria University of Wellington and NSF (OCE-1130359). We acknowledge UC Berkeley Gump Research Station for logistic support. This is Contribution Number 212 from UC Berkeley’s Richard B. Gump South Pacific Research Station, Moorea, French Polynesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L Mydlarz.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Shima, J.S., McNaughtan, D. & Strong, A.T. Vermetid gastropods mediate within-colony variation in coral growth to reduce rugosity. Mar Biol 162, 1523–1530 (2015). https://doi.org/10.1007/s00227-015-2688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2688-7