Abstract
A conjoint analysis of gut contents and stable C and N isotopes was applied to determine the main food sources and feeding habits of dominant amphipods in an eelgrass bed (Zostera marina) in Gwangyang Bay, Korea. Gut content observations demonstrated that, while Gammaropsis japonicus and Jassa slatteryi are herbivorous, feeding on epiphytes and detritus, Pontogeneia rostrata and Monocorophium acherusicum are omnivorous, feeding on mesozooplankton fragments and detritus. Stable isotope data confirmed that epiphytes, detritus, and mesozooplankton fragments were major food sources for amphipods in the eelgrass bed. Isotopic mixing model calculations clearly showed an interspecific difference in diet composition. A high isotopic dissimilarity between amphipod taxa demonstrated interspecific trophic diversity, reflecting their herbivorous (G. japonicus and J. slatteryi) and omnivorous (P. rostrata and M. acherusicum) feeding habits and confirmed the detrivorous feeding habits of caprellids. Such trophic diversity at interspecific level of the amphipod species indicates that they use different food resources within their microhabitats and play species-specific functional roles as mediators in trophic pathways from producers to higher-level consumers of the eelgrass ecosystem. Finally, our findings suggest that information on the species-specific trophic ecology of amphipods is needed to better understand their potential role in the trophic dynamics and carbon flow of seagrass bed ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In eelgrass beds, the peracarid crustaceans and especially the Amphipoda are by far the most species-rich group (Fenchel et al. 1975; Wijnsma et al. 1999; Dittmann 2000; Graeve et al. 2001) and are considered to be among the most important secondary producers (Carrasco and Arcos 1984; Jeong et al. 2006). They play a principal role in the eelgrass bed food web, acting as a trophic mediator from primary producers to higher-order consumers (Graeve et al. 2001). Indeed, concerning total energy flow, they are among the key taxa in seagrass bed ecosystems (Stoner and Livingston 1980; Edgar and Shaw 1995; Taylor 1998). A qualitative and quantitative characterization of the trophic role of amphipods and of the entire food web could contribute significantly to a more accurate description of the trophic structure and nutrient fluxes in seagrass beds. Because these key macrofaunal species can consume suspended matter, epiphytes and/or eelgrass, their effects on an eelgrass bed can be either positive or negative (Hughes et al. 2004; Valentine and Duffy 2006). Furthermore, the population dynamics and productivity of these species depend mainly on the availability of alternative food resources (i.e., suspended and/or deposited detrital materials and dissolved organic matters) derived from the eelgrass bed (Yu et al. 2003; Jeong et al. 2006).
Energy transfer from primary producers to consumers in seagrass ecosystems is known to be through three main pathways of consumption: living seagrass tissues, leaf detritus, and related constituents and epiphytes (Buia et al. 2000). Seagrass beds are highly productive ecosystems (Duarte and Chiscano 1999). However, as the direct consumption of seagrass leaves by herbivores is generally low, much of this production eventually enters the detrital food chain through mechanical and biological processes, resulting in particulate detrital materials and dissolved organic carbon (Moriarty et al. 1985; Ziegler and Benner 1998; Stabenau et al. 2004). In contrast, various studies on trophic networks in seagrass beds have shown strong food web linkages between epiphytic algae (also benthic microalgae) and consumers (Jernakoff et al. 1996; Lepoint et al. 2000; Moncreiff and Sullivan 2001; Jaschinski et al. 2008; Jephson et al. 2008). The above-mentioned studies suggest that major trophic pathways may vary among seagrass ecosystems.
In the eelgrass (Zostera marina) bed of Namhae-do on the southern coast of Korea, four gammaridean amphipods (Gammaropsis japonicus, Jassa slatteryi, Pontogeneia rostrata, and Monocorophium acherusicum) and caprellidean amphipods have high biomass and production (Jeong et al. 2004, 2006). Their annual secondary production and biomass are highest among all amphipods inhabiting temperate seagrass beds (Takeuchi 1989; Takeuchi and Hirano 1991, 1992; Jeong et al. 2006, 2009). Their density is controlled by predation pressure from various fish species, such as Pholis nebulosa and Pseudoblennius cottoides (Yun et al. 2002). Some demersal and generalist fish (e.g., the flatfish Limanda yokohamae and the snailfish Liparis tanakai) utilize mainly amphipods as diet during a critical period in their juvenile development (Kwak and Huh 2003a, b). These are indicative of the important role of amphipods as a link from lower to higher trophic levels in the eelgrass ecosystem (Takeuchi 1989; Moksnes et al. 2008).
Amphipods are the most diverse group of crustaceans with respect to life styles, trophic types, habitats and size spectra (Caine 1980; De Broyer and Jazdzewski 1996), but the ecofunctional and trophodynamic roles of epibenthic amphipods are still poorly known. Moreover, the trophic roles and functional types have been studied in fewer than about 10 % of amphipod species, with very few quantitative approaches so far (Dauby et al. 2001). For example, for the most important superfamilies of gammarids (namely the Corophiidae and Eusiroidea), morphofunctional feeding types often cannot be deduced with certainty from the morphology of feeding appendages, because they show opportunistic dietary choices for various diet produced from local sources (Ledru 2000; Sheader et al. 2004). The feeding preferences of caprellid amphipods are also not well understood, with wide discrepancies appearing in the literature, which is restricted with a few exceptions to general invertebrate texts (Dewey 1970). Many authors have stated that caprellids fed mainly by scraping diatoms off the substrata to which they are attached (see Saunders 1966). In this respect, knowledge about the feeding ecology of important trophic mediators such as the amphipods, is crucial in understanding the trophic pathways in seagrass systems. Based on their gut contents, the epibenthic amphopids inhabiting seagrass beds have been regarded as generalist feeders, relying mainly on epiphytes and detritus derived from the seagrass (Macko et al. 1982; Stephenson et al. 1986; Yu et al. 2003; Jephson et al. 2008). However, because most studies have lumped amphipods into a single functional group (Lepoint et al. 2000, Vizzini et al. 2002), little is known about species-specific feeding ecology and hence their major food sources in most seagrass ecosystems (Douglass et al. 2011; Farlin et al. 2011).
Various aspects of amphipod feeding activity have been studied, including their diet (Fenchel et al. 1975; Biernbaum 1979; Nielsen and Kofoed 1982; Icely and Nott 1985; Stuart et al. 1985) using several methods such as gut content and lipid analyses (Sargent and Whittle 1981; Phleger et al. 1998). However, there are several limitations associated with employing these methods in determining their feeding (see Gurney et al. 2001 for a detailed description). Alternatively, stable isotope analysis has proved a powerful tool for discriminating between potential food sources, providing an accurate time-integrated measure of food actually assimilated during the feeding history of consumers (Fry and Sherr 1984; Michener and Schell 1994). The technique is based on the assumption that stable isotope ratios of consumer tissues reflect those of their diets, with predictable enrichment of heavier isotopes. Carbon isotope ratios can be used to identify food sources for consumers because there is little fractionation between prey and predator (Fry and Sherr 1984). Nitrogen isotope ratios can be utilized to estimate consumer trophic position because of the stepwise trophic level enrichment in 15N (Kling et al. 1992; Hobson 1993; Vander Zanden and Rasmussen 2001).
In the present study, we used gut contents and carbon and nitrogen stable isotope ratios (δ13C and δ15N) for the following purposes: (1) to examine interspecific difference in diet composition of four amphipod species (G. japonicus, J. slatteryi, P. rostrata, and M. acherusicum) in an eelgrass bed on the southern coast of Korea; (2) to assess the relative importance of potential food resources (epiphytes on the surface of eelgrass blades, eelgrass tissues, suspended particulate organic matter (POM), and sedimentary organic matter) for the diet of each amphipod species; and (3) to elucidate the potential role of amphipods in diverse functional roles—not as one single functional group—in trophic pathways through food webs of the eelgrass bed ecosystems.
Materials and methods
Study site
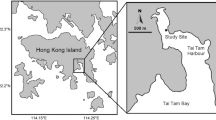
This study was carried out in an eelgrass (Zostera marina) bed in the northwestern part of Namhae-do, Gwangyang Bay, Korea (Fig. 1). The sampling site was composed of muddy sand. The semi-enclosed inward part of the bight communicates with the rest of the bay through a shallow channel. The bight has no marked runoff of freshwater. During the rainy season, however, low salinities (about 10) were caused by runoff from the Seomjin River near the sampling site. The water depth in the inward part does not exceed 5 m, and this location (about 3000 m2) is covered by monospecific stands of Z. marina along the shoreline.
Sample collection
All samples for analyses were taken in May 2008 by SCUBA diving and collected in the same area (100–150 m2) at a distance of 20 m from the shore and at a depth of 3.5 m, in order to minimize the spatial differences in the potential food sources. Composite specimens of eelgrass were taken (containing green leaves, senescent brown leaves, fallen decomposing leaves and roots). Fallen, decomposing leaves were collected from the bottom. The leaves were examined using a microscope for detection of seagrass debris and epibionts and were then carefully cleaned of epiphytes using a razor blade. Epiphytic diatoms (hereafter, epiphytes) and macroalgae (red seaweed) on the surface of eelgrass blades were collected from mature green leaves using a razor blade.
Phytoplankton were collected from the water column with a tow net (20-μm mesh size) directly above the eelgrass bed. From these samples, particles and animals were carefully removed under a microscope, after prefiltered using a 63-μm sieve. Suspended zooplankton were sampled using a tow net (250-μm mesh size). The suspended zooplankton samples from the eelgrass bed consisted mainly of Acartia sp.. Harpacticoid copepods were collected with a handheld net (250-μm mesh size) using SCUBA diving equipment (see Jeong et al. 2006 for a detailed description). Suspended particulate organic matter (POM) was collected from the water column with a vacuum pump and was obtained by filtering 10 l of seawater, which was prefiltered through a 100-μm sieve to remove large particles and zooplankton, onto prewashed and precombusted (450 °C, 4 h) Whatman GF/F filters (0.70-μm nominal pore size). Sedimentary organic matter (SOM) was collected from the top 0.5 cm of sediment. Three replicate samples were taken by hand corer of 5 cm diameter.
Amphipod samples were taken with handheld net (150-μm mesh size) with SCUBA diving and were separated from the eelgrass by rinsing with filtered seawater and sorted under a dissecting microscope. All amphipod samples for gut content and stable isotope analyses consisted of at least 10 individuals of the same species that had been collected concurrently at the same site. Sex or maturity of the amphipods in the present study was not considered. Only amphipods greater than 5 mm in length were used for gut content analyses.
Gut content analysis
The gut of each gammarid amphipod was carefully dissected and immersed in glycerol on a glass slide. Gut fullness was assessed by microscope. Individual food items were identified and counted under a differential interference microscope (Zeiss Axioskop, Göttingen, Germany) and a cold-field emission scanning electron microscope (SEM, Hitachi S-3000 N, Tokyo, Japan). The food items were identified to the lowest possible taxonomic category, using the works of Yu et al. (2003). Dietary composition of each gammarid amphipod species using the point method (Wear and Haddon 1987). This method is suitable for assessing the diet of crustaceans including amphipods whose gut contents are macerated and different to quantify (Takahashi and Kawaguchi 1998; Oh et al. 2001). Prior to observation by SEM, the gut contents were dehydrated using a graded ethanol and t-butyl alcohol series and then freeze-dried. Samples were mounted on stubs and sputter coated with platinum.
The data were presented as percentage composition by number (%N) and frequency of occurrence (%O). Percentage composition by number represents the proportion of a particular item relative to the total number of all items counted in the gut and provides information on each individual’s feeding behavior. The percentage occurrence and relative abundance for each food type was estimated using the following equations:
where n i is the number of amphipods with food i in the gut, N is the total number of amphipods with gut contents, S i is the number of items of food i, and S t is the total number of food items. Caprellid amphipods were not considered.
Stable isotope analysis
Amphipods were kept in filtered seawater overnight to evacuate their gut contents before being frozen. After defrosting, all food source and amphipod samples were rinsed in distilled water, dried at 60 °C for 48 h and ground to a fine powder using a mortar and pestle. Prior to isotopic analysis, all samples were treated, in silver cups, with a small amount of 1 N HCl to remove carbonates until the sample ceased to effervesce; samples were then dried again. Because acid washing can affect the nitrogen ratios of organic material (Bunn et al. 1995), samples for nitrogen isotope analysis were not acidified.
The carbon and nitrogen isotopic compositions of the powdered samples were determined using a continuous-flow isotope ratio mass spectrometer (CF-IRMS, Isoprime, GV Instruments, Manchester, UK) coupled with an elemental analyzer (EuroVector 3000 Series, Milan, Italy). For isotope analysis, the samples were weighed (0.5–1.0 g) into tin capsules and then oxidized at high temperature (1,030 °C) in the elemental analyzer. δ13C and δ15N values are expressed in parts per thousand (‰) relative to the conventional standard PDB (Pee Dee Belemnite) for carbon and atmospheric N2 for nitrogen, respectively, according to the following formula: δX = [(R sample/R standard) – 1] × 103, where X is 13C or 15N and R is the 13C/12C or 15N/14N. Sucrose (ANU C12H22O11; NIST, Gaithersburg, MD, USA) and ammonium sulfate ([NH4]2SO4; NIST) were used for the internal δ13C and δ15N calibration, respectively, and were analyzed twice after every six samples. The standard deviation of repeated measurements of the laboratory standard was 0.1 ‰ for δ13C and 0.15 ‰ for δ15N.
Multiple-source mixing model and statistical analyses
IsoSource software was used to determine the relative contribution of each source to the mixed signature of amphipods (Phillips and Gregg 2003; Phillips et al. 2005). Epiphytes on the surface of eelgrass blades, eelgrass tissues, POM, and SOM were considered as potential trophic resources for the amphipods. Consumers are generally enriched in heavier isotopes (13C and 15N) relative to their diets (i.e., isotopic fractionation). For the isotopic mixing model calculations, the isotope values of amphipods were corrected with the average fractionation estimates of −0.7 ‰ for δ13C and 1 ‰ for δ15N known for amphipods (Macko et al. 1982; Stephenson et al. 1986; see also “Discussion”). These trophic fractionation values were lower than the average estimates (−0.41 ‰ ± 1.14 for δ13C; 2.5 ‰ ± 2.5 for δ15N) suggested for herbivores (Vander Zanden and Rasmussen 2001; McCutchan et al. 2003). Source increment was set at 1 %. Tolerance was initially set at 0.1 % (Phillips and Gregg 2003). If mixture isotope values were outside the set thresholds, we incrementally increased the tolerance value to maximum of 0.5 %.
Prior to statistical analyses, data were tested for homogeneity of variances and normal distribution. Bonferroni corrected t tests allowed comparison between taxa within food sources and animals.
Results
Gut content analysis
Eight main components were distinguished in the gut analysis of amphipod species (Table 1). The most common food items in the gut of Gammaropsis japonicus and Jassa slatteryi were found to be epiphytic diatoms (Fig. 2a, b), which were found in 100 % of the guts examined and constituted more than 70 % of the total gut content. These two species had similar diatom contents of 71.8 and 74.5 %, respectively. The other categories contributed only minor proportions to the diet (<20 % of total abundance each). Epiphytic diatoms were present either intact or as detritus and were more frequent in the guts, which were completely filled with frustules. The relative abundance of the main diatoms found in gut contents was not measured in the present study. However, of the diatoms identified in guts, Cocconeis sp. was the most abundant (>50 % of total diatom count for each individual). This diatom is attached to the blades of eelgrass by a mucilaginous film and is ingested by benthic amphipods, which scrape the eelgrass substratum to feed. Detritus was also a dominant food item in the guts of amphipods (Fig. 2c, d). It was found in all guts analyzed (100 % presence in all four amphipod species) but constituted less than 35 % of the total abundance of food items in the guts (Table 1). Detritus fragments were the second major contributors to the diet of these amphipods and were mainly derived from plant litter and eelgrass leaves (Fig. 2c). The size of detritus ranged from about 10–50 μm. The detrital fragments were broken to pieces by the action of the strong teeth, as shown by the decrease in detritus size. Broken piece of these fragments were intermittently associated with diatoms (Fig. 2d). Macroalgae showed the lowest percentage occurrence in the guts of amphipods (0–50 %; Table 1). The contributions of macroalgae ranged from zero in M. acherusicum to 5.8 % in G. japonicus. These detrital components were made up of filamentous particles about 0.5 μm diameter. Red algae are the most common macroalgal form found off the southern coast of Korea (Huh et al. 1998) and seemed to be more frequent in guts of amphipods during spring. This corresponded to a period of higher production of epiphytic algae and eelgrass leaf area makes a considerable contribution to this process (Jeong et al. 2004).
Gut contents of amphipods in this study area. a, Gammaropsis japonicus, diatom Cocconeis sp.; b Jassa slatteryi, diatoms fragments; c, Pontogeneia rostrata, detritus; d, P. rostrata, diatoms fragments and detritus; e, P. rostrata, appendicular of Acartia sp.; f, Monocorophium acherusicum, crustacean fragments
The contributions of mesozooplankton and/or zoobenthos fragments (harpacticoid copepods, unidentified crustaceans, Acartia sp.) to the gut material of M. acherusicum (63.7 %) were remarkably higher than those of P. rostrata (39.6 %). A high contribution was observed for crustacean fragments and Acartia sp. (Fig. 2e, f). Crustacean fragments were the dominant food item found in the gut of M. acherusicum (31.6 % of total gut contents, Table 1). These fragments were small detritus (<150 μm, Fig. 2f). Gut analysis did not allow further identification of these components, which were not entirely assimilated because they were found in the hindgut. The calanoid copepod Acartia sp. occurred in high abundance in the guts of P. rostrata and M. acherusicum (Table 1). Acartia sp. fragments accounted for 25.6 % of the total abundance of food items in the guts of P. rostrata, constituting the second major food item (Fig. 2e). The fragments were also consistently present in the mid- and hindguts of P. rostrata and M. acherusicum. Harpacticoid copepods and dinoflagellate cysts represented 5.8–9.9 % of total gut contents in P. rostrata and M. acherusicum and were minor contributors to the diet (<10 % of the total abundance for each; Table 1).
Stable isotopes signatures in food sources and amphipods
Whereas the δ13C values of the food sources in the eelgrass bed exhibited a wide range (−21.0 ± 0.4 ‰ in POM to −9.5 ± 0.1 ‰ in green leaves of eelgrass), the δ15N values showed a much narrower range between epiphytes (mean 7.2 ± 0.1 ‰) and suspended zooplankton (mean 11.3 ± 0.6 ‰, Fig. 3). Decomposing leaves and roots of eelgrass showed very similar δ13C values (mean −10.7 ± 0.2 ‰ and −10.8 ± 0.4 ‰, respectively). The δ13C values of green and brown leaves of seagrass were very similar (mean −9.5 ± 0.1 ‰ and −10.1 ± 0.2 ‰, respectively), values being slightly enriched in δ13C compared with the former two counterparts. The δ15N values of eelgrass blades ranged from 10.3 ± 0.1 ‰ to 10.7 ± 0.2 ‰ and were substantially enriched relative to epiphytes, macroalgae, and harpacticoid copepods (Bonferroni test, p < 0.05, Table 2). There were no significant differences in the δ13C and δ15N values between eelgrass tissues, the values being similar to those reported previously (Fujiwara and Highsmith 1997; Kharlamenko et al. 2001). Overall, eelgrass δ13C and δ15N values were significantly higher than those from other sources (Bonferroni test, p < 0.001).
Mean (±SD) δ13C versus δ15N values of amphipods and potential food items in this Zostera marina bed. Gl green leaves, Fl fallen decomposing leaves, Sl senescent brown leaves, Rt roots, Har harpacticoid copepods, Cap caprellids, Ma macroalgae, POM particulate organic maters, SOM sedimentary organic matter, SuZ suspended zooplankton
Epiphytic algae on eelgrass blades consisted mainly of diatoms: Cocconeis sp. Epiphytes were 13C-depleted compared with the eelgrass blades on which they grew; the δ13C values (mean −15.2 ± 0.4 ‰) were ~3.0 ‰ lower than those for eelgrass blades (Bonferroni test, p < 0.001; Table 2) and showed intermediate values between those of POM, phytoplankton and eelgrass (Fig. 3). The δ15N value (mean 7.2 ± 0.1 ‰) of epiphytes showed the lowest value among the food sources. The δ13C values (mean −15.1 ± 0.5 ‰) of macroalgae were similar to those of epiphytes collected from the surface of eelgrass blades. The δ15N values of macroalgae showed intermediate values (mean 8.6 ± 0.2 ‰) among the food sources. The δ13C values of POM (mean −21.0 ± 0.4 ‰) were similar to those of phytoplankton (−19.0 ± 0.8 ‰). These δ13C values were within the range typical of suspended POM collected from the southern coastal bays of the Korean Peninsula (Kang et al. 2003). The δ15N values of POM and phytoplankton (means 9.8 ± 0.8 ‰ and 8.8 ± 0.5 ‰, respectively) were also within the range (mean 8.6–11.7 ‰) reported for those in near shore waters of adjacent coastal bays (Kang et al. 2003). The δ13C values (mean −11.6 ± 0.4 ‰) of harpacticoid copepods (the main dietary component of benthic mezograzers in this study) were consistently 13C-enriched compared with suspended zooplankton (Bonferroni test, p < 0.001, Table 2), whereas the δ15N values were similar to each other (Bonferroni test, p > 0.05).
Amphipods exhibited very low intraspecific variations (<1 ‰) in δ13C and δ15N (Fig. 3). A wide δ13C range (~4.2 ‰) of interspecific difference was found between G. japonicus and M. acherusicum. Among the amphipods, δ13C values of G. japonicus and J. slatteryi (means −14.2 ± 0.6 ‰ and −14.8 ± 0.7 ‰, respectively) had nearly identical values, whereas P. rostrata and M. acherusicum (means −16.0 ± 0.5 and −18.4 ± 0.4 ‰, respectively) had lower δ13C values than G. japonicus and J. slatteryi (Bonferroni test, p < 0.05; Table 2). Caprellid amphipods showed lower δ13C values (mean −18.4 ± 0.5 ‰) than those of the former four amphipods (Bonferroni test, p < 0.05; Table 2). The δ15N values between G. japonicus and J. slatteryi (means 9.1 ± 0.1 ‰ and 9.5 ± 0.1 ‰, respectively) and between P. rostrata and M. acherusicum (means 10.8 ± 0.3 ‰ and 11.4 ± 0.5 ‰, respectively) were not significantly different, but the values between these two groups (G. japonicus and J. slatteryi vs. P. rostrata and M. acherusicum) were substantially different (Bonferroni test, p < 0.05; Table 2).
Mixing model estimates of dietary proportions
The mixing model estimates showed variations in the major dietary components of amphipods (Table 3). The results are in accordance with the gut content observations. The results of model calculations indicated that epiphytes on the surface of eelgrass blades were the major food source of G. japonicus (68–69 %) and J. slatteryi (50–59 %). In contrast, POM and SOM were estimated to be more important nutritional source for P. rostrata (0–57 and 0–67 %, respectively), M. acherusicum (60–86 and 0–20 %, respectively), and caprellids (10–50 and 23–70 %, respectively). Z. marina was also of considerable importance in the diet of all amphipod species analyzed (31–32, 18–23, 31–36, and 13–22 % for G. japonicus, J. slatteryi, P. rostrata, and M. acherusicum, respectively).
Discussion
We found that these amphipods had a species-specific diet in this eelgrass system, a result that is contrast to the longstanding belief that amphipods can be treated as several functional groups in benthic macrophyte systems. The significant differences in the δ13C and δ15N values between the two groups reflected the difference in dietary composition in accordance with gut contents.
G. japonicus and J. slatteryi had δ13C values close to that of epiphytes (Fig. 3). The similarity in δ13C between these species and epiphytes might be explained by the amphipod gut contents, which comprised mainly epiphytic diatoms. This result indicates that these amphipods exploited epiphytic microalgae on eelgrass blades. The presence of epiphytes on host eelgrass increases habitat complexity and food supply for amphipods. It is generally accepted that eelgrass supports a large number of epiphytic organisms as accessible primary producers for grazing consumers (Kharlamenko et al. 2001). In the study area, the greatest development of epiphytes occurs between spring and summer with a chlorophyll a peak from April to July, and this is dependent on eelgrass biomass (Jeong et al. 2004). As a result, it is expected that the production of amphipods will depend on the presence of epiphytes, which provide higher resource availability (Edgar 1983; Martin-Smith 1993).
It has been demonstrated that some species of grazing amphipods actually feed on eelgrass blades and that sometimes they are the main consumers of eelgrass among all animals studied in eelgrass ecosystems (Stephenson et al. 1986; Boström and Mattila 2005). However, in the present study, eelgrass tissues were not found in the gut of any of the four amphipods (Table 1). Fry and Sherr (1984) suggested that seagrass tissues appear to be a minor food source for consumers, whereas seagrass-derived detritus and epiphytes seem to play the most important trophic role. On the dual isotope plot, δ13C values of G. japonicus and J. slatteryi were aligned to those of epiphytes and macroalgae but still far from the values of POM (also SOM and phytoplankton; Fig. 3). In contrast, the δ15N values of G. japonicus and J. slatteryi were aligned to those of POM and were very close to the values of macroalgae but 2 ‰ higher than those of epiphytes. Diet–animal tissue isotopic fractionation is critical in determining the relative contribution of different food sources to an animal’s diet. Trophic fractionation has been reported to be significantly lower in herbivores than in carnivores for δ13C and δ15N (−0.41 ± 1.14 ‰ vs. 0.91 ± 1.04 ‰ and 2.5 ± 2.5 ‰ vs. 3.4 ± 0.4 ‰, respectively); the fractionation estimates bring more variable in herbivores than in carnivores (Vander Zanden and Rasmussen 2001; Vanderklift and Ponsard 2003). Because of the elevated variance for herbivores in isotopic fractionation between prey and predator, the average fractionation estimates obtained from the literature can hide large variations between intra- and interspecific population comparisons. In marine amphipods, trophic fractionation values are typically low and highly variable (Lepoint et al. 2000; Vizzini et al. 2002; Farlin et al. 2011; Michel 2011). Therefore, as recommended by Caut et al. (2008), the fractionation values measured by in vitro grazing experiments of amphipods—rather than the above-mentioned general fractionation estimates—were used as trophic enrichment factors in this study.
Considering the trophic fractionation of about −0.7 ‰ for δ13C and 1 ‰ for δ15N (Macko et al. 1982; Stephenson et al. 1986), the isotopic composition of these two herbivorous amphipods (G. japonicus and J. slatteryi) indicates that they might not feed directly on eelgrass tissues. Similar δ15N values between these two herbivorous amphipods and macroalgae may indicate a low contribution of macroalgae to the amphipods, as confirmed by a low abundance (5.8 and 2.8 %, respectively) of macroalgae in their gut contents. Accordingly, the δ13C and δ15N values suggest that the amphipod food chains are based on epiphytes. Indeed, linear mixing model calculations based on mass balance equations using the δ13C and δ15N signatures of four sources of organic matter showed that epiphytes were definitely the major dietary component for G. japonicus and J. slatteryi (68–69 and 50–59 %, respectively; Table 3). The results of isotopic mixing model calculations were consistent with the conclusion from gut content analysis, confirming the herbivorous feeding habits of these species.
In contrast, P. rostrata and M. acherusicum had more 13C-depleted but 15N-enriched values compared with the herbivorous amphipods that feed exclusively on epiphytes (Fig. 3). Greater 13C-depleted values in these two amphipods than in the herbivorous amphipods indicate an increased contribution of POM or SOM to their ultimate dietary sources. Moreover, their higher δ15N values (1.3–2.3 ‰) than those of the herbivorous amphipods indicate their higher trophic positions. The scanning electron microscope study of gut contents clearly showed that P. rostrata and M. acherusicum utilize a mixed diet derived from carbon sources, such as zooplankton, epiphytes and vegetal detritus (Table 1). Gut content analysis proved the omnivorous feeding habit of P. rostrata, showing a predominance of detritus in its food items (32.8 %, Table 1). Isotopic mixing model calculations indicated that POM and SOM constitute a major dietary component of P. rostrata (0–57 % for POM and 0–67 % for SOM; Table 3). P. rostrata was shown to feed mainly on planktonic and/or benthic microalgae as an epiphyte grazer and detritus feeder (Brawley and Fei 1987). In the gut content analysis of P. rostrata, macroalgae were a minor component of the amphipod diets. On the other hand, in the P. rostrata diet, carcasses of zooplankton represented almost half of its food items (39.6 %; Table 1). Some members of the family Eusiridae, such as P. rostrata, show an opportunistic dietary choice for a carnivorous diet (crustacean fragments) produced from local sources (Ledru 2000; Sheader et al. 2004). They are also known to be grazing omnivores that often move on the sediment surface to feed on deposited prey and detrital materials derived from the carcasses of zooplankton (Biernbaum and Wenner 1993; Yu 2002). In this study, SOM consisted of detritus containing carcasses of suspended zooplankton and benthic diatoms and the contents are known to be highest during spring (Jeong et al. 2004). This suggests that deposited prey can constitute an important component of major food sources of P. rostrata inhabiting eelgrass beds.
For M. acherusicum, several studies demonstrated that it feeds on plant debris or benthic macroalgae as a burrower and adjusts its feeding strategy according to ambient food availability (Whitlatch 1981; Murdoch et al. 1986; Jeong et al. 2004). The composition of the diet of amphipods might reflect to some degree the relative abundance and availability of food items (Yu et al. 2003). In the present study, the gut content of M. acherusicum showed that it had a mainly carnivorous diet (crustacean fragments; Table 1). Most copepods and benthic amphipods inhabiting temperate eelgrass beds breed during spring and then die (Park 2006; Jeong et al. 2009). Carcasses of copepods might account for a significant part of the sinking fluxes of organic matter in food chains in benthic microhabitats. According to Yu et al. (2003), meiofaunal fragments were the dominant food item of carnivorous amphipods and the density of food items—such as copepods and detritus—could be an important factor according for variations in the dietary composition of benthic amphipods. In this study, the high contents of POM and SOM were likely to reflect increased carcasses of dead individuals after copepod breeding, suggesting their considerable contribution to the dietary composition of M. acherusicum. Our isotopic results, along with gut content analysis, support this hypothesis. A major contribution of carrion-derived organic matter to the diet—such as the crustacean remains—has often been found in some benthic amphipods (Dauby et al. 2001; Nyssen et al. 2002).
With a few exceptions, caprellid feeding is not well covered or understood in general invertebrate textbooks (Dewey 1970). Guerra-Garcia and Tierno de Figueroa (2009) reported that caprellids are mainly detritivores (detritus represented 86 % of the caprellid diet), but some species can be considered as obligate predators and feed mainly on small crustaceans (copepods and other amphipods). In this present study, isotopic signatures of caprellid amphipods were the most 13C-depleted, showing a great similarity to SOM and POM (Fig. 3). The δ15N value of caprellid amphipods was located among the four gammaridean amphipods. Isotopic mixing model calculations clearly showed that SOM and POM were major dietary components for caprellids, with feasible contributions of 23–70 and 10–50 %, respectively (Table 3). These results were consistent with the conclusion from gut content analysis, confirming the detrivorous feeding habits of caprellids.
Our results are inconsistent with functional roles among amphipod taxa in the eelgrass ecosystem as suggested by Bell (1991) and Duffy and Harvilicz (2001). Instead, our conjoint analysis of stable isotopes and gut contents offers evidence of diverse feeding habits among amphipod species (Duffy 1990; Nyssen et al. 2002; Farlin et al. 2011). Such a trophic diversity of these amphipod species at the interspecific level indicates that they use different food resources within the microhabitats and play species-specific functional roles in trophic pathways from producers to higher-level consumers of the eelgrass ecosystem. The data obtained in this study demonstrated that, whereas herbivorous amphipods (G. japonicus and J. slatteryi) might serve as important trophic mediators in epiphytic microalgal-based carbon pathway, omnivorous taxa (P. rostrata and M. acherusicum) could play an important role in forming trophic links in a phytoplankton-based pathway. Aspects of positive or negative interactions of the seagrass–epiphyte–amphipod system by epiphyte removal or from amphipods grazing directly on seagrass leaves have been developed by Jernakoff et al. (1996). Poore et al. (2009) reported that grazing of herbivorous amphipods did not affect the growth rates of macroalgae, the cover of its epiphytes, or the structure of algal assemblages, suggesting no evidence of grazer control on algal biomass and epiphytes. The studies are beyond the scope of this study. However, it is expected that the species specificity in the functional role of amphipods may result in different contributions among species to the seagrass–epiphytes–amphipod interactions. This study suggests that information on species-specific trophic ecology rather than on one general trophic category of amphipods is needed to better understand their potential role in the trophic dynamics and carbon flow of seagrass bed ecosystems.
In conclusion, the combination of analyzing gut contents and a stable isotope technique proved to be valuable tool in detecting feeding strategies and trophic diversity of the amphipod community in seagrass systems. As confirmed by gut contents, our isotopic data strongly suggest that epiphytes, detritus, and mesozooplankton fragments are major food sources for amphipods in the Z. marina bed in Gwangyang Bay. Furthermore, a great isotopic dissimilarity between amphipod taxa demonstrated interspecific trophic diversity, reflecting their herbivorous (G. japonicus and J. slatteryi) and omnivorous (P. rostrata and M. acherusicum) feeding habits. However, the contribution of these dietary components to the diets of amphipods might vary depending on the temporal availability of food sources. These variations can influence the seasonal changes in food selectivity and production by amphipods. In this respect, more detailed information on the seasonal or temporal availabilities of food sources will help us to better understand the feeding ecology of amphipods.
References
Bell SS (1991) Amphipods as insect equivalents? An alternative view. Ecology 72:350–354
Biernbaum CK (1979) Influence of sedimentary factors on the distribution of benthic amphipods of Fishers Island Sound. J Exp Mar Biol Ecol 38:201–223
Biernbaum CK, Wenner EL (1993) Trapping of necrophagous crustaceans on the upper continental slope of south Carolina USA. J Crust Biol 13:601–608
Boström C, Mattila J (2005) Effects of isopod grazing: an experimental comparison in temperate (Idotea balthica, Baltic Sea, Finland) and subtropical (Erichsonella attenuate, Gulf of Mexico, USA) ecosystems. Crustaceana 78:185–200
Brawley SH, Fei XG (1987) Studes of mesoherbivory in aquaria and in an unbarricaded mariculture farm on the Chinese coast. J Phycol 23:613–623
Buia MC, Gambi MC, Zupo V (2000) Structure and functioning of Mediterranean seagrass ecosystems: an overview. Biol Mar Medit 7:167–190
Bunn SE, Loneragan NR, Kempster MA (1995) Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: implication for food web studies using multiple stable isotopes. Limnol Oceanogr 40:622–625
Caine EA (1980) Ecology of two littoral species of caprellid amphipods (Crustacea) from Washington, USA. Mar Biol 56:327–335
Carrasco FD, Arcos DF (1984) Life history and production of a cold-temperate population of the sublittoral amphipod Ampelisca araucana. Mar Ecol Prog Ser 14:245–252
Caut S, Angulo E, Courchamp F (2008) Caution on isotopic model use for analyses of consumer diet. Can J Zool 86:438–445
Dauby P, Scailteur Y, De Broyer C (2001) Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia 443:69–86
De Broyer C, Jazdzewski K (1996) Biodiversity of the Southern Ocean: towards a new synthesis for the Amphipoda (Crustacea). Boll Mus Civ Stor Nat Verona 20:547–568
Dewey RA (1970) The feeding of Caprella equilibra Say, 1818 (Amphipoda: Crustacea). Dissertation, Faculty of San Diego State College, San Diego
Dittmann S (2000) Zonation of benthic communities in a tropical flat of north-east Australia. J Sea Res 43:33–51
Douglass JG, Duffy JE, Canuel EA (2011) Food web structure in a Chesapeake Bay eelgrass bed as determined through gut contents and 13C and 15N isotope analysis. Estuar Coast 34:701–711
Duarte CM, Chiscano CL (1999) Seagrass biomass and productivity: a reassessment. Aquat Bot 65:159–174
Duffy JE (1990) Amphipods on seaweeds: partners or pests? Oecologia 83:267–276
Duffy JE, Harvilicz AM (2001) Species-specific impacts of grazing amphipods in an eelgrass-bed community. Mar Ecol Prog Ser 223:201–211
Edgar GJ (1983) The ecology of south-east Tasmanian phytal animal communities. II. Seasonal change in plant and animal populations. J Exp Mar Biol Ecol 70:159–179
Edgar GJ, Shaw C (1995) The production and trophic ecology of shallow-water fish assemblages in southern Australia. II. Diets of fishes and trophic relationships between fishes and benthos at Western Port. Victoria. J Exp Mar Biol Ecol 194:83–106
Farlin JP, Lewis LS, Anderson TW, Lai CT (2011) Functional diversity in amphipods revealed by stable isotopes in an eelgrass ecosystem. Mar Ecol Prog Ser 420:277–281
Fenchel T, Kofoed HL, Lappalainen A (1975) Particle size-selection of two deposit feeders: the amphipod Corophium volutator and the prosobranch Hydrobia ulvae. Mar Biol 30:119–128
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Fujiwara M, Highsmith R (1997) Harpacticoid Copepods: potential link between inbound adult salmon and outbound juvenile salmon. Mar Ecol Prog Ser 158:205–216
Graeve M, Dauby P, Scailteur Y (2001) Combined lipid, fatty acid and digestive tract content analyses: a penetrating approach to estimate feeding modes of Antarctic amphipods. Polar Biol 24:853–862
Guerra-Garcia JM, Tierno de Figueroa JM (2009) What do caprellids (Crustacea: Amphipoda) feed on? Mar Biol 156:1881–1890
Gurney LJ, Froneman PW, Pakhomov EA, McQuaid CD (2001) Trophic positions of three euphausiid species from the Prince Edward Islands (Southern Ocean): implications for the pelagic food web structure. Mar Ecol Prog Ser 217:167–174
Hobson KA (1993) Trophic relationships among high arctic seabirds: insights from tissue-dependent stable-isotope models. Mar Ecol Prog Ser 95:7–18
Hughes AR, Bando KJ, Rodriguez LF, Williams SL (2004) Relative effects of grazers and nutrients on seagrasses: a meta-analysis approach. Mar Ecol Prog Ser 282:87–99
Huh SH, Kwak SN, Nam KW (1998) Seasonal variations of eelgrass (Zostera marina) and epiphytic algae in eelgrass beds in Kwangyang Bay. J Korean Fish Soc 31:56–62
Icely JD, Nott JA (1985) Feeding and digestion in Corophium volutator (Crustacea: Amphipoda). Mar Biol 89:183–195
Jaschinski S, Brepohl DC, Sommer U (2008) Carbon sources and trophic structure in an eelgrass Zostera marina bed, based on stable isotope and fatty acid analyses. Mar Ecol Prog Ser 358:103–114
Jeong SJ, Yu OH, Suh HL (2004) Seasonal variation and feeding habits of amphipods inhabiting Zostera marina beds in Gwangyang Bay, Korea. J Korean Fish Soc 37:122–128
Jeong SJ, Yu OH, Suh H-L (2006) Secondary production of Jassa slatteryi (Amphipoda, Ischyroceridae) on a seagrass bed (Zostera marina L.) in southern Korea. Mar Ecol Prog Ser 309:205–211
Jeong SJ, Yu OH, Suh H-L (2009) Reproductive patterns and secondary production of Gammaropsis japonicus (Crustacea, Amphipoda) on the seagrass Zostera marina of Korea. Hydrobiologia 623:63–76
Jephson T, Nyström P, Moksnes P-O, Baden SP (2008) Trophic interactions within Zostera marina beds along the Swedish coast. Mar Ecol Prog Ser 369:63–76
Jernakoff P, Brearley A, Nielsen J (1996) Factors affecting grazer-epiphyte interactions in temperate seagrass meadows. Oceanogr Mar Biol Annu Rev 34:109–162
Kang CK, Kim JB, Lee KS, Kim JB, Lee PY, Hong JS (2003) Trophic importance of benthic microalgae to macrozoobenthos in coastal bay system in Korea: dual stable C and N isotope analyses. Mar Ecol Prog Ser 259:79–92
Kharlamenko VI, Kiyashko SI, Imbs AB, Vyshkvartzev DI (2001) Identification of food sources of invertebrates from the seagrass Zostera marina community using carbon and sulfur stable isotope ratio and fatty acid analyses. Mar Ecol Prog Ser 220:103–117
Kling GW, Fry B, O’Brien WJ (1992) Stable isotopes and plankton trophic structure in arctic lakes. Ecology 73:561–566
Kwak SN, Huh SH (2003a) Feeding habits of juvenile Liparis tanakai in the eelgrass, Zostera marina bed in Kwangyang Bay. J Kor Fish Soc 36:372–377 (Korean with English abstract)
Kwak SN, Huh SH (2003b) Feeding habit of Limanda yokohamae in the eelgrass (Zostera marina) bed in Kwangyang Bay. J Kor Fish Soc 36:522–527 (Korean with English abstract)
Ledru S (2000) Trophic niche and nutritional biochemistry of invertebrates inhabiting East Pacific Rise hydrothermal vent sites. Université de Paris-Sud, France, Dissertation
Lepoint G, Nyssen F, Gobert S, Dauby P, Bouquegneau JM (2000) Relative impact of a seagrass bed and its adjacent epilithic algal community in consumer diets. Mar Biol 136:513–518
Macko SA, Lee WY, Parker PL (1982) Nitrogen and carbon isotopic fractionation by two species of marine amphipods: laboratory and field studies. J Exp Mar Biol Ecol 63:145–149
Martin-Smith KM (1993) Abundance of mobile epifauna: the role of habitat complexity and predation by fishes. J Exp Mar Biol Ecol 174:243–260
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulfur. Oikos 102:378–390
Michel L (2011) Multidisciplinary study of trophic diversity and functional role of amphipod crustaceans associated to Posidonia oceanica meadows. University of Liège, Belgium, Dissertation
Michener RH, Schell DM (1994) Stable isotopes ratios as tracers in marine aquatic food webs. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental sciences. Blackwell Scientific Publications, Oxford, pp 138–157
Moksnes P-O, Gullström M, Tryman K, Baden S (2008) Trophic cascades in a temperate seagrass community. Oikos 117:763–777
Moncreiff CA, Sullivan MJ (2001) Trophic importance of epiphytic algae in subtropical seagrass beds: evidence from multiple stable isotope analyses. Mar Ecol Prog Ser 215:93–106
Moriarty DJW, Pollard PC, Hunt WC, Moriarty CM, Wassenberg TJ (1985) Productivity of bacteria and microalgae and the effect of grazing by holothurians in sediments on a coral reef flat. Mar Biol 85:293–300
Murdoch MH, Barlocher F, Laltoo ML (1986) Population dynamlcs and nutrition of Corophium volutator (Pallas) in the Cumberland Basin (Bay of Fundy). J Exp Mar Biol Eco1 3:235–249
Nielsen MV, Kofoed LH (1982) Selective feeding and epipsammic browsing by the deposit-feeding amphipod Corophium volutator. Mar Ecol Prog Ser 10:81–88
Nyssen F, Bery T, Lepoint G, Bouquegeneau JM, De Broyer C, Dauby P (2002) A stable isotope approach to the eastern Weddell Sea trophic web: focus on benthic amphipods. Polar Biol 25:280–287
Oh CW, Hartnoll RG, Nash RDM (2001) Feeding ecology of the common shrimp crangon crangon in Port Erin Bay, Isle of Man, Irish Sea. Mar Ecol Prog Ser 214:211–223
Park JI (2006) The effects of Zostera marina on the distribution of planktonic copepods. Dessertation, Chonnam National University, Korea
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Phillips DL, Newsome SD, Gregg JW (2005) Combining sources in stable isotope mixing models: alternative methods. Oecologia 144:520–527
Phleger CF, Nichols PD, Virtue P (1998) Lipids and trophodynamics of Antarctic zooplankton. Lipids 32:1093–1100
Poore AGB, Campbell AH, Steinberg PD (2009) Natural densities of mesograzers fail to limit growth of macroalgae or their epiphytes in a temperate algal bed. J Ecol 97:164–175
Sargent JR, Whittle KJ (1981) Lipids and hydrocarbons in the marine food web. In: Longhurst A (ed) Analysis of marine ecosystems. Academic Press, London, pp 491–533
Saunders CG (1966) Dietary analysis of caprellids (Amphipoda). Crustaceana 10:314–316
Sheader M, Van Dover CL, Thurston MH (2004) Reproductive ecology of Bouvierella curtirama (Amphipoda: Eusiridae) from chemically distinct vents in the Lucky Strike vent field, Mid-Atlantic Ridge. Mar Biol 144:503–514
Stabenau ER, Zepp RG, Bartels E, Zika RG (2004) Role of the seagrass Thalassia testudinum as a source of chromophoric dissolved organic matter in coastal south Florida. Mar Ecol Prog Ser 282:59–72
Stephenson RL, Tan FC, Mann KH (1986) Use of stable carbon isotope ratios to compare plant material and potential consumers in a seagrass bed and a kelp bed in Nova Scotia, Canada. Mar Ecol Prog Ser 30:1–7
Stoner AW, Livingston RJ (1980) Distributional ecology and food habits of the banded blenny Parclinus fasciatus (Clinidae): a resident in a mobile habitat. Mar Ecol 56:239–246
Stuart V, Head EJH, Mann KH (1985) Seasonal changes in the digestive enzyme levels of the amphipod Corophium volutator (Pallas) in relation to diet. J Exp Mar Biol Ecol 88:243–256
Takahashi K, Kawaguchi K (1998) Diet and feeding rhythm of the sand–burrowing mysids Archaeomysis kokuboi and A. japonica in Otsuchi Bay, northeastern Japan. Mar Ecol Prog Ser 162:191–199
Takeuchi I (1989) Taxonomic and ecological studies of the Caprellidea (Crustacea, Amphipoda) inhabiting the Sargassum zone. Ph.D. thesis, Graduate School of Agriculture, The University of Tokyo, Tokyo (in Japanese)
Takeuchi I, Hirano R (1991) Growth and reproduction of Caprella danilevskii (Crustacea: Amphipoda) reared in the laboratory. Mar Biol 110:391–397
Takeuchi I, Hirano R (1992) Duration and size of embryos in epifaunal amphipods Caprella danilevskii Czerniavski and C. okadai Arimoto (Crustacea: Amphipoda: Caprellidea). J Exp Mar Biol Ecol 164:161–169
Taylor RB (1998) Density, biomass and productivity of animals in four subtidal rocky reef habitats: the importance of small mobile invertebrates. Mar Ecol Prog Ser 172:37–51
Valentine JF, Duffy JE (2006) The central role grazing in seagrass ecology. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 463–501
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Vizzini S, Sarà G, Michener RH, Mazzola A (2002) The role and contribution of the seagrass Posidonia oceanica (L.) Delile organic matter for secondary consumers as revealed by carbon and nitrogen stable isotope analysis. Acta Oecol 23:277–285
Wear RG, Haddon M (1987) Natural diet of the crab Ovalipes catharus (Crustacea, Portunidae) around central and northern New Zealand. Mar Ecol Prog Ser 35:39–49
Whitlatch RB (1981) Animal-sediment relationships in intertidal marine benthic habitats: determinants of deposit-feeding species diversity. J Exp Mar Biol Ecol 53:31–45
Wijnsma G, Wolff WJ, Meijboom A, Duiven P, de Vlas J (1999) Species richness and distribution of benthic tidal flat fauna of the Banc d’Arguin, Mauritania. Oceanol Acta 22:233–243
Yu OH (2002) Population ecology and production of the benthic amphipods (Curstacea) in a temperate sandy shore, southern Korea. Dessertation, Chonnam National University, Korea
Yu OH, Suh H-L, Shirayama Y (2003) Feeding ecology of three amphipod species Synchelidium lenorostralum, S. trioostegitum and Gitanopsis japonica in the surf zone of a sandy shore. Mar Ecol Prog Ser 258:189–199
Yun SG, Byun SH, Kwak SN, Huh SH (2002) Seasonal variation of caprellids (Crustacea: Amphipoda) on blades of Zostera marina bed in Kwangyang Bay, Korea. J Kor Fish Soc 35:105–109 (Korean with English abstract)
Ziegler S, Benner R (1998) Ecosystem metabolism in a subtropical seagrass dominated lagoon. Mar Ecol Prog Ser 173:1–12
Acknowledgments
This research was a part of the project titled “Long-term change of structure and function in marine ecosystems of Korea” funded by the Ministry of Land, Transport and Maritime Affairs, Korea. We thank H. J. Park and J. H. Kwak for their assistance in the field and data processing. We would like to thank three anonymous referees for reviewing our manuscript. Special thanks are given to Dr. Martin Thiel (Facultad Ciencias del Mar, Universidad Catolica del Norte, Chile) for critical review and constructive comments for our earlier draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Jeong, S.J., Suh, HL. & Kang, CK. Trophic diversity in amphipods within a temperate eelgrass ecosystem as determined by gut contents and C and N isotope analysis. Mar Biol 159, 1943–1954 (2012). https://doi.org/10.1007/s00227-012-1981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1981-y