Abstract
Japanese eels spawn mainly during June–August. The larvae (leptocephali) then drift for 3–5 months before metamorphosing into glass eels. The recruitment season generally starts in southern East Asia in November and in northern areas in April the following year, a lag of ~5 months. However, analysis of otolith daily growth rings revealed only a 1–2-month difference in the mean leptocephalus stage between southern and northern East Asian samples. Experiments and field observation indicate that glass eels may starve, lose body weight, and remain in early pigmentation stage for a few months in cold waters. The time lag in recruitment can be accounted for by a longer leptocephalus stage combined with a low temperature-driven delay to upstream migration in winter. The leptocephalus duration and oceanic currents determine the dispersal locations up to the glass eel phase, while temperatures determine the timing of upstream migration time at each location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese eel (Anguilla japonica Temminck and Schlegel, 1846) is a temperate catadromous fish with a complex lifecycle and wide distribution (Tsukamoto 1992, 2006; Tesch 2003). Previous studies using microsatellite DNA loci indicated no significant genetic differences, either spatially or temporally, among populations of Japanese eels in East Asia (Chang et al. 2007; Han et al. 2008, 2010a). Differences in habitat utilization or larval duration were not found to contribute to population structuring (Han et al. 2010b, c). These results strongly suggest the existence of a single panmictic population of Japanese eels.
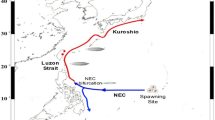
The spawning area for Japanese eels lies to the west of Mariana Island, where silver eels congregate to spawn between June and August (Tsukamoto et al. 1989, 1992; Ishikawa et al. 2001; Tsukamoto 1992, 2006, 2009; Zenimoto et al. 2009). After the fertilized eggs hatch, the larvae (leptocephali) drift from the spawning site, first in the North Equatorial Current (NEC) and then via the Kuroshio and its branch waters for several months to reach the coastal waters of East Asia. They then metamorphose into glass eels and enter the river/estuarine growth habitats mainly in Taiwan, China, Korea, and Japan (Cheng and Tzeng 1996; Tesch 2003).
We conducted interviews with glass eel fishermen and traders to gain a greater understanding of recruitment dynamics. We discerned that glass eel recruitment to estuaries occurs in the following order in East Asia. First, few Japanese glass eels are found around the northeastern coast of Luzon Island but abundant glass eels occur in Taiwan in late October, with the main fishing season being between November and February (Fig. 1). After 2–4 weeks, recruitment occurs in the Fujian Province of China and the Pacific coast of southern Japan, where the main fishing season is between December and February. In the Zhejiang and Guangdong Provinces of China, Jeju Island, and Pacific coast of central Japan, the main fishing seasons occur between January and March. Later, from February to April, the recruitments concentrate in the southern Jiangsu Province of China (around Changjiang River estuary), the southern coast of Korea, and east coast of Japan. In the northern Jiangsu Province of China and the west coast of Korea, the main fishing seasons are between March and May. The final recruitments occur at the northwestern coast of Korea and Yalu River between April and June. The start of the recruitment season therefore differs by up to 5 months between southern and northern East Asia.
Map showing the distribution of the Japanese glass eel (bold lines) and the NEC, Kuroshio, and its branch waters around the East Asian coasts. The number shows the sampling location of the glass eels in previous and present studies (listed in Table 1). The main recruitment months of the glass eel in each location in East Asia are indicated in the boxes. TW Taiwan
Despite this difference, back-calculated otolith increments of leptocephalus samples near the spawning area indicated that eels spawn within a period between June and August (Tsukamoto et al. 1989, 1992; Ishikawa et al. 2001; Tsukamoto 2009; Zenimoto et al. 2009), suggesting that the glass eels recruited during the main catch seasons in each location of East Asia would have hatched during this period. We reviewed studies of otolith daily growth rings of glass eels obtained from East Asian areas: these show that the time difference in the leptocephalus duration between Taiwan and Korea is around 1 month (105.3 ± 13.5 d for eels collected in Taiwan, vs 130.6 ± 3.3 d for eels collected in Korea: Table 1), much less than the difference in the start date of the recruitment seasons. Since the mean duration of metamorphosis from leptocephalus to glass eel is around 20–40 days, irrespective of the location (Table 1), the recruitment delay between Taiwan and Korea samples must therefore stem from variations in the duration of the postleptocephalus stage (i.e., glass eel stage). It has been found that the glass eel feeding, swimming, pigmentation, and otolith growth would be hampered under low temperature (Dou et al. 2003; Fukuda et al. 2009). It has also been found that the pigmentation of European glass eel (Anguilla anguilla) slows down in high salinity water (Briand et al. 2005). Our study aimed to test the effects of temperature and salinity on growth and pigmentation of Japanese glass eels and to clarify the mismatches in the mean age and recruitment time lag between glass eels recruited from southern and northern East Asian regions.
Materials and methods
Otolith sampling
Japanese glass eels were collected by fishermen from the Tanshui River estuary of Taiwan in February 2008 (Table 1). Sagittal otoliths (n = 45) were analyzed for daily growth increments by scanning electron microscopy (SEM) according to the previously described method (Tzeng 1990). SEM photographs were taken at TechComm, College of Life Science, National Taiwan University (Fig. 2). Faint images were excluded from the analysis. Supposed daily increments in the otoliths were counted from the first ring outside the core to the end of the metamorphosis zone. The image of otolith increments on the glass eel zone was obscure and thus was not counted.
Map showing the 4 main zones of the glass eel otolith (up-right), and a representative scanning electron micrograph illustrating daily growth increments of the sagittal otolith of a glass eel collected in the Tanshui River estuary in February 2008. C core zone, LZ leptocephalus zone, M metamorphosis zone, GZ glass eel zone
Meta-analysis of glass eel otolith studies
The ages (in days) of Japanese glass eels were derived on the basis of the presumed otolith daily increments (Tsukamoto 1989; Table 1). In some studies (Shinoda and Tsukamoto 2009; Tzeng 1990, 2003; Tzeng and Tsai 1992), 2 or 5 days were added to the number of otolith increments for the adjustment of the yolk-sac stage (Lecomte-Finiger 1992) in the core zone. These added days were deducted for consistency.
Temperature and salinity experiments
For temperature and salinity tests, glass eels collected using a boat net from the coastal waters (21°C, 34.5 psu) of Ilan, in northeastern Taiwan in December 2009, were transferred to a box with natural seawater and immediately brought to the laboratory of the Institute of Fisheries Science, Taipei, Taiwan. Twenty individuals were initially subsampled, and their total length (to the nearest 0.1 mm), body weight (to the nearest 0.001 g), and pigmentation stage were recorded. To identify the effect of salinity on the stage of pigmentation, individuals (n = 80) were divided and kept in the dark in 4 different salinity tanks (0, 17, 26, and 35 psu, 20 for each) at 18°C without feeding and salinity acclimation. The aforementioned parameters were recorded again after 1 month. To identify the effect of temperature on pigmentation stage, individuals (n = 90) were divided and kept in the dark in 4 different temperature tanks (4°C, 10°C, 15°C, and 20°C) containing seawater (35 psu) without feeding. The 4°C tank contained 30 individuals and the other 3 tanks contained 20 individuals each. Time-series sampling was conducted during the 3-month experiment period to identify changes in pigmentation status and somatic growth at different temperature conditions. Ten individuals were randomly sampled from each tank at 30 days. After 2 months, the remaining individuals in the 10°C, 15°C, and 20°C tanks were sampled, whereas only 9 individuals from the 4°C tank were randomly sampled. The remaining 10 individuals in the 4°C tank were kept for 1 month longer before measurement. The same temperature and salinity experiments were repeated for glass eels collected in the same location in January 2010. Pigmentation stage (Stages VA, VB, VIA1, VIA2, VIA3, VIA4, and VIB) was determined according to European eel criteria (Tesch 2003).
Data analysis
Data of total length and body weight were checked by Kolmogorov–Smirnov tests and were fitted the normal distribution. Differences in the total length and body weight between the experimental groups were tested using analysis of variance (ANOVA) followed by Tukey’s Honestly Significant Differences (HSD) multiple-comparison test. SPSS (ver. 12, IBM, NY USA) software was used for the statistical analysis. The differences were considered significant when P < 0.05.
Currents and temperature patterns of East Asian coastal waters
The East Asian continental shelf has relatively shallow seas and is strongly affected by monsoons and Kuroshio invasion in the winter (Chen 2009). The surface currents in the East Asian continental shelf during winter (November to March) have been modified based on Chen’s findings (2009). The graphs of monthly mean sea surface temperature (SST) of the East Asian coastal waters with a spatial resolution of 1.1 km were provided by the Remote Sensing Laboratory, National Taiwan Ocean University (http://140.121.161.31/RSL/index.e.html). The data were received from the advanced very high resolution radiometer (AVHRR) sensors on the TIROS-N series satellite of the National Oceanic and Atmospheric Administration (NOAA).
Results
Leptocephalus duration
The SEM images of the polished otoliths showed 4 main zones (McCleave 2008): core zone, leptocephalus zone, metamorphosis zone, and glass eel zone (Fig. 2). The metamorphosis zone, in which the increments become diffuse and the strontium:calcium ratio decreases sharply (Arai et al. 1997; Wang and Tzeng 1998), separates the leptocephalus zone and the glass eel zone (Fig. 2). The glass eel zone, which starts at a check and ends at the edge of the otolith, corresponds to the glass eel stage after metamorphosis (Fig. 2). In our study, the mean durations of the leptocephalus and metamorphosis stages were 118.0 ± 13.6 and 22.1 ± 2.3 days, respectively. In other studies, the mean duration of the leptocephalus stage varied between regions. In Taiwan, the leptocephalus stage lasted between 80 and 120 days, and between 120 and 140 days in the case of samples from Japan, Korea, and China (Table 1). The duration of metamorphosis of the glass eel was approximately 20–40 days irrespective of location (Table 1). The mean duration of the glass eel stage varied widely from 7 days (Kawakami et al. 1999) to >40 days (Cheng and Tzeng 1996; Table 1).
Hatching date
The data of 14 studies were analyzed to assess spawning seasons according to location. The back-calculated numbers of otolith increments of glass eels collected from November to June of the following year revealed that eel spawning time extended from April through January of the following year (Table 1). However, the estimated dates of the spawning season based on field sampling of leptocephali are from June to August. The spawning dates that were estimated from glass eel samples collected in Taiwan and the Pacific coasts of Japan lie mainly between May and September and are in good agreement with the estimates from field leptocephali samples (Table 1). Glass eel samples from northern China and later recruits, however, showed obvious inconsistencies in estimated spawning time (Table 1).
Temperature and salinity tests of glass eel
In the salinity experiments, there were no significant differences in the mean total length or body weight among the different salinity groups compared with the values in the initial group (P > 0.05 for all; Table 2). Only 1 individual died during the experimental period in the 0 psu group. The pigmentation stages progressed from stage V in the initial group to stage VI in each group after 1 month of rearing at 18°C. More than 70% of the glass eels were at stages VIA2 and VIA3 (the pigmentation occurring caudally and dorsally) in each group (Table 2). In the temperature experiments, no significant differences in mean total length or body weight were noted between the different temperature groups and the initial group after 1 month of test (P > 0.05 for all; Table 3). After 2 months, the mean total lengths in the 20°C and 15°C tanks and the mean body weight in the 20°C tank were significantly lower than the corresponding value in the initial group (Table 3). After 3 months, the mean body weight in the 4°C group was significantly lower than that in the case of the initial group (Table 3). In the 4°C group, the early pigmentation stage (VA and VB) was noted at 1 and 2 months of rearing, and 60% of the individuals were still at the early VA and VB stages even after 3 months (Table 3). Higher temperature and longer rearing times resulted in a higher percentage of individuals with advanced pigmentation stage (Table 3). Glass eels in the 4°C tank always stayed at the bottom with little activity. The higher the water temperature, the more active the eels were.
Surface currents and temperature in East Asia coastal waters in winter
Kuroshio originates in the westward-flowing NEC, turns northward east of the Philippines, and flows past Taiwan into the East China Sea. When Kuroshio impinges on the East Asian continental shelf, portions of Kuroshio branch out onto the coastal waters (Fig. 1). The mean SST images of the East Asian coastal waters in November 2008, January 2009, March 2009, and May 2009 are shown in Fig. 3. In the winter, the warm waters of Kuroshio and the cold coastal waters form the visible thermal fronts (Chen 2009). In November, the cold coastal waters invade southwardly, cooling the waters of Fujian coast and western Taiwan (Fig. 3a). In January, the mean air temperature is the lowest in East Asia (Japan Meteorological Agency). The SST ranges are as follows: at all coastal waters of Korea and northern coast of the Jiangsu Province of China, 0–5°C; at the southern coast of the Zhejiang Province of China and in the Japan Sea coast, 10–15°C; and at the Pacific Ocean coast of Japan, 10–20°C (Fig. 3b). In March, the northeastern monsoon winds start to withdraw and the mean SST around the Changjiang River estuary and south coast of Korea increases to >8°C (Fig. 3c). In May, the mean SST in all coastal waters of East Asia is >8°C (Fig. 3d).
Discussion
Japanese eels spawn in restricted areas (12–17°N and 141–143°E) during a specific time span (from June through August) (Ishikawa et al. 2001; Tsukamoto 1992, 2009). For 3 months, the larvae are passively transported via the NEC to east Philippines (Zenimoto et al. 2009), and they later enter Kuroshio and its branch waters toward their habitats. In this study, we find that (1) the start of the recruitment season for glass eels differs by up to 5 months between southern and northern East Asia; (2) analysis of otolith daily growth rings revealed a 1–2-month difference in the mean leptocephalus stage between southern and northern East Asian samples; (3) the glass eels could remain in the early pigmentation stage, while losing some body weight, under low seawater temperatures; and (4) during winter, the mean SST in the northern coastal waters/estuaries of China and Korea is usually <5°C. We hypothesize that the recruitment time lag of the glass eel to northern East Asia could be due to a longer leptocephalus stage (1–2 months) combined with the low water temperature-driven delay (1–3 months) to upstream migration.
Factors affecting recruitment of glass eels
Metamorphosis from a leptocephalus into a glass eel in seawater is associated with a transition from “pelagic” to “benthic” behavior (Jegstrup and Rosenkilde 2003; Tesch 2003). Once metamorphosed, they lose their willow leaf body shape that is suitable for long-distance drifting on oceanic currents. Glass eels show benthic sheltering behavior during the day. They colonize the coastal and estuarine waters by active swimming and by tidal stream transport at night (McCleave and Wippelhauser 1987; Tesch 2003). In this study, temperature, not salinity, had a strong effect on glass eel pigmentation. In the winter, the mean SST in the coastal waters of Korea and northern China is usually <5°C, a temperature that would inhibit glass eel feeding, swimming, and pigmentation. Temperature-dependent pigmentation development is found not only in Japanese eels (Dou et al. 2003; Fukuda et al. 2009) but also in European eels (Bertin 1956; Boëtius and Boëtius 1989; Briand et al. 2005). In March, the mean SST around the Changjiang River estuary and the south coasts of Korea usually rises above 8–10°C, a temperature at which glass eels become active and start their upstream migration (Zhang et al. 1981; this study). In May, the mean SST in the coasts of the northern Jiangsu Province to the Yalu River estuary and that of the west coast of Korea is >8–10°C, which is in complete agreement with the real recruitment seasons of Japanese glass eels in these locations. Thus, the considerable time lag (3–5 months) in the main recruitment season between Taiwan and northern China/Korea suggests that once metamorphosed, the glass eels may stay offshore and remain in their early pigmentation stage for a few months before starting the upstream migration. The low temperature-driven pigmentation retardation also explains that glass eels caught earlier during the main recruitment season show early pigmentation stages of VA and VB, irrespective of their location in East Asia (Han et al. 2010a).
In the field samples, the mean numbers of glass eel per kilogram was around 5,000–5,500 in Taiwan and Japan, around 6,500 individuals/kg in Zhejiang, Shanghai and the southern coast of Korea, and around 7,000 individuals/kg in the northern areas of the Jiangsu Province. There were approximately 7,500–8,500 individuals/kg in the Yalu River estuary (Chen et al., personal communication). The later the recruitment occurs, the smaller the mean individual size. In the present study, after 3 months of experiment without feeding, the mean body weight in the 4°C group was significantly lower than that in the initial group. This supports the hypothesis that the wild glass eels in cold waters may stay offshore and lose body weight due to starvation for a few months. Upstream migration occurs only when the water temperature rises above 8–10°C. In European eels, the recruitment time is earlier in France/Spain than in North Europe (Tesch 2003). The recruitment dynamics of European glass eels may be similar to those of Japanese glass eels.
Duration and timing of larval events
The back-calculated timing of spawning (as calculated from otolith increment studies) does not agree, in all cases, with field observations of the spawning period (Table 1). This apparent mismatch in the spawning times estimated from glass eel otoliths and from leptocephalus sampling has also been reported for the American eel (A. rostrata) and European eel (A. anguilla; McCleave 2008). This has two explanations: either eels spawn outside of the periods indicated by field studies or the age of the glass eels in some otolith increment studies has been underestimated.
The spawning dates estimated from otolith analyses of glass eels in Taiwan and the Pacific Ocean coast of Japan occur between May and September and are in good agreement with the observed spawning dates. Since the leptocephali that metamorphose near the coastal waters of Taiwan and the Pacific Ocean site of Japan in winter could quickly approach estuaries due to the suitable upstream migration temperature, it is likely that the estimation of glass eel age at these locations is quite accurate. However, the later recruitments of glass eels from northern China and Korea, with a longer glass eel stage, may be associated with significantly underestimated postleptocephalus duration, which give rise to marked delay in the estimated spawning time.
According to our meta-analysis, the mean leptocephalus duration of the glass eels from Taiwan was in the range of 80–120 days, whereas that of those from China, Japan, and Korea was approximately 120–140 days, i.e., a difference of 30–60 days. This may account for the mean time lag noted during the transport of leptocephali from Taiwan to Japan via Kuroshio (Cheng and Tzeng 1996) and to Korea and China, possibly by the branched currents of the Kuroshio. However, since the mean time lag in the main recruitment seasons of the glass eel between Taiwan and northern China/Korea is 3–5 months, the difference in the glass eel age between Taiwan and northern China/Korea must therefore stem from variations of up to 3 months in the duration of the glass eel stage before recruitment. Despite this, the reported variations in the glass eel stages range only between 7 and 40 days, considerably less than the expected difference. One factor to take into account is that otolith growth in the glass eel stage has been reported to be affected by temperature. In fact, it ceases at <10°C under experimental conditions (Umezawa and Tsukamoto 1991; Fukuda et al. 2009). Furthermore, starvation of the glass eel (Fukuda et al. 2009) and possible reabsorption of marginal otoliths (Cieri and McCleave 2000) may also lead to underestimates of the duration of the glass eel stage. Furthermore, the daily increments at glass eel zone of the otolith are often obscure. We argue therefore that the discrepancies in the estimated date of the spawning period are probably due to underestimation of the otolith increments in the glass eel zone brought about by inconsistencies in otolith growth rate and structure.
Japanese eel stocks are declining markedly, and the annual glass eel catch has varied considerably over the past few decades (Dekker 2004; Han et al. 2009). Therefore, understanding the dispersal dynamics of the Japanese glass eel is important from the fisheries management perspective. Since the leptocephali are passively transported by oceanic currents, and glass eel recruitment in Taiwan begins one to several months earlier than in other areas, the dynamics of glass eel recruitment in Taiwan may serve as a useful indicator of the subsequent glass eel recruitment in other locations.
References
Arai T, Otake T, Tsukamoto K (1997) Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 161:17–22
Bertin L (1956) Eels: a biological study. Cleaver-Hume Press Ltd., London
Boëtius I, Boëtius J (1989) Ascending elvers, Anguilla anguilla, from five European localities. Analyses of pigmentation stages, condition, chemical composition and energy reserves. Dana 7:1–12
Briand C, Fatin D, Cicotti E, Lambert P (2005) A stage structure model to predict the effect of temperature and salinity on glass eel Anguilla anguilla pigmentation development. J Fish Biol 67:993–1009
Chang KC, Han YS, Tzeng WN (2007) Population genetic structure among intra-annual arrival waves of the Japanese eel Anguilla japonica in northern Taiwan. Zool Stud 46:583–590
Chen CTA (2009) Chemical and physical fronts in the Bohai, Yellow and East China seas. J Mar Sys 78:394–410
Cheng PW, Tzeng WN (1996) Timing of metamorphosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 131:87–96
Cieri MD, McCleave JD (2000) Validation of daily otolith increments in glass-phase American eels Anguilla rostrata (Lesueur) during estuarine residency. J Exp Mar Biol Ecol 257:219–227
Dekker W (2004) Slipping through our hand. Population dynamics of the European eel. PhD dissertation. Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam
Dou S, Miller MJ, Tsukamoto K (2003) Growth, pigmentation and activity of juvenile Japanese eels in relation to temperature and fish size. J Fish Biol 63:152–165
Fukuda N, Kuroki M, Shinoda A, Yamada Y, Okamura A, Aoyama J, Tsukamoto K (2009) Influence of water temperature and feeding regime on otolith growth in Anguilla japonica glass eels and elvers: does otolith growth cease at low temperatures? J Fish Biol 74:1915–1933
Han YS, Sun YL, Liao YF, Shen KN, Liao IC, Tzeng WN (2008) Temporal analysis of population genetic composition in the overexploited Japanese eel Anguilla japonica. Mar Biol 155:613–621
Han YS, Tzeng WN, Liao IC (2009) Time series analysis of Taiwanese catch data of Japanese glass eels Anguilla japonica: possible effects of the reproductive cycle and El Niño events. Zool Stud 48:632–639
Han YS, Hung CL, Liao YF, Tzeng WN (2010a) Population genetic structure of the Japanese eel Anguilla japonica: panmixia in spatial and temporal scales. Mar Ecol Prog Ser 41:221–232
Han YS, Hung CL, Iizuka Y, Chang HC, Chang BJ, Ho HC, Shiao JC (2010b) Does larval duration contribute to population genetic isolation of the Japanese eel Anguilla japonica? Fish Manag Ecol 17:366–368
Han YS, Iizuka Y, Tzeng WN (2010c) Variable habitat use by Japanese eel: a behavioral plasticity or a heritable character? Zool Stud 49:392–397
Ishikawa S, Suzuki K, Inagaki T, Watanabe S, Kimura Y, Okamura A, Otake T, Mochioka N, Suzuki Y, Hasumoto H, Oya M, Miller MJ, Lee TW, Fricke H, Tsukamoto K (2001) Spawning time and place of the Japanese eel Anguilla japonica in the North Equatorial Current of the western North Pacific Ocean. Fish Sci 67:1097–1103
Jegstrup IM, Rosenkilde P (2003) Regulation of post-larval development in the European eel: thyroid hormone level, progress of pigmentation and changes in behaviour. J Fish Biol 63:168–175
Kawakami Y, Mochioka N, Nakazono A (1999) Immigration patterns of glass eels Anguilla japonica entering river in northern Kyushu, Japan. Bull Mar Sci 64:315–327
Lecomte-Finiger R (1992) Growth history and age at recruitment of European glass eels (Anguilla anguilla) as revealed by otolith microstructure. Mar Biol 114:205–210
Li CH (1998) Interrelationship among daily age, body length, birth date and sampling location at recruitment of Anguilla japonica elvers in the Chinese coast. Acta Oceanologica Sinica 20:107–113
Li B, Xie YH, Lu YX (1992) The study on daily otolith rings of young Japanese eel Anguilla japonica. Zool Res 13:201–207
McCleave JD (2008) Contrasts between spawning times of Anguilla species estimated from larval sampling at sea and from otolith analysis of recruiting glass eels. Mar Biol 155:249–262
McCleave JD, Wippelhauser GS (1987) Behavioural aspects of selective tidal stream transport in juvenile American eels. Am Fish Soc Symp 1:138–150
Moon HT (2002) The early life history of eel Anguilla japonica determined by otolith microstructure and catch data of glass eels. PhD Thesis, Chungnam National University
Shinoda A, Tsukamoto K (2009) The early life history and migration of Japanese eel larvae. Am Fish Soc Symp 69:879–881
Tabeta O, Tanaka K, Yamada J, Tzeng WN (1987) Aspects of the early life history of the Japanese eel Anguilla japonica determined from otolith microstructure. Nippon Suisan Gakkaishi 53:1727–1734
Tesch FW (2003) The eel. Blackwell Science, Oxford, pp 100–103
Tsukamoto K (1989) Otolith daily increments in the Japanese eel. Nippon Suisan Gakkaishi 55:1017–1022
Tsukamoto K (1990) Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. J Fish Biol 36:659–671
Tsukamoto K (1992) Discovery of the spawning area for Japanese eel. Nature 356:789–791
Tsukamoto K (2006) Spawning of eels near a seamount: tiny transparent larvae of the Japanese eel collected in the open ocean reveal a strategic spawning site. Nature 493:929
Tsukamoto K (2009) Oceanic migration and spawning of anguillid eels. J Fish Biol 74:1833–1852
Tsukamoto K, Umezawa A, Tabeta O, Mochioka N, Kajihara T (1989) Age and birth date of Anguilla japonica leptocephali collected in western north Pacific in September 1986. Nippon Suisan Gakkaishi 55:1023–1028
Tsukamoto K, Umezawa A, Ozawa T (1992) Age and growth of Anguilla japonica leptocephali collected in western north Pacific in July 1990. Nippon Suisan Gakkaishi 58:457–459
Tzeng WN (1990) Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan estuary as inferred from otolith growth increments. Mar Biol 107:75–81
Tzeng WN (2003) The processes of onshore migration of the Japanese eel Anguilla japonica as revealed by otolith microstructure. In: Aida K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 181–190
Tzeng WN, Tsai YS (1992) Otolith microstructure and daily age of Anguilla japonica Temminck and Schlegel elvers from the estuaries of Taiwan with reference to unit stock and larval migration. J Fish Biol 40:845–857
Umezawa A, Tsukamoto K (1990) Age and birth date of the glass eel, Anguilla japonica, collected in Taiwan. Nippon Suisan Gakkaishi 56:1199–1202
Umezawa A, Tsukamoto K (1991) Factors influencing otolith increment formation in Japanese eel, Anguilla japonica T. and S., elvers. J Fish Biol 39:211–223
Wang CH, Tzeng WN (1998) Interpretation of geographic variation in size of American eel Anguilla rostrata elvers on the Atlantic coast of North America using their life history and otolith ageing. Mar Ecol Prog Ser 168:35–43
Xie YH, Li B, Fu LJ, Tang ZP, Xie H (1997) Population structure of Anguilla japonica elvers from the estuaries of Chinese coast. J Fish Sci China 4:33–38
Zenimoto K, Kitagawa T, Miyazaki S, Sasai Y, Sasaki H, Kimura S (2009) The effects of seasonal and interannual variability of oceanic structure in the western Pacific North Equatorial Current on larval transport of the Japanese eel Anguilla japonica. J Fish Biol 74:1878–1890
Zhang YW, Xiao CY, Zhang SY (1981) Upstream migration and distribution of the anguillid eels in China. Sinozoologia 1:117–121 (In Chinese)
Acknowledgments
This study was funded by the National Taiwan University (98R0314); National Science Council of the Executive Yuan, Taiwan (NSC 99-2923-B-002-005-MY3 and NSC 99-2313-B-002-021-MY3). I want to thank the eel traders Mr. WC Chen, JH Yao, and YF Chen for providing information about glass eels, and the students and assistants of the Institute of Fisheries Science, National Taiwan University, for their help with eel sampling. I would also like to thank two anonymous reviewers and the editor for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Righton.
Rights and permissions
About this article
Cite this article
Han, YS. Temperature-dependent recruitment delay of the Japanese glass eel Anguilla japonica in East Asia. Mar Biol 158, 2349–2358 (2011). https://doi.org/10.1007/s00227-011-1739-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1739-y