Abstract
This study examines the degree of isolation among 0-group Atlantic cod in a region where a number of resident adult congregations have been found. The location of key nursery areas in Scottish coastal waters was identified from trawl surveys of settled 0-group cod around the Scottish coast. Segregation among four of these key areas; within the Clyde, Shetland Isles, Moray Firth and Buchan was investigated using otolith elemental composition. Isotope concentrations in the otoliths of cod were analysed by solution-based inductively coupled plasma mass spectrometry of the whole otolith. Discriminant analyses showed a clear separation between nursery areas based on the concentrations of three elements (manganese, barium and magnesium). The concentration of these elements was not affected by the age or size of individuals. Classification accuracy exceeded 90% for all four sites. Evidence of temporal persistence in the relative spatial differences in elemental concentrations between two sites for 2 year-classes was found. The results suggest limited exchange between sites during some period of the early life history. The significance of this limited exchange is discussed in relation to recent evidence for adult fidelity and the utility of closed areas for protecting nursery grounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whilst the use of closed areas to conserve temperate marine fish stocks has not been widely accepted as an effective alternative to present fisheries management regimes, there is general agreement that protection of areas where key life history stages congregate could be a valuable management tool. For example, the policy to close two spawning areas of haddock on Georges Bank was widely supported by fishers as a viable management measure with instrinsic biological value (Halliday 1988). Similarly, the protection of nursery areas for juveniles has received general support and achieved some success (Pickett et al. 2004). However, the reduction in fishing mortality arising from the closure of a specific area will depend on the level of site fidelity of fish throughout the period they are vulnerable to fishing gears. Hence, the utility of area closures is largely dependent on the degree to which fish remain resident within the area proposed (Polunin 2002).
Restoration of Atlantic cod stocks (Gadus morhua L.) is a priority throughout much of the species range (FAO 1995). This species exhibits a diversity of migratory behaviours, ranging from site attached residents to wide ranging dispersers (Robichaud and Rose 2004). Evidence from genetic studies indicates that sedentary residents and those that home to their spawning site can represent more or less reproductively isolated groups of fish (Ruzzante et al. 2000; Hutchinson et al. 2001; Knutsen et al. 2003). As has been found in many other coastal areas (Godø and Sunnanå 1984; Jakobsen 1987; Lawson and Rose 2000; Ruzzante et al. 1997, 2000), a number of relatively discrete resident spawning groups of Atlantic cod have been identified in the coastal waters of the North Sea and west of Scotland (Neat et al. 2006; Wright et al. 2006). However, as natal philopatry is not always found in conjunction with spawning fidelity, it is important to understand the level of segregation of recruits as well as adults (Forrester et al. 2002; Krizter and Sale 2004). As with most other regions, there is no reliable information on the nursery areas, which supply resident spawning groups in the North Sea and west of Scotland or the level of exchange of juveniles between nursery areas. In this study, nursery areas were defined as areas of habitat which supported significantly higher juvenile densities than other areas. Passive transport of early life history stages could lead to a substantial advection of cod eggs and larvae from Shetland south to the Scottish east coast (Heath and Gallego 1997). However, it is not known whether there is an extensive interchange of early settled juveniles. Whilst studies of 0-group cod within bays and sea lochs indicate that they can exhibit a high degree of site fidelity (Hawkins et al. 1985; Grant and Brown 1998) there does appear to be the potential for wider dispersal following settlement in more open nursery areas (Lough et al. 1989). Therefore, it is important to examine the geographical isolation and movements of settled 0-group cod among nursery areas in the coastal region.
Although tag-recapture studies have been conducted on 0-group cod in partially enclosed water masses, their small size, high rate of mortality and sample numbers needed, makes this approach unrealistic for large-scale studies. Therefore, alternative methods for determining the degree of inter-mixing would be desirable. Whilst genetic differentiation should form the basis for any inferences concerning population segregation, low levels of gene flow may prevent the detection of genetic differences, even when inter-mixing is sufficiently low to treat groups as distinct at time scales relevant to fisheries management (Begg and Waldman 1999; Kinlan and Gaines 2003; Palumbi 2003). Thus, non-genetic means of differentiation can play an important role in identifying populations under such circumstances (Brooke 1981; Swain et al. 2004).
Otolith elemental composition is increasingly being used as an aid to stock identification (Campana 1999; Thresher 1999; Campana and Thorrold 2001). This approach is based on two assumptions; firstly, that the elemental composition of the otolith reflects the physico-chemical characteristics of the environment to which the fish was exposed (Secor et al. 1995; Bath et al. 2000) and secondly, that the material deposited on the otolith is metabolically inert after deposition, and is not susceptible to resorption or remobilisation (Mugiya et al. 1991). Hence, otolith elemental concentrations may represent elemental fingerprints that reflect differences in the chemical composition of the individuals’ habitat. Otolith elemental fingerprint analysis has been widely used to detect differences between groups of fish (Thorrold et al. 1998a; Edmonds et al. 1991; Rooker et al. 2001) including Atlantic cod (Campana et al. 1994). However, elemental compositions can vary with size and age and may not be temporally stable between year-classes (Milton et al. 1997; Campana et al. 2000). Key therefore, to the present study, was to account for potential effects of size and age and sample over more than a single year.
The aim of the paper was to examine the degree of segregation among nursery areas of Atlantic cod in Scottish waters, by investigating the otolith elemental signature from fish collected from four different areas of high 0-group density. We also aim to examine the issue of temporal stability within this signature, by investigating the elemental properties of otoliths from two areas across 2 years of sampling. Such spatial differentiation among juveniles is necessary before future research designed to identify the origins of adult cod (based on the comparison of the juvenile portion of the adult otolith with the juvenile signature) can be undertaken. The results are considered in relation to recent evidence for resident spawning groups in cod.
Materials and methods
Juvenile distributions
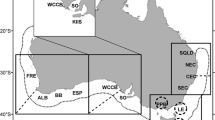
Information on the distribution of 0-group cod was collected by chartered fishing vessels from inshore surveys conducted on the west and east coasts of Scotland (including the Shetland Isles) in November–December 2001 and in August–September 2002–2004. The 2001 inshore surveys were conducted in partnership with the fishing industry, and used a variety of gears including both whitefish and Nephrops (Nephrops norvegicus) trawls. The 2002–2004 inshore surveys used a BT158 bottom trawl to sample at least two locations per ICES statistical rectangle (1° longitude × 0.5° latitude). Data from the Scottish third quarter surveys of the North Sea and fourth quarter survey of ICES region VIa were used to provide information for offshore areas. These offshore surveys only sample 1–2 trawl stations per ICES statistical rectangle. The Scottish 3rd quarter surveys of the North Sea and 4th quarter survey of ICES region VIa west coast use the GOV, a high-headline bottom trawl fitted with a 20 mm cod end liner.
The total length of specimens was measured to the nearest centimeter. Numbers of juveniles caught smaller than the minimum length of 1-group (20 cm TL) were raised to a swept volume. The swept volume (V) for each trawl was calculated based on:
From this, the number of 0-group cod m−3 of seabed was calculated. As data were collected over a period of several weeks numbers in the swept volume were inflated to represent density on a single survey date. The ICES estimate of natural mortality (M) for 0-group (0.8) was used to inflate or reduce numbers according to:
where Ne is the estimated number of 0-group on the to 31 August, Nc is the number of 0-group caught and t is the number of days from the 31 August. Due to the different scales of sampling for all surveys and years, densities from the inshore surveys (locations within 1° longitude of the coastline) were averaged into a quarter ICES grid resolution (15 × 15 nm, or 0.5° longitude × 0.25° latitude) whereas the more offshore locations with lower sampling intensity were averaged to one ICES grid resolution. Both grids were then overlayed onto the same quarter grid scale using ARC GIS. Since we had no information on juvenile vertical distribution and for the purposes of presentation, numbers per swept volume were expressed for a height of 1 m and an area of a km2.
Juvenile sample collection
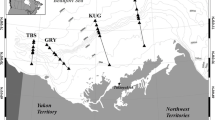
Four candidate nursery areas to the west (Clyde), north (Shetland) and east (Moray Firth and Buchan) of Scotland were chosen for sampling, reflecting significant regions of demersal juvenile distribution (Fig. 1). The main sampling period was August and September 2003 when 0-group cod were taken from single locations in inshore waters around Shetland, the Moray Firth and Buchan area, onboard FRV Clupea. Further sampling was conducted in the Clyde area onboard the RV Aplysia of the University Marine Biological Station Millport (UMBSM). Juvenile cod collected in the Clyde and Shetland in November and December 2001 provided the opportunity for a temporal comparison of elemental composition in these areas. In each sample, information on total fish length (±1 mm) and total and eviscerated wet weight (±0.1 g) were collected. Cod sagittae were removed with plastic forceps and preserved dry either in paper packets (2001) or plastic microtubes (2003). Subsequent analysis was limited to 0-group specimens less than 26 cm total length (mean 14.3 cm); see Table 1.
Elemental analysis
In preparation for elemental analysis, sagittal otoliths were placed in acid washed vials filled with 18 mΩ doubly deionized water, and sonicated for a few minutes. Any remaining adhering tissue was removed with acid washed ceramic tipped forceps. The otoliths then underwent a series of sonification and rinsing steps, using deionized water throughout. Otoliths were air-dried in their caps under a Class 100 laminar flow hood before being weighed to the nearest 0.01 mg and re-stored within their vials. All ICP-MS work was conducted by the Center of Chemical Analysis (IceTec) in Iceland.
Otoliths were dissolved with HNO3 (final concentration ∼1%) in a microwave oven to ensure complete dissolution of the otolith matrix. As solution strength affects the measurement, the samples were matrix matched by diluting the samples to different volumes of solution based on weight. In this way, the samples were diluted to a strength with less than 10% variation (overall ∼4% calculated as standard deviation). From these variable volume solutions a final dilution of the samples for measurement was made.
Ca and Sr were analysed by ICP Spectro optical spectrophotometer (Spectroflame ICP Model D, Spectro Analytical Instruments), and a suite of nine elements (14 isotopes) were analysed using a LECO Renaissance ICP time-of-flight mass spectrometer (Model ETP AF833H, incorporating an electron multiplier detector and using Argon as the carrier gas). When more than one isotope was available for the same element, those with least interference were used. A total of 10 reagent blanks were processed (and analysed) concurrently with the samples for blank corrections and to calculate limits of detection. Measurement accuracy and precision was monitored by measuring a NIES certified reference material No.22 and an in-house MC2 otolith reference material (derived from Icelandic cod otoliths) regularly throughout the runs. Linear regression correction was used to compensate for dynamic drift during a run, based on blank and matrix-matched internal standard (69Ga, 115In and 140Ce) measurements.
Precision and accuracy for Ca and Sr deviated by <4% from the average value of determination of the NIES certified sample throughout the measurement. The mean concentration for most isotopes greatly exceeded (>10×) the LOD, except for 63Cu, 7Li and 66Zn. Estimates of precision, measured as the average standard deviation in five determinations of MC2 laboratory reference sample concentrations, suggested a low deviation for most elements, ranging from 1.7–9%. Precision was above 10% for Cu, Li and Zn. The accuracy in terms of absolute deviation from the MC2 laboratory reference sample was <7.4% for 138Ba, 25Mg and 86Sr, <21% for 7Li, 55Mn and 63Cu and >50% for 66Zn. Useable data was thus obtained for four elements, based on measurements of the isotopes 138Ba, 25Mg, 55Mn and 86Sr.
Statistical analysis
Initial inspection of the relationship between juvenile density and depth indicated a non-linear trend and so local regression (loess) with a span selected by cross validation was applied to the survey data. This nonparametric smoothing technique is a generalization of running means, which gets a predicted value at each point by fitting a weighted linear regression. The span controls the speed with which the influence of points decreases with distance from the point of interest. Data were first ln transformed to reduce the effect of large outliers on the fit.
Isotope concentrations were tested for the assumption of normality and homogeneity of variance using the Kolmogorov–Smirnov test for goodness of fit, and the Hartley Fmax test for homogeneity of variance (Sokal and Rolf 1995). Variables that did not satisfy the criteria were ln transformed prior to further analysis. To determine whether there were significant differences among areas for multi-element concentrations (Ba, Mn and Mg) a multivariate analysis of variance (MANOVA) was used with area as the independent variable. Assuming samples were found to be grouped by geographical area, we were interested in determining which of the elemental variables contributed most to this grouping. A technique frequently used for such problems is discriminant analysis (DA) (Huberty 1994; Campana 1999). DA applies a dimension reduction on the data similar to principal component analysis, such that groups are separated as much as possible. Furthermore, it allows the response variables that contribute the most to this separation to be identified. A backward selection procedure was used to find which of the elements provided the highest discrimination between samples in the four areas based on the decrease in the total sum of all Mahalanobis distances. DA calculates the percent of misclassified observations in each given group. This number tends to be optimistic because the classified data are the same as used to build the classification function. A cross-validation procedure was therefore applied in which each observation was omitted one at a time and the classification function recalculated using the remaining data, before classifying the omitted observation. The absolute error of the classification function was estimated as the difference between the actual contribution of juveniles to the total otolith chemistry data and the estimated mean contribution. One thousand simulations of the element data were used to estimate the variability of the classification estimator. The procedure used for estimating this variability was bootstrapping the data with replacement (see Millar 1990).
As inter-annual variation in water chemistry can affect the chemical composition of otoliths (Milton et al. 1997; Campana et al. 2000), temporal persistence in otolith elemental composition was considered from a comparison between the elemental signature for two sites (Shetland and the Clyde) in 2 years (2001 and 2003) using a two-way MANOVA with year and area as independent variables and Ba, Mn and Mg as dependent variables. All analyses were conducted using S-PLUS v 6.2 (Insightful Corp.).
Otolith microstructure analysis
As the study used whole otoliths it was important to consider the possible confounding effects of size and/or age on otolith chemistry results. The daily age of individuals (N = 119) was determined from the lapilli otoliths (Table 1). Otoliths were prepared from a representative sub-sample of the length-measured samples collected in 2003 from the four main nursery areas around the coast of Scotland: Moray Firth, Buchan, Shetland and the Clyde. Lapilli were mounted in thermosetting plastic and ground on both sides using silicon carbide paper (grit 2,500), cleared in immersion oil and daily increments were counted along the axis demonstrating the most unambiguous incremental sequence, from primordium to the outer edge. Increment counts were repeated at least three times, and the median value was used to represent age, thus reducing the influence of single aberrant counts.
Results
Distribution of juvenile cod
The density distribution of 0-group cod in recent years (Fig. 1) indicates that 0-group cod were generally scarce around much of the Scottish coast. The median density for the region was only 10 cod.km−2 (Table 2). All densities exceeding this value occurred within 60 km of the coast, and occupied only 26.3% of the total area swept and also account for approximately 97% of all 0-group cod. Substantially elevated densities of >100 cod.km−2 were only found within the Clyde, Moray Firth, and isolated sites off Mull in the Minch, west of Scotland, Shetland and St Andrews Bay, east of Scotland. Densities >100 cod.km−2 comprised just 4.1% of the surveyed region, but around 77% of all 0-group caught. Concentrations >1,000 cod.km−2 were restricted to at most two of the quarter ICES statistical rectangles. Presence in tows was related to depth with around 60% of tows in depths <90 m containing 0-group cod compared to less than 10% in depths >180 m. In the sites where cod were present, density was negatively related to depth from 11 to 175 m (LOESS smoother, r2 = 0.3; n = 463; P < 0.01; Fig. 2). Differences in density may also have been related to exposure. Exposed coastlines were generally found to be areas of low juvenile abundance, while several well sheltered areas (especially around Shetland) were found to have high juvenile abundance. Thus, from all available data, juvenile cod were largely located in very discrete concentrations around the Scottish coast.
Variability in otolith chemistry between nursery grounds
No significant interaction between isotope concentration and length or daily age were found at the P < 0.05 significance level. Quadratic discriminant analysis (QDA) was used to test for differences in otolith chemistry between nursery areas, since the assumption of equality of covariance matrices in linear discriminant analysis was not met (χ2 18 = 166.1; P < 0.001; based on final three elements used). An initial QDA on all elements indicated that there was a significant discrimination along the first and second axes (canoconical correlations 0.95, 0.71; P < 0.001). The backward selection procedure was continued until the hypothesis test indicated that there was no further significant discrimination along the second axis. The backward stepwise discriminant analysis indicated that the elements Mn and then Ba contributed most to group separation. During the backward selection procedure 86Sr was deselected. The hypothesis test indicated that no more variables should be omitted or else no significant discrimination (at the 0.1 significance level) along the second axis could be made. The estimated discriminant scores along the first two axes are presented in Fig. 3. The first two series of discriminant scores explained 90.5 and 9.4% of the total variance, respectively. There was a clear discrimination between Clyde, Shetland and Moray Firth (1,3,6) along the first axis and between samples from Buchan and all remaining areas along the second axis. The percentage of samples correctly assigned to their groups was 92, 98, 98 and 94% for Moray Firth, Shetland, Clyde and Buchan, respectively (Table 3). The overall percentage assignment only declined by 1.5% when cross validation was applied. The bootstrapped estimate of contribution by sample area indicates that the classification estimator provided an accurate assignment (Table 4). The estimates of percentage contribution from the different areas ranged from 24.1 to 25.7% and thus were similar to the 25% from each sampled area. This corresponds to an estimated error rate of ≤2%.
The Clyde and Shetland otoliths were characterised by having the highest and lowest levels of Mn, respectively. The Moray Firth and Buchan otoliths were characterised by having the highest and lowest levels of Ba, respectively. Whilst there were virtually no classification errors, those that occurred tended to be associated with adjacent sample areas. Evidence for temporal persistence in otolith chemistry between the two areas that were sampled in both years (Shetland and the Clyde) was found (Fig. 4). There was no overlap in the concentration of Mn between the two regions in either year with higher concentrations in the Clyde. Similarly, Ba and Mg concentrations significantly differed between the two areas in both years (MANOVA; P < 0.01). However, there were significant differences between the 2 years for Ba and Mg (MANOVA; P < 0.001) but not Mn (MANOVA; P = 0.124).
Discussion
Habitats that support high juvenile densities, demonstrate high growth and survival rates and contribute disproportionally to the production of adult recruits have often been referred to as nurseries (see Beck et al. 2001 for review). The surveys conducted for this study demonstrate that whilst 0-group cod occur extensively over much of the west Scottish shelf region and northern North Sea, the majority are confined to a few small coastal areas. A similar patchy distribution of juvenile cod have been reported in inshore waters off Norway (Olsen and Soldal 1989), Newfoundland (Dalley and Anderson 1997; Grant and Brown 1998), Nova Scotia (Tupper and Boutilier 1995), Sweden (Pihl and Wennhage 2002) and the southern North Sea (Riley and Parnell 1984). Inshore studies off the west of Scotland have previously suggested that areas such as bays and lochs may provide important nursery grounds for cod, since reported juvenile densities tend to be very high (Hawkins et al. 1985, Kamenos et al. 2004). However, by mapping an entire stock region, the present study is the first to confirm that the shallow (depths < 20 m) and near shore areas are the most important areas for cod. The high densities in these areas were more than two orders of magnitude higher than the average and as such these could be defined as nursery areas (see Beck et al. 2001). Moreover, the estimated abundances in the inshore areas are probably underestimated by our surveys, as the nature of potentially important habitat (e.g., weed covered gravel, eelgrass and stones; Lough et al. 1989; Tupper and Boutilier 1995) are often inaccessible to bottom trawl surveys.
The distribution of Atlantic cod spawning grounds is known to extend over much of the region investigated in this study, with concentrations off Shetland, the Butt of Lewis, in the Minch and the Clyde (Hislop 1986; Wright et al. 2006). The Moray Firth and other eastern coastal areas of Scotland were reported to be spawning grounds in the mid-1950s (Raitt 1967). However, egg surveys indicate that spawning became more localised by 1992 (Heath et al. 1994) and virtually absent by 2004 (Fox et al. 2005). Tag-recapture experiments in the Clyde, Moray Firth and the Minch conducted in the 1960s and 1970s provides evidence of year round residency of adult cod in these regions (Wright et al. 2006). Similarly, Neat et al. (2006) found evidence of residency in cod in Shetland coastal waters based on data storage tag results. Many of the high density areas of 0-group cod found in the present study occur within the regions of resident adult cod suggesting that there may be a link between the resident spawning areas and these nursery areas.
Elements such as Ca, Mg, Sr, Mn and Ba have been routinely identified as important in studies of stock differentiation (Thorrold et al. 1998b; Thresher 1999; Gillanders 2002; Bergenius 2005) whilst elements under strong physiological regulation (e.g., Na, K, S, P and Cl) are rarely of value (Thresher et al. 1994; Proctor et al. 1995). The present study obtained a high classification accuracy (92–94%) using only three elements; Mn, Mg and Ba. Water chemistry, temperature, diet, metabolic rates and genetics may all generate variability in otolith chemistry (Campana 1999; Thresher 1999). For many elements, geographical variation in water concentration is typical, with variability in coastal and inland waters generally much larger than that of the open ocean (Balls et al. 1993). Marine concentrations of Ba tend to increase with depth (IPCS 1990), which is consistent with our findings, as the highest concentrations of otolith Ba originated from the deepest site sampled. However, concentrations of Ba in otoliths have also been found to be influenced by temperature and salinity, in addition to ambient elemental concentrations (e.g., Elsdon and Gillanders 2002, 2003; Martin et al. 2004). Concentrations of other metals increase in inshore areas, with generally inverse relationships between metal concentration and salinity (Balls et al. 1993). The variation in Mn levels between our studied nursery areas may reflect such differences in water salinity. Particulates from inshore areas have been found to be markedly enriched with Mn relative to adjacent offshore areas (Balls 1986), and are thought to reflect areas of lower salinity caused by freshwater inflow from surrounding rivers. Regardless of cause, the marked regional variation in elemental concentration between sites provides a reliable indicator of the different nursery areas.
The few studies that have examined temporal stability in otolith chemistry indicate that stock-specific signatures can vary among years (Patterson et al. 1999; Campana et al. 2000). Nevertheless, the present study’s comparison has indicated a persistent regional signature in otolith chemistry between 2001 and 2003. Although the absolute elemental concentration of Ba differed between the 2 years analysed, the fact that there was no overlap in the ranges of both Mn and Ba between areas suggests they could provide a consistent spatial marker of origin for cod in Scottish waters.
Atlantic cod are known to settle from a pelagic phase in early summer (Tupper and Boutilier 1995), with settlement peaking in July in western Scottish waters (Magill and Sayer 2004). Thus, the otolith elemental composition of settled cod in August–September could reflect a range of habitats and water masses inhabited during both the larval and settled phase, although, as accretion rate increases with age, it will most likely reflect that of the settled juvenile habitat. According to simulations of passive transport of cod eggs and larvae, there is the potential for substantial transport of these early life stages from Shetland to the Moray Firth and Buchan regions (Heath and Gallego 1997). Given the proximity of many of the nursery grounds found in this study to known spawning areas of high adult fidelity (Wright et al. 2006) it is important to know whether advected larvae are liable to make a return migration once settled or are lost to their natal spawning areas. A tag-recapture experiment on 1-year-old cod in the southern North Sea suggested that juveniles tend to remain within 100 km of their release site (Riley and Parnell 1984) and older cod tend to exhibit a similar level of fidelity (Wright et al. 2006). Hence, if there is natal fidelity there may be an expectation for a return migration following settlement. However, an active migration of 0-group has not been supported by previous small scale tagging studies, although these were limited to semi-enclosed sea areas (Hawkins et al. 1985; Grant and Brown 1998). Work at Loch Torridon on the Scottish west coast indicated that 0-group cod did not move more than a few 100 m for several weeks after settlement and modal length analysis suggested that they remained close to their settlement areas until they became adults (Hawkins et al. 1985). The regional differences in otolith elemental composition found in the present study may reflect this period of site attachment. Certainly, the high classification success indicates there can be little or no inter-mixing between nursery patches more than 200 km apart. This scale of segregation is consistent with the distribution of spawning congregations and adult residency indicated from adult movements (Neat et al. 2006; Wright et al. 2006). Further work is clearly needed in order to determine whether the similarity in spatial segregation of juvenile and adult cod is reflective of resident populations or whether there is inter-mixing between the settled 0-group and adult phases. Combining the elemental signatures in the otoliths found in the present study with the elemental signatures from otolith cores of adult fish could provide a test of this hypothesis, and could help define the contribution of individuals from juvenile habitats to the adult population.
The growing evidence for resident Atlantic cod populations from this and other studies has important implications to fisheries management. The maintenance of intra-specific diversity forms part of the Rio convention on biodiversity (Ryman et al. 1995). Since catch control measures applied at the level of the stock do not affect where fleet effort is concentrated, significant depletions of local resident populations might go undetected by fishery based stock monitoring programmes. If the comparative isolation of the nursery areas identified in this study and the site fidelity evident from adult tag recapture studies (Wright et al. 2006; Neat et al. 2006) reflects locally resident populations then fishing in these areas could have a serious local impact. This is particularly an issue given the currently highly localised nature of 0-group concentrations. Although conserving sub-structure of fished stocks does pose serious problems to management based on total allowable catches, temporarily closing areas might be an effective management measure in re-building small resident populations (Polunin 2002). This is particularly true for areas where juveniles concentrate, since large by-catches of undersized fish have no commercial advantage to fishermen. Investigations of distribution and structuring of the type made in the present study are clearly useful in identifying areas worth protecting.
References
Balls PW (1986) Composition of suspended particulate matter from Scottish coastal waters—geochemical implications for the transport of trace metal contaminants. Sci Total Environ 57:171–180
Balls P, Cofino W, Schmidt D, Topping G, Wilson S (1993) ICES baseline survey of trace metals in European shelf waters. ICES J Mar Sci 50:435–444
Bath GE, Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (2000) Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim Cosmochim Acta 64:1705–1714
Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51(8):633–641
Begg GA, Waldman JR (1999) An holistic approach to fish stock identification. Fish Res 43:35–44
Bergenius MAJ, Mapstone BD, Begg GA, Murchie CD (2005). The use of otolith chemistry to determine stock structure of three epinepheline serranid coral reef fishes on the great barrier reef, Australia. Fish Res 72:253–270
Brooke HE (1981) The conundrum of the stock concept—area nature and nurture definable in fishery science? Can J Fish Aquat Sci 38:1479–1480
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci 58:30–38
Campana SE, Fowler AJ, Jones CM (1994) Otolith elemental fingerprinting for stock identification on Atlantic cod (Gadus morhua) using laser ablation ICPMS. Can J Fish Aquat Sci 51:1942–1950
Campana SE, Chouinard GA, Hanson JM, Frechet A, Brattey J (2000) Otolith elemental fingerprints as biological tracers of fish stocks. Fish Res 46:343–357
Dalley EL, Anderson JT (1997) Age-dependent distribution of demersal juvenile Atlantic cod (Gadus morhua) in inshore/offshore northeast Newfoundland. Can J Fish Aquat Sci 54:168–176
Edmonds JS, Caputi N, Morita M (1991) Stock discrimination by trace-element analysis of otoliths of orange roughy (Hoplostethus atlanticus), a deep-water marine teleost. Aust J Mar Freshw Res 42:383–389
Elsdon TS, Gillanders BM (2002) Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Can J Fish Aquat Sci 59:1796–1808
Elsdon TS, Gillanders BM (2003) Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri. Mar Ecol Prog Ser 260:263–272
FAO (1995) Code of conduct for responsible fisheries. FAO, Rome, pp 41
Forrester GE, Vance RR, Steele MA (2002) Simulating large-scale population dynamics using small-scale data. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Deigo, pp 275–301
Fox C, Taylor M, Dickey-Collas M, van Damme CJG, Bolle L, Daan N, Rohlf N, Kraus G, Munk P, Fossum P, Bailey N (2005) Initial results from the 2004 ichthyoplankton survey of the North Sea. ICES CM 2005/AA:04
Gillanders BM (2002) Temporal and spatial variability in elemental composition of otoliths: implications for determining stock identity and connectivity of populations. Can J Fish Aquat Sci 59:669–679
Godø OR, Sunnanå K (1984) Spawning area and distribution of 0-group cod, Gadus morhua L, on the Møre coast. In: Dahl E, Danielssen DS, Moksness E, Solemdal P (eds) The propagation of cod Gadus morhua L. Flod Rapp 1:519–532
Grant SM, Brown JA (1998) Nearshore settlement and localized populations of age 0 Atlantic cod (Gadus morhua) in shallow coastal waters of Newfoundland. Can J Fish Aquat Sci 55:1317–1327
Halliday RG (1988) Use of seasonal spawning area closures in the management of haddock fisheries in the northwest Atlantic. NAFO Sci Counc Stud 12:27–36
Hawkins AD, Soofiani NM, Smith GW (1985) Growth and feeding of juvenile cod (Gadus morhua L.). J Cons Int Explor Mer 42:11–32
Heath M, Gallego A (1997) From the biology of the individual to the dynamics of the population: bridging the gap in fish early life stages. J Fish Biol (Suppl. A):1–29
Heath M, Rankine P, Cargill L (1994) Distribution of cod and haddock eggs in the North Sea in 1992 in relation to oceanographic features and compared with distributions in 1952–1957. In: Jakobsson J, Astthorsson OS, Beverton RJH, Bjoernsson B, Daan N, Frank KT, Meincke J, Rothschild B, Sundby S, Tilseth S (eds) ICES marine science symposia, Reykjavik, pp 438–439
Hislop JRG (1986) The demersal fishery in the Clyde Sea Area. In: Proceedings of the Royal Society of Edinburgh section B 90:423–437
Huberty CJ (1994) Applied discriminant analysis. Wiley-Interscience, New York
Hutchinson WF, Carvalho GR, Rogers SI (2001) Marked genetic structuring in localised spawning populations of cod Gadus morhua in the North Sea and adjoining waters, as revealed by microsatellites. Mar Ecol Prog Ser 223:251–260
IPCS (1990) Barium international programme on chemical safety: environmental health criteria 107. World Health Organisation, Geneva
Jakobsen T (1987) Coastal cod in northern Norway. Fish Res 5:223–234
Kamenos NA, Moore PG, Hall-Spencer JM (2004) Small-scale distribution of juvenile gadoids in shallow inshore waters; what role does maerl play? ICES J Mar Sci 61:422–429
Kinlan BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Knutsen H, Jorde PE, André C, Stenseth NC (2003) Fine-scaled geographical population structuring in a highly mobile marine species: the Atlantic cod. Mol Ecol 12:385–394
Kritzer JP, Sale PF (2004) Metapopulation ecology in the sea: from Levins’ model to marine ecology and fisheries science. Fish Fish 5:131–140
Lawson GL, Rose GA (2000) Small-scale spatial and temporal patterns in spawning of Atlantic cod (Gadus morhua) in coastal Newfoundland waters. Can J Fish Aquat Sci 57:1011
Lough RG, Valentine PC, Potter DC, Auditore PJ, Bolz GR, Neilson JD, Perry RI (1989) Ecology and distribution of juvenile cod and haddock in relation to sediment type and bottom currents on eastern Georges Bank. Mar Ecol Prog Ser 56:1–12
Magill SH, Sayer MDJ (2004) Abundance of juvenile cod (Gadus morhua L.) in the rocky subtidal as influenced by winter water temperature. J Mar Biol Assoc UK 84:439–442
Martin GB, Thorrold SR, Jones CM (2004) Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Can J Fish Aquat Sci 61:34–42
Millar R B (1990). Comparison of methods for estimating mixed stock fishery composition. Can J Fish Aquat Sci 47:2235–2241
Milton DA, Chenery SR, Farmer MJ, Blaber SJM (1997) Identifying the spawning estuaries of the tropical shad, terubok Tenualosa toli, using otolith microchemistry. Mar Ecol Prog Ser 153:283–291
Mugiya Y, Hakomori T, Hatsutori K (1991) Trace metal incorporation into otoliths and scales in the goldfish, Carassius auratus. Comp Biochem Physiol C 99:327–331
Neat FC, Wright PJ, Zuur AF, Gibb IM, Gibb FM, Tulett D, Righton DA, Turner RJ (2006) Residency and depth movements of a coastal group of Atlantic cod (Gadus morhua L.). Mar Biol 148:643–654
Olsen S, Soldal AV (1989) Observations on inshore distribution and behaviour of 0-group Northeast Arctic cod. Rapp Procès-Verb Réun Cons Int Explor Mer 191:296–302
Palumbi SR (2003) Population genetics, demographic connectivity and the design of marine protected areas. Ecol Appl 13: S146–S158
Patterson HM, Thorrold SR, Shenker JM (1999) Analysis of otolith chemistry in Nassau grouper (Epinephelus striatus) from the Bahamas and Belize using solution-based ICP-MS. Coral Reefs 18:171–178
Pickett GD, Kelley DF, Pawson MG (2004) The patterns of recruitment of sea bass, Dicentrarchus labrax L. from nursery areas in England and Wales and implications for fisheries management. Fish Res 68:329–342
Pihl L, Wennhage H (2002) Structure and diversity of fish assemblages on rocky and soft bottom shores on the Swedish west coast. J Fish Biol 61:148–166
Polunin NVC (2002) Marine protected areas, fish and fisheries. In: Hart PJB, Reynolds JD (eds) Handbook of fish biology and fisheries. Blackwell, Oxford, pp 293–318
Proctor CH, Thresher RE, Gunn JS, Mills DJ, Harrowfield IR, Sie SH (1995) Stock structure of the southern bluefin tuna Thunnus maccoyi: an investigation based on probe microanalysis of otolith composition. Mar Biol 122:511–526
Raitt DFS (1967) Cod spawning in Scottish waters. Preliminary Investigations. ICES CM 1967/F:29
Riley JD, Parnell WG (1984) The distribution of young cod. In: Dahl E, Danielssen DS, Moksness E, Solemdal P (eds) The Propagation of Cod Gadus morhua L. An international symposium. Flodevigen rapportser, Arendal (Norway)
Robichaud D, Rose GA (2004) Migratory behaviour and range in Atlantic cod: inference from a century of tagging. Fish Fish 5:185–214
Rooker JR, Secor DH, Zdanowicz VS, Itoh T (2001) Discrimination of northern bluefin tuna from nursery areas in the Pacific Ocean using otolith chemistry. Mar Ecol Prog Ser 218:275–282
Ruzzante DE, Taggart CT, Cook D, Goddard SV (1997) Genetic differentiation between inshore and offshore Atlantic cod (Gadus morhua) off Newfoundland: a test and evidence of temporal stability. Can J Fish Aquat Sci 54:2700–2708
Ruzzante DE, Wroblewski JS, Taggart CT, Smedbol RK, Cook D, Goddard SV (2000) Bay-scale population structure in coastal Atlantic cod in Labrador and Newfoundland, Canada. J Fish Biol 56:408–431
Ryman N, Utter F, Laikre L (1995) Protection of intraspecific biodiversity of exploited fishes. Rev Fish Biol Fish 5:417–446
Secor DH, Henderson-Arzapalo A, Piccoli PM (1995) Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? J Exp Mar Biol Ecol 192:15–34
Sokal RR, Rohlf FJ (1995) Biometry. W.H. Freeman, New York
Swain DP, Hutchings JA, Foote CJ (2004) Environmental versus genetic influences on identification characters. In: Cadrin SX, Friedland KD, Waldman J (eds) Stock identification methods. Academic, New York
Thorrold SR, Jones CM, Swart PK, Targett TE (1998a) Accurate classification of juvenile weakfish Cynoscion regali to estuarine nursery areas based on chemical signatures in otoliths. Mar Ecol Prog Ser 173:253–265
Thorrold SR, Jones CM, Campana SE, McLAren JW, Lam JWH (1998b) Trace element signatures in otoliths record natal river of juvenile American shad (Alsoa sapidissima). Limnol Oceanogr 43:1826–1835
Thresher RE (1999) Elemental composition of otoliths as a stock delimiter in fishes. Fish Res 43:165–204
Thresher RE, Proctor CH, Gunn JS, Harrowfield IR (1994) An evaluation of electron probe microanalysis of otoliths for stock delineation and identification of nursery areas in a southern temperate groundfish, Nemadactylus macropterus (Cheilodactylidae). Fish Bull 92:817–840
Tupper M, Boutilier RG (1995) Effects of habitat on settlement, growth and postsettlement survival of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 52:1834–1841
Wright PJ, Galley E, Gibb IM, Neat FC (2006) Fidelity of adult cod to spawning grounds in Scottish waters. Fish Res 77:148–15
Acknowledgments
We are indebted to Hermann Thordarson from the Center of Chemical Analysis, Technological Institute of Iceland (IceTec) for elemental analyses. We also gratefully acknowledge the help of the following in sample collection: Suzanne Ware (previously of UMBSM); skippers, crew and scientific staff onboard the RV Aplysia, FRV Clupea and FRV Scotia and FVs Karona, Radiant Star, Ocean Gem and Silver Dawn. This work was conducted as part of a contract with the European Union METACOD Q5RS-2001-00953 and the Scottish Executive projects MF0756 and MF0465. It does not necessarily reflect the views of the European Commission and in no way anticipates any future opinion of the Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Atkinson, Cambridge.
Rights and permissions
About this article
Cite this article
Gibb, F.M., Gibb, I.M. & Wright, P.J. Isolation of Atlantic cod (Gadus morhua) nursery areas. Mar Biol 151, 1185–1194 (2007). https://doi.org/10.1007/s00227-006-0565-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0565-0