Abstract
The morphological characteristics and the population genetic structures of the fissiparous seastar Coscinasterias acutispina were investigated for eight sites in the Sea of Japan in order to clarify the presence of sexual and asexual reproduction. Morphological observation based on arm length showed that fission was common at all eight sites examined, indicating the likely production of clonal individuals. A random amplified polymorphic DNA (RAPD) marker was used to detect clones arising by fission and to assess gene flow among sites. A simulation approach using RAPD data revealed the presence of clonal individuals at almost all sites, suggesting the existence of asexual reproduction. The result of phylogenetic analysis according to RAPD genotype showed no relationship between genetic and geographic distances. Considering the limited movement ability of seastar species during the adult phase, these observations suggest the existence of marked gene flow among sites, due to dispersal of planktonic larvae produced by sexual reproduction. These observations suggest that multi-locus genotypic compositions depend on the relative amounts of recruitment from sexual and asexual reproduction in each population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some animals, such as sessile and sedimentary marine invertebrates, have an asexual mode of reproduction as well as a sexual mode, although sexual reproduction predominates in most other animals (Hughes 1989). However, the evolutionary processes leading to the possession of both modes in a single species, and its adaptive significance, have been scarcely explained. In order to clarify these issues, the relative contributions of asexual and sexual reproduction to population maintenance need to be elucidated.
Fission is a well known feature of three echinoderm classes, the Asteroidea, Ophiuroidea, and Holothuroidea, and has been recognized since the nineteenth century (Emson and Wilkie 1980). In the Asteroidea, fission dividing the animal into two equal parts usually takes place across the disk during the benthic adult phase (Emson and Wilkie 1980). The genus Coscinasterias comprising four species (C. tenuispina, C. acutispina, C. calamaria, and C. muricata), all of which are fissiparous, is common in shallow continental waters worldwide (Clark and Downey 1992). These fissiparous seastars simultaneously possess functional sexual reproductive ability as defined by the presence of mature sperm or oocytes (Emson and Wilkie 1980).
One of these species, C. acutispina (Stimpson) is broadly distributed in the north-western Pacific, especially around the Japanese Archipelago and coastal region of southern China (Edmondson 1935; Huang 1994). Yamazi (1950) reported the process of organ regeneration after fission in a C. acutispina population collected from Japan. Fujita (1999) investigated the fission pattern of this species and indicated that a higher frequency of fission occurred during summer, and/or in smaller body size classes, as had already been demonstrated by Crozier (1920) and Emson (1978) for other fissiparous seastars. On the other hand, most animals of this species had a mature gonad, indicating the potential for sexual reproduction (Noumura and Kanatani 1962; Komatsu et al. 1999). Komatsu et al. (1998) reported that larvae of this species obtained by artificial fertilization metamorphosed into juveniles via a planktonic phase. However, the study which discussed the existence of both asexual fissiparity and sexual larval recruitment in natural populations of this species had little been reported (Fujita 1999; Fujita et al. 2001). To investigate this issue, population genetic analyses using allozyme markers have recently been conducted on fissiparous seastars. In C. calamaria populations of Western Australia, Johnson and Threlfall (1987) observed that the range of multilocus genotypes showed the proportions unexpected from random mixing, confirming the highly clonal structure of local populations. Sköld et al. (2002) obtained similar results in part of a C. muricata population of New Zealand to those in C. calamaria. These studies demonstrated that a genetic marker offers available information on the contribution of asexual and sexual reproduction to the maintenance of local populations in fissiparous seastars. Genetic makers based on the polymerase chain reaction (PCR; Saiki et al. 1988), which can easily reveal genetic polymorphism, have become widely used for analysis of population genetic structure. One of these markers is random amplified polymorphic DNA marker (RAPD; Williams et al. 1990), which can be easily applied to a wide range of plant and animal taxa, allowing low cost of the technique, requirement of only nanograms of template DNA, and examination of an essentially unlimited number of loci, while RAPD marker has potentially some problems such as repeatability (Lynch and Milligan 1994). Recently, RAPD markers have been used frequently to examine population structure and to detect clones in asexual species (e.g. Ceplitis 2001; Karako et al. 2002).
In the present study, we investigated the geographical variation of fissiparity frequency in C. acutispina based on morphological features and detection of clones by RAPD analysis. Furthermore, we evaluated gene flow among populations, based on population genetic structures of this species from the Sea of Japan, in order to clarify the frequency of sexual reproduction in the natural populations.
Materials and methods
Sample collection and morphological analysis
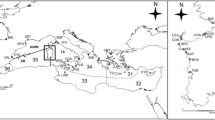
Samples of C. acutispina (Stimpson) were collected during July and August 2003 from eight sites in the intertidal and/or shallow subtidal zone of the Sea of Japan (Table 1; Fig. 1). For each individual, the length of every arm was measured. The arms were categorized into two groups, long and short, being divided by the latest fission line (Fujita 1999). The largest arm length within the long arm group (R) and that within the short arm group (re) were recorded to examine the previous occurrence of fission (see Johnson and Threlfall 1987). We regarded an individual with a re/R ratio under 0.9 as expectedly undergone fission.
Locations of the eight sites used for sampling of Coscinasterias acutispina along Honshu and Sado islands bordering the Sea of Japan. Site codes are from Table 1
DNA extraction and polymerase chain reaction (PCR)
We randomly chose approximately 15 individuals from each population for RAPD analysis (Table 1). A single arm or tube foot was clipped from each individual, and fixed in 99.5% ethanol for DNA extraction. Total DNA was extracted using the Chelex method (Singer-Sam et al. 1989). Tissues in 500 μl of 5% Chelex buffer (Chelex® 100; Sigma) were incubated at 95°C for 30 min, and then vortexed for 30 s by a tube mixer. The supernatant was stored at −30°C and used for PCR amplification as template DNA.
For RAPD amplification carried out using GenAmp PCR System 9700 (Applied Biosystems), 10 ng of genomic DNA was amplified in 20 μl of a reaction mixture containing 0.2 mM dNTPs, 5 μM primer, 0.625 U Ex Taq DNA polymerase (TaKaRa ) and 1×Ex Taq buffer (TaKaRa). The samples were subjected to preheating at 94°C for 2 min, followed by 45 cycles of denaturation at 94°C for 30 s, annealing at 36°C for 30 s and extension at 72°C for 1 min. After the last cycle, a final extension step of 94°C for 5 min was used to complete the reaction. Reaction products were separated by electrophoresis in 1.5% NuSieve 3:1 Agarose gel (TaKaRa) in TAE buffer, stained with ethidium bromide and photographed under UV illumination.
In order to find suitable primers for the analysis, we screened 40 random decamer primers (OPA and OPB primer sets; Operon technologies), and selected five that gave clear, variable and reproducible band patterns: OPA-1 (5′-CAGGCCCTTC-3′); OPA-2 (5′-TGCCGAGCTG-3′); OPA-19 (5′-CAAACGTCGG-3′); OPB-15 (5′-GGAGGGTGTT-3′) and OPB-17 (5′-AGGGAACGGA-3′). In addition, repeatable banding patterns were indicated by periodic re-amplification of individuals.
Data analysis
RAPD analysis was performed for 128 specimens collected from eight sites in the Sea of Japan (Table 1). The detected banding patterns were then translated into a 0/1-matrix (1 for presence of a RAPD band, 0 for absence), and used for subsequent analyses based on a two-allele model.
We examined the existence of clones by comparing the RAPD banding patterns. We assessed clones on the basis of P SEX-values, i.e., the likelihood of finding at least as many identical multilocus genotypes as those observed in a panmictic population (Ivey and Richards 2001) from the allele frequencies at each site. The MLGsim program (Stenberg et al. 2003) was used to compute the P SEX-values and test for their significance.
The genetic population structure inferred from the RAPD markers was assessed by phylogenetic relationships among RAPD genotypes. Phylogenetic relationships among RAPD genotypes were assessed by using the neighbor-joining cluster algorithm based on genetic distance D (Nei and Li 1979) in PAUP* 4.0b10 (Swofford 2000).
Results
On the basis of arm length (re/R) ratio, fissiparity was considered to occur at all eight sites. A high proportion of individuals bearing fission scar (re/R<0.9) was also revealed at each site, ranging from 81.2% at Aikawa to 95.2% at Wajima (Fig. 2). Most animals sampled from Uozu, where is only subtidal site surveyed in the present study, seemed to have low asymmetry.
re/R frequency distribution of arm length at the eight sites in the Sea of Japan. Site codes are from Table 1. Numerical values for each site mean the proportion of individuals that had undergone fission (%), which were regarded as having a re/R ratio of < 0.9
The five RAPD primers examined generated 20 clear and reproducible bands. The number of polymorphic bands per primer varied from 4 to 7 with an average of 5.6. These primers detected 42 RAPD genotypes in a total of 128 individuals collected from the eight sites (Table 2). The number of differences for scored bands between all possible pairs of RAPD genotypes varied from 1 to 13. Identical RAPD genotypes among individuals were found at every site. A simulation approach based on P-SEX values detected clones at six sites (Aikawa, Uozu, Himi, Kurosaki, Noto and Mihonoseki) (Table 2). We were unable to examine this simulation for Takeno, since this site was organized into only one RAPD genotype. Some RAPD genotypes shared among sites were observed between Uozu and Kurosaki (RAPD genotype 4), and Kurosaki and Wajima (7, 11, 22), the others being unique to each site.
The neighbor-joining network based on genetic distance demonstrated the phylogenetic relationship of the 42 RAPD genotypes (Fig. 3). Genetic groups comprising the RAPD genotypes from geographically close sites were scarcely detected on the network.
Unrooted phylogram illustrating all RAPD genotypes in Coscinasterias acutispina, using the neighbor-joining cluster algorithm based on D (Nei and Li 1979). First codes indicate the RAPD genotype number. Figures following numbers are the site codes listed in Table 1. The numbers of individuals with the same genotype are shown in parentheses
Discussion
Morphological analysis suggested that fission occurred at every site examined, indicating the likely presence of clonal individuals. However, number of fissioned individuals on the basis of re/R might underestimate, because arm asymmetry was reduced in large individuals which had the rapid growth of regenerated arms (Yamazi 1950). Most individuals which had nearly to one on the re/R ratio seemed to larger body size (data not shown). Therefore, actually fissioned individuals might be still more than observed one in this study. In addition, most samples from Uozu, where is only subtidal habitat for this species in this study site as reported by Fujita et al. (2001), recently might be not undergo fission. The similar results which subtidal populations had the lower proportion of recently divided individuals than intertidal population were observed in C. calamaria (Johnson and Threlfall 1987; Barker et al. 1991) and C. muricata (Sköld et al. 2002). Thus, fission occurrence in C. acutispina might be negatively influenced by subtidal environment which differs from intertidal environment in food conditions, temperature stress, and sediment stability (Mladenov 1996).
The present RAPD analysis which revealed 48 RAPD genotypes from 128 specimens may be better discrimination than previous analyses. Johnson and Threlfall (1987) distinguished 48 genotypes from 430 animals of C. calamaria, being a lower number of the detected multi locus genotypes per individuals comparing with the present study.
The present analysis found multiple individuals that had an identical genotype for 19 RAPD genotypes at each of the all eight sites. The detection of clones using genetic markers needs to reveal both an identical genotype between individuals and a high level of divergence between different genotypes (Suyama et al. 2000). In the present study, the minimum proportion of pairwise differences between different genotypes was 0.37%, which was scarcely enough to recognize the occurrence of clones in the C. acutispina specimens examined. However, the calculation of P SEX-values based on a simulation approach demonstrated the presence of clonal individuals at almost all sites, except for Wajima and Takeno. At Wajima, which exhibited the highest frequency of possible fission, a high degree of genetic diversity was revealed, thus clones might not have been detected by this simulation, indicating the need for further analysis using more samples. On the other hand, at Takeno, there was a single genotype (RAPD type 19), implying that all sampled individuals might have been derived from a single clone.
As the results of both morphological and RAPD analyses, asexual reproduction highly occurred at each of the eight sites, while recent fissiparity has differenced with each site. This was in contrast to previous study of C. muricata, which indicated that several fjord populations do not undergo fission at all (Sköld et al. 2002, Sköld et al. 2003). According to these studies, almost all fjord individuals had large body size. On general, fissiparous seastars have a lower incidence of fission as body size grown. It will be necessary to compare the environments and growth states of intertidal population to subtidal population in order to properly characterize spatial fission variability in C. acutispina.
The phylogenetic relationships among RAPD genotypes had disagreement with the geographic relationships in the present study. This result should indicate the existence of gene flow among the sites. Additionally, this pattern might be unexplained by isolation by distance. It should be impossible for adult individuals of C. acutispina, which typically inhabit hard substrata, to move among sites through sandy areas. Nishida and Lucas (1988) observed genetic homogeneity among populations of the crown-of-thorns seastar Acanthaster planci, which has a planktonic larval phase lasting at least several weeks (Yamaguchi 1977; Lucas 1982), throughout the Pacific (>1,000 km scale) due to larval dispersion. Since C. acutispina has a longer planktonic phase (a few months; Komatsu et al. 1998), the existence of gene flow among eight sites, the longest distance between them being about 550 km, should be validity.
Japanese turban shell Turbo cornutus populations from the Sea of Japan were genetically homogeneity, being probably generated by the Tsushima Current (Kojima et al. 1997). Kojima et al. (1997) revealed that genetic differentiation was detected between populations of the Sea of Japan and those of the Seto Inland Sea where is close geographically. Therefore, population genetic structure of C. acutispina should be examined in wider range of sea area.
Consequently, the gene flow estimated in the present study might be due to the dispersal of planktonic larvae produced by sexual reproduction. Thus we conclude that populations of C. acutispina in the Sea of Japan might possess abilities for both asexual and sexual reproduction, and be maintained thorough a combination of both. In the future study, the contributions of both reproductive modes to population maintenance should be surveyed on the temporal variability in asexual and sexual recruitment on the basis of the detection of clones and gene flow by genetic marker such as RAPD presently established.
References
Barker MF, Scheibling R, Mladenov PV (1991) Seasonal changes in population structure of the fissiparous asteroids Allostichaser insignis (Farquhar) and Coscinasterias calamaria (Gray). In: Scalera-Liaci L, Canicatti C (eds) Echinoderm research 1991. Balkema, Rotterdam, pp 191–196
Ceplitis A (2001) The importance of sexual and asexual reproduction in the recent evolution of Allium vineale. Evolution 55:1581–1591
Clark AM, Downey ME (1992) Starfishes of the Atlantic. Chapman and Hall, London
Crozier WJ (1920) Notes on some problems of adaptation. 2. On the temporal relations of asexual propagation and gametic reproduction in Coscinasterias tenuispina. Biol Bull Mar Biol Lab Woods Hole 39:122–128
Edmondson HC (1935) Autotomy and regeneration in Hawaiian starfishes. Bernice P Bishop Mus, Occasional pap 11:3–20
Emson RH (1978) Some aspects of fission in Allostichaster polyplax. In: McLusky DS, Berry AJ (eds) Physiology and behavior of marine organisms. Pergamon, Oxford, pp 321–329
Emson RH, Wilkie IC (1980) Fission and autotomy in echinoderms. Mar Biol Ann Rev 18:155–250
Fujita T (1999) Population structure of a fissiparous seastar Coscinasterias acutispina. In: Carnevali C, Bonasoro F (eds) Echinoderm research 1998. Balkema, Rotterdam, pp 465–470
Fujita D, Seto Y, Moriyama Y, Komatsu M (2001) Coscinasterias acutispina: Distribution and ecology in Toyama Bay. In: Barker MF (eds) Echinoderms 2000. Swets and Zeitlinger, Lisse, pp 169–174
Huang Z (1994) Marine species and their distributions in China’s Seas. China Ocean Press, Beijing
Hughes RN (1989) A functional biology of clonal animals. Chapman and Hall, London
Ivey CT, Richards JH (2001) Genotypic diversity and clonal structure of Everglades sawgrass, Cladium jamaicense (Crperaceae). Int J Plant Sci 162:1327–1335
Johnson MS, Threlfall TJ (1987) Fissiparity and population genetics of Coscinasterias calamaria. Mar Biol 93:517–525
Karako S, Achituv Y, Perl-Treves R, Katcoff D (2002) Asterina burtoni (Asteroidea; Echinodermata) in the Mediterranean and the Red Sea: does asexual reproduction facilitate colonization? Mar Ecol Prog Ser 234:139–145
Kojima S, Segawa R, Hayashi I (1997) Genetic differentiation among populations of the Japanese turban shell Turbo (Batillus) cornutus corresponding to warm currents. Mar Ecol Prog Ser 150:149–155
Komatsu M, Moriyama Y, Hirano Y (1998) Sexual reproduction of the fissiparous seastar, Coscinasterias acutispina (Stimpson). Zool Sci 15 (Suppl):61
Komatsu M, Moriyama Y, Hirano Y (1999) A sex ratio and an annual reproductive cycle in population of the fissiparous seastar, Coscinasterias acutispina (Stimpson). Zool Sci 16 (Suppl):55
Lucas JS (1982) Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J Exp Mar Biol Ecol 65:173–193
Lynch M, Milligan B (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nishida M, Lucas JS (1988) Genetic differences between geographic populations of the Crown-of-thorns starfish throughout the Pacific region. Mar Biol 98:359–368
Noumura T, Kanatani H (1962) Induction of spawning by radial nerve extracts in some starfishes. J Faculty Sci, Tokyo Univ 9:397–402
Mladenov PV (1996) Environmental factors influencing asexual reproductive processes in echinoderms. Oeceanol Acta 19:227–235
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer—directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 259:487–491
Singer-Sam J, Tanguay RH, Riggs AD (1989) Use of Chelex to improve the PCR signal from a small number of cells Amplifications. A forum for PCR users, issue 3 (September):11
Sköld M, Barker MF, Mladenov PV (2002) Spatial variability in sexual and asexual reproduction of the fissiparous seastar Coscinasterias muricata: the role of food and fluctuating temperature. Mar Ecol Prog Ser 233:143–155
Sköld M, Wing SR, Mladenov PV (2003) Genetic subdivision of a seastar with high dispersal capability in relation to physical barriers in a fjordic seascape. Mar Ecol Prog Ser 250:163–174
Stenberg P, Lundmark M, Saura A (2003) MLGsim: a program for detecting clones using a simulation approach. Mol Ecol Notes 3:329–331
Suyama Y, Obayashi K, Hayashi I (2000) Clonal structure in a dwarf bamboo (Sasa senanensis) population inferred from amplified fragment length polymorphism (AFLP) fingerprints. Mol Ecol 9:901–906
Swofford DL (2000) PAUP*phylogenetic analysis using parsimony (*and other methods), Version 4. Sinauer Associates, Sunderland
Williams JGK, Kubelik AR, Lival KJ, Rafaski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Yamaguchi M (1977) Larval behavior and geographic distribution of coral reef asteroids in the Indo-West Pacific. Micronesica 13:283–296
Yamazi I (1950) Autotomy and regeneration in Japanese seastars and ophiurans I: Observation on a seastar Coscinasterias acutispina (Stimpson) and four species of ophiurans. Annot Zool Jap 23:175–186
Acknowledgements
We thank Dr. M. Nozaki of the Niigata University, Dr. D. Fujita of the Tokyo University of Marine Science and Technology and S. Honjo of the Takeno Snorkel Center, for collecting our samples. We are also grateful to Dr. A. Kijima of the Tohoku University, Dr. R. Yokoyama of the Toyama University and Dr. Y. Seto of the Prefectural office of Toyama, for their helpful suggestions. We also thank the members of the Regulatory Biology Group at the Toyama University and Mr. T. Haramoto for their kind support during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Haramoto, S., Komatsu, M. & Yamazaki, Y. Population genetic structures of the fissiparous seastar Coscinasterias acutispina in the Sea of Japan. Mar Biol 149, 813–820 (2006). https://doi.org/10.1007/s00227-005-0163-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0163-6