Abstract
The protandric simultaneous hermaphrodite shrimp Lysmata wurdemanni (Gibbes 1850) has a pure searching mating system, i.e., males are continually searching for receptive females and copulation is brief. To examine whether size-based advantage in male–male competition occurs and whether the mating ability of male-phase (M) shrimp equals that of euhermaphrodite-phase shrimp serving as males (Em), mating performance, including mating frequency and precopulatory behavior, of M and Em shrimp was compared using two M:Em ratios. Two experiments were carried out from March 2004 to August 2004 at Florida Institute of Technology’s Vero Beach Marine Laboratory using laboratory-cultured shrimp that originated from Port Aransas, TX, USA. In the two experiments, one parturial euhermaphrodite-phase shrimp acting as a female (Ef) was maintained with one M and two Em shrimp (one with and one without an egg mass), and two M and two Em shrimp, respectively. The M shrimp used were always smaller than the Em shrimp. Experiment 1 showed that there was no significant difference in mating ability between Em with and without egg mass. In both experiments, the M shrimp gained mating partners more frequently than the Em shrimp did. In the experiment with two M and two Em shrimp, mating frequencies of the small M and large M shrimp were similar. Precopulatory behaviors of the M shrimp were more active than those of the Em shrimp. Mating between the small M and larger Ef shrimp was sometimes successful even when the size difference was 20.0 mm total length (TL). Mating between a larger M shrimp and smaller Ef shrimp sometimes failed when the size difference was only 13.0 mm TL. Mating frequency of M shrimp over that of Em shrimp with Ef shrimp increased significantly with increasing density and operational sex ratio. The advantage of M over Em shrimp in obtaining mating partners is probably a result of sexual selection and adaptation, and may partially explain the observed delayed sex change in some L. wurdemanni, i.e., some male-phase shrimp grow very large and never become hermaphrodites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection arises because some individuals have higher mating success than others of the same sex (Darwin 1871). Two elements, male–male competition and mate choice, are involved in sexual selection, which are termed intra-sexual and inter-sexual selection, respectively (Anderson 1994). Intra-sexual selection has been recognized for a long time as an important selective agent in a variety of taxa including insects, crustaceans, fish, and mammals (Anderson 1994; Jormalainen 1998 for reviews). Among males, mating success may depend on their ability to monopolize resources needed by the females for mating. That large males in a population often have a mating advantage over their smaller rivals has been widely reported. For example, one of the most common mating patterns in a natural population is assortative mating by size (Arak 1983; Ridley 1983; Crespi 1989). However, it has been suggested that small and agile males may have higher mating success when competition is based on searching capabilities (reviewed by Anderson 1994; Bauer and Abdalla 2001).
Caridean shrimp are a particularly suitable group among decapod crustaceans for studying mating behavior and sexual selection, as there are several different mating systems (reviewed by Correa and Thiel 2003): (1) In the monogamy system, a male-female pair lasts for a long time; (2) in the neighborhood-of-dominance system, male mating success depends largely on their ability to aggressively overtake and defend receptive females. Pair formation only lasts for a short time, during which dominant males attend, fertilize, and guard females and then the mates separate; (3) in the pure search system, male mating success depends primarily on their ability to find and mate with as many receptive females as possible. Upon encounter, the males transfer sperm in simple, brief acts after which the pair immediately separate; (4) In the search and attend system, adult males live solitarily on hosts, but change hosts frequently in search of females. Upon encounter, males stay on the hosts and each mate returns to a solitary life-style after mating.
Species of the genus Lysmata have a unique protandric simultaneous hermaphroditic reproductive system among decapod crustaceans. Shrimp mature first as a functional male having male external characteristics: (1) cincinnulli on the endopods of pleopods 1 and, (2) appendices masculinae on the endopods of pleopods 2 (Bauer and Holt 1998). In Lysmata wurdemanni, most males pass through four transitional phases (i.e., four transitional molts) to become a euhermaphrodite-phase (E) (termed female-phase by Bauer and Holt 1998) with both male and female functions, with the external male characteristics gradually disappearing (Zhang and Lin 2005, in press). The size of sex change is variable, with the minimum around 24.0 mm in total length (TL) (Lin and Zhang 2001). Large shrimp (>24.0 mm TL) still in male-phase (M) have been found in the wild (Bauer and Holt 1998) and in laboratory environments (unpublished data). Social mediation has been reported to be the main factor influencing sex change in L. wurdemanni (Lin and Zhang 2001; Baeza and Bauer 2004). Additionally, some abiotic factors such as temperature and photoperiod, may affect the sex change (Bauer 2002a; Baldwin and Bauer 2003), and other factors may also be involved. For example, in the protandric shrimp Pandalus latirostri, male–male competition may be responsible for the delayed sex change (Chiba et al. 2003).
Male mating tactics in L. wurdemanni can be classified as pure searching, since they are continuously “on the prowl” for a receptive female. When one is encountered, copulation occurs almost immediately after a brief interaction (Wickler and Seibt 1981; Bauer and Holt 1998; Bauer 2002a; Bauer 2004; Zhang and Lin 2004). This kind of mating is often referred to as “promiscuous”. It has also been described as “scramble competition polygamy” in insects (Thornhill and Alcock 1983) and “encounter rate polygamy” in fiddler crabs (Christy 1987). Whether size-based male–male competition occurs in pure searching species is not clear. The size advantage model predicts that sex change is adaptive in protandric hermaphroditic species in which reproductive success is correlated with increasing body size in females, but not in males (Ghiselin 1969). A previous study has indicated that euhermaphrodite-phase L. wurdemanni acting as males (Em) have the same ability to copulate with parturial euhermaphrodite-phase shrimp (Ef) as large M shrimp do; however, Em and large M shrimp copulate with Ef shrimp more frequently than do small M shrimp (Bauer 2002a). This implies that there might indeed be size-based male–male competition in this species. Other factors such as density or operational sex ratio should also be considered. L. wurdemanni aggregate in small tide pools and rock jetties in the wild (Bauer and Holt 1998; Bauer 2000), so the densities are quite high. Effects of density and/or operational sex ratio on reproductive activities, such as sexual interaction and mating success, have been demonstrated in insects (Greenfield and Shelley 1985; French and Cade 1989; Cade and Cade 1992; Sirot and Brockmann 2001), some crustaceans (Ridley and Thompson 1985; Debuse et al. 1999), and fish (Jirotkul 1999a, b; Cleveland et al. 2002).
The goals of the present study were to test whether males (M) and euhermaphrodite acting as males (Em) in L. wurdemanni have equal ability to obtain a mating partner, and whether size-based advantage in male–male competition occurs in this protandric simultaneous hermaphroditic shrimp. These goals were accomplished by comparing the mating performance, including mating frequency and precopulatory behaviors of M and Em shrimp. The null hypotheses were that there is no difference in mating ability (obtaining mates) between M and Em shrimp, and no size-based male–male competition in the species.
Materials and methods
The study was conducted at Florida Institute of Technology’s Vero Beach Marine Laboratory. The shrimp (L. wurdemanni [Gibbes 1850]) used in this study were raised in the laboratory from broodstock originally collected from Port Aransas, TX, USA in 2002. The larvae were grown to postlarvae and sexual maturity following protocols described by Zhang et al (1998) and Calado et al (2003). The shrimp were fed with frozen Artemia sp. Water temperature was maintained at 26–27°C, salinity at 35‰, on a 14-h light:10-h dark cycle with an artificial light source. A complete water change was made twice daily. All shrimp used for mating experiments, both M and Em, were housed individually at least 4 days before the experiments to ensure that they did not copulate with other E shrimp. M and E shrimp were identified according to Zhang and Lin (2005, in press).
We first determined the minimum size of M shrimp that were capable of mating. For this experiment, one M and one Ef shrimp that was about to molt were housed in a 10-l bucket (with a 346-cm2 bottom area). The Ef shrimp used in this experiment were 23.0–24.0 mm TL. The M shrimp of 16.0 mm TL were tested first, because shrimp larger than 16.0 mm TL can mate with Ef shrimp of 24.0 mm TL successfully (unpublished data). If the M shrimp mated successfully, a smaller size of M shrimp (reduced incrementally by 1.0 mm TL) was tested until the minimum size was found. Each test consisted of five replicate M shrimp of the same size. When an Ef shrimp spawned, at least 30 embryos were checked to confirm that successful fertilization had occurred. Experimental conditions, including temperature, water salinity, light were the same as above.
The effect of size (TL) difference between M/Em and Ef shrimp on mating success was also examined. First a difference of 10.0 mm TL between M/Em (smaller) and Ef shrimp (larger) was tested using the same procedures described above. If mating was successful, the TL difference between M/Em and Ef shrimp for subsequent tests was increased by 1.0 mm TL each time until mating was unsuccessful. One M/Em shrimp and one Ef shrimp that was about to molt were maintained in a 10-l bucket. Each test (size difference) had five replicates. A similar experiment with large Em shrimp and small Ef shrimp was also conducted.
To examine mating competition, two experiments were conducted. Experiment 1 tested the hypothesis that there is no difference in mating capability between M shrimp and Em shrimp with and without an egg mass. Both experiments examined whether the size of mating partner affects mating competition and the relationship between mating competition and sex ratio. In the first, one Ef shrimp that was close to parturial molting (i.e., reproductive molting; newly molted Ef shrimp may mate, followed by spawning), one M, and one Em shrimp with egg mass and one Em shrimp without egg mass were kept in a 10-l bucket. In the second experiment, two (one large and one small) M and two (one large and one small) Em shrimp were placed with an Ef shrimp in a 10-l bucket. The Em shrimp used in this experiment was either with or without an egg mass because there was no significant (Student’s t test, t=0.82, NS) difference in mating ability between the Em shrimp with and without an egg mass in the first experiment (see Results). Thirty replicates were carried out for each experiment and mating behaviors were recorded with a Sony handycam video recorder using fluorescent illumination between 21:00 hours and 01:00 hours. Mating frequencies of M and Em shrimp with the Ef shrimp were measured and compared. Mating frequencies of small and large shrimp, either M or Em shrimp were compared. If the Ef shrimp copulated with more than one M shrimp, the first successful copulation was used in the data analysis. When copulation duration was >2 s, mating was considered successful, since male-role shrimp can transfer spermatophores within this time and eggs would be fertilized successfully (Zhang and Lin 2004). Encounter activity of the M and Em shrimp during premolt of Ef shrimp were analyzed. A positive response was defined as the M/Em shrimp showing obvious precopulatory behaviors (such as approach and follow) toward the parturial Ef shrimp. During the observations, water was not changed. M and Em shrimp were used only once.
Results
The present study shows that the minimum size of M shrimp able to mate successfully was 13.0 mm TL. Three of the five pairs mated successfully when the M shrimp were 13.0 mm TL. All five pairs failed to mate when the M shrimp were 12.0 mm TL.
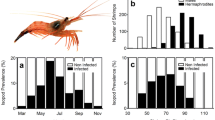
If the male-role shrimp was smaller than the receptive Ef shrimp, mating could still be successful even when the size difference reached 20.0 mm TL. At this difference two of the five pairs mated successfully (i.e., eggs were fertilized). However, if the male-role shrimp was larger than the Ef shrimp, mating failed in three of five pairs when the size difference was only 13.0 mm TL (Fig. 1).
Lysmata wurdemanni. Effect of size (TL) difference between M (male-phase)/Em (euhermaphrodite-phase serving as male) and Ef (euhermaphrodite-phase serving as female) shrimp on number of mating success. The height of each bar represents the number of successful matings out of the five pairs at the specified TL differences
In each of the two competition experiments, the Em shrimp were always larger than the M shrimp (Table 1). There was no difference (Student’s t test, t=0.82, NS) between the sizes of the Em shrimp with and without egg masses (Table 1). In the 1 M:2 Em experiment, 16 of the 30 matings occurred between the M and Ef shrimp. Of the remaining, eight Em shrimp with egg masses and six Em shrimp without egg masses mated with the Ef shrimp. There was no significant difference in mating ability (Chi-square test: χ2=1.14, NS) between the Em with and without egg masses. However, the mating frequency of the M shrimp with the Ef shrimp was significantly higher (Chi-square test: χ2=5.4, P<0.025) than that of the Em shrimp (Fig. 2). In the 2 M:2 Em experiment, 24 of the 30 matings were between the M shrimp and the Ef shrimp. The M shrimp mated more frequently (Chi-square test: χ2=10.8, P<0.005) with the Ef shrimp than their rival Em shrimp did. Of the six Em shrimp that mated with the Ef shrimp, two were large and four were small. However, among the M shrimp, although larger M shrimp (14) had more opportunity to mate with the Ef than smaller M shrimp (10), the difference was not significant (Chi-square test: χ2=0.67, NS). Mating frequency of M shrimp with Ef shrimp over that of Em shrimp with Ef shrimp increased significantly (Chi-square test, χ2=8.571, P<0.01) with increasing density and operational sex ratio (Fig. 2).
Lysmata wurdemanni. Comparative mating success of sexual phases with two sex ratios. Comparison based on assumption that M (male-phase) and Em (euhermaphrodite-phase serving as male) shrimp have the same access to the Ef (euhermaphrodite-phase serving as female) shrimp for mating. Observed (filled bars) and expected (open bars) numbers of the matings (out of 30) with ratio of A.1 M:2 Em:1 Ef and B. 2 M:2 Em:1 Ef are shown. At both sex ratios, the M shrimp mated more frequently with the E shrimp than the Em shrimp did (Chi-square test: χ2 =5.4, P<0.05 for 1 M : 2 Em treatment; χ2 =10.8, P<0.005 for 2 M:2 Em treatment)
The proportion of M shrimp displaying positive precopulatory behavior towards the Ef shrimp (79/90, 87.7%) was significantly higher (Chi-square test: χ2 =28.94, P<0.001) than that of the Em shrimp (72/120, 60.0%). No apparent competition behavior was observed among the male-role shrimp. The rule for copulation in the shrimp seems to be first come, first mate. When more than one male-role shrimp actively chased a newly molted Ef shrimp, the most active shrimp tended to do the mating. Although there was no apparent direct interaction (behavioral competition) among the male-role shrimp, a newly molted Ef shrimp reacted to the active chase. The Ef shrimp flipped to escape the male-role shrimp when (an)other male-role shrimp was (were) nearby and tried to mate. Under this circumstance, the first male-role shrimp generally succeeded in mating with the Ef shrimp, with the sperm transfer completed within ~2 s. Three Ef shrimp copulated twice in each of the two experiments. The second mating occurred within 30 s after the first.
Discussion
The hypothesis that there is no difference in mating ability between the M and Em L. wurdemanni was rejected based on the experimental data presented here. Smaller males (M) obtained more mating partners than the larger ones (Em). A greater proportion of the M shrimp displayed active precopulation behaviors than the Em shrimp. The mating frequency of the M shrimp with Ef shrimp was significantly higher than that of the Em shrimp. This differs from a previous study in which the Em shrimp were competitive with M shrimp of various sizes in obtaining copulations with Ef shrimp (Bauer 2002a). Different densities and operational sex ratios used in the present and Bauer’s (2002a) studies may be responsible for the difference. Bauer (2002a) did the experiment in 20-cm wide × 25-cm long × 15-cm high aquaria. Within each aquarium one Ef shrimp and two male-role shrimp, either M or Em, were housed. Density of the male-role shrimp was lower (2/500 cm2, equivalent to 40/m2) than in the present study (3 or 4/346 cm2, equivalent to 87 or 116/m2, respectively). Operational sex ratios were 3:1 and 4:1 in the present study, respectively, compared to 2:1 used by Bauer (2002a). The present study shows that mating frequency of M shrimp with Ef shrimp over that of Em shrimp with Ef shrimp increased significantly (Chi square test, χ2 =8.571, P<0.01) at the higher density and operational sex ratio. The present study shows that L. wurdemanni males mature at a minimum size of about 13.0 mm TL, and M/Em shrimp can mate with smaller and larger Ef shrimp even when the size difference reaches 13.0 and 20.0 mm TL, respectively. In the wild, the proportion of MP shrimp bigger than 13.0 mm TL is generally >50% during the reproductive season (Bauer 2002b). With M and Em shrimp combined, the population is highly male-biased. Hence, mating frequencies of M shrimp in the wild may be even higher.
Another difference between the present study and that of Bauer (2002a) is that size of M shrimp did not affect mating frequency. Bauer (2002a) found that large M shrimp gained significantly more matings than small M shrimp. Sizes of the M shrimp in the present study (large MP: 29.2±2.9 mm TL, 7.3±0.9 mm carapace length [CL], small M: 25.3±2.5 mm TL, 5.5±0.6 mm CL) were similar to those used in Bauer’s (2002a) experiment (large M: 7.4 mm CL, small M: 5.4 mm CL). However, the density and sex ratio were different between the two studies. Mating in L. wurdemanni seems to be first come, first mate, although the mating ability of M and Em shrimp was different. Size of male-role shrimp is not correlated with mating success. Multiple mating in this species occurs occasionally. Only three of the 30 Ef shrimp mated more than once (twice). The Ef shrimp do not display the “cleaning activities” to reject a spermapophore as in some other shrimp, for example a caridean shrimp Heptacarpus sitchensis (Bauer 1976). Moreover, a newly molted Ef shrimp (especially after copulation) tended to escape if more than one male-role shrimp were nearby. Escape behavior may also be a response to protect itself from predation during the most vulnerable postmolt period, as reported for another caridean shrimp Palaemonetes pugio (Berg and Sandifer 1984).
Male mating tactics are phenotypically plastic with respect to population density in many insects (Greenfield and Shelley 1985; French and Cade 1989; Cade and Cade 1992) and fish (Jirotkul 1999b). In species with a male-biased operational sex ratio, interference behavior between males has been reported in crustaceans (Jormalainen et al. 1994; Debuse et al. 1999), fish (reviewed by Clutton-Brock and Parker 1992; Jirotkul 1999a) and other taxa (reviewed by Clutton-Brock and Parker 1992). In L. wurdemanni, although there is no obvious direct male–male interaction (behavioral competition) in obtaining the receptive Ef shrimp for copulation, chase by more than one male-role shrimp stimulated the recently molted Ef shrimp to escape from being held by the male-role shrimp. Frequency of male chase increased with increasing density. Because M shrimp were more active than Em shrimp before copulation, they would have more of a chance than Em shrimp to hold the molted E for mating.
Explanations for why some large M shrimp do not change sex to E include social control (Lin and Zhang 2001; Baeza and Bauer 2004). The present study showed that the M shrimp apparently have advantages in mating over Em shrimp. This suggests that sexual selection or adaptation may also be important in controlling the sex change and causing variations of size at sex change in L. wurdemanni. The present study showed that the proportion of M shrimp that displayed active precopulatory behavior was significantly higher than for Em shrimp. An out-crossing simultaneous hermaphrodite only has an energetic advantage when the population density is low. If reproductive encounters are high then selection should favor gonochorists (Heath 1977). Strictly speaking, L. wurdemanni is not a pure functional simultaneous hermaphroditic species since some shrimp may remain as males (Bauer and Holt 1998; Lin and Zhang 2001). On the other hand, selection should favor simultaneous hermaphrodites that are able to control investment in male and female function in response to environmental conditions (Charnov and Bull 1977). So E shrimp may adapt to channel more energy to female activities and functions when M shrimp are abundant. The proportion of M shrimp in the population is often around 50% (Bauer 2002b) and some of the M shrimp are much larger than many of the E shrimp (Bauer and Holt 1998). That sex allocation in simultaneous hermaphroditic species is mating group size dependent has been predicted (reviewed by Charnov 1982) and demonstrated (e.g. Raimondi and Martin 1991). Individuals of the hermaphroditic species Catomerus polymerus allocate proportionately more of their reproductive resources to female function when they are in small mating groups than in large ones (Raimondi and Martin 1991). Moreover, mating may be costly to the male-role shrimp in precopulatory behavior and physiology. In the simultaneous hermaphroditic pond snail, Lymnaea stagnalis, copulation significantly reduces the egg-laying rate (Visser et al. 1994). Premating struggling female water striders (Aquarius remigis) consumed an average 126% more energy compared to the nonstruggling females (Watson et al 1998).
This study has demonstrated that operational sex ratio is an important factor influencing inter-male competition in L. wurdemanni. We suggest that the cost of precopulatory behavior may also strongly affect the intensity of sexual selection. M shrimp may delay changing sex because they may be selected when density is high. Future studies should focus on comparing sex change and mating behavior of low and high density species in the genus Lysmata to further understand the evolution of mating system and simultaneous hermaphroditism.
References
Anderson M (ed) (1994) Sexual selection. Princeton University Press, Princeton
Arak A (1983) male–male competition and mate choice in anuran amphibian. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 181–210
Baeza JA, Bauer RT (2004) Experimental test of socially mediated sex change in a protandric simultaneous hermaphrodite, the shrimp Lysmata wurdemanni (Caridea: Hippolytidae). Behav Ecol Sociobiol 55:544–550
Baldwin AP, Bauer RT (2003) Growth, survivorship, life span, and sex change in the hermaphroditic shrimp Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae). Mar Biol 143:157–166
Bauer RT (2002a) Tests of hypotheses on the adaptive of an extended male phase in the hermaphroditic shrimp Lysmata wurdemanni (Caridea: Hippolytidae). Biol Bull 203:347–357
Bauer RT (2002b) Reproductive ecology of a protandric simultaneous hermaphrodite, the shrimp, Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae). J Crust Biol 22:742–749
Bauer RT (ed) (2004) Remarkable shrimp: Adaptations and natural history of the carideans. University of Oklahoma Press, Norman
Bauer RT, Abdalla JH (2001) Male mating tactics in the shrimp Palaemonetes pugio (Decapoda, Caridea): Precopulatory mate guarding vs. pure searching. Ethology 107:185–199
Bauer RT, Holt GJ (1998) Simultaneous hermaphroditism in the marine shrimp Lysmata wurdemanni (Caridea: Hippolytidae): an undescribed sexual system in the decapod Crustacea. Mar Biol 132:223–235
Berg AV, Sandifer PA (1984) Mating behavior of the grass shrimp Palaemonetes pugio Holthuis (Decapoda, Caridea). J Crust Biol 4:417–424
Cade WH, Cade ES (1992) Male mating success, calling and searching behavior at high and low densities in the field cricket, Gryllus integer. Anim Behav 43:49–56
Calado R, Narciso L, Morais S, Rhyne AL, Lin J (2003) A rearing system for the culture of ornamental decapod crustacean larvae. Aquaculture 218:329–339
Charnov EL (ed) (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL, Bull J (1977) When is sex environmentally determined? Nature 266:828–930
Chiba S, Goshima S, Shinomiya Y (2003) male–male competition selects for delayed sex change in the protandrous shrimp Pandalus latirostris. Mar Biol 142:1153–1157
Christy JH (1987) Competitive mating, mate choice and mating associations of brachyuran crabs. Bull Mar Sci 41:177–191
Cleveland AL, Itzkowtiz M, Haley M (2002) Male variation in mating success after female numbers are reduced. J Fish Biol 60:179–177
Clutton-Brock TH, Parker GA (1992) Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456
Correa C, Thiel M (2003) Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena Historia Natural 76:187–203
Crespi BJ (1989) Causes of assortative mating in arthropods. Anim Behav 38:980–1000
Darwin C (ed) (1871) The descent of man, and selection in relation to sex. Murray, London
Debuse VJ, Addison JT, Reynolds JD (1999) The effects of sex ratio on sexual competition in the European lobster. Anim Behav 58:973–981
French BW, Cade WH (1989) Sexual selection at varying population densities in male field crickets Gryllus veletus and G. pennsylvanicus. J Insect Behav 2:115–121
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Greenfield MD, Shelley TE (1985) Alternative mating strategies in a desert grasshopper: evidence of density-dependence. Anim Behav 33:1192–1210
Heath D J (1977) Simultaneous hermaphroditism: cost and benefit. J Theor Biol 64:363–373
Jirotkul M (1999a) Operational sex ratio influences female preference and male–male competition in guppies. Anim Behav 58:287–294
Jirotkul M (1999b) Population density influences male–male competition in guppies. Anim Behav 58:1169–1175
Jormalainen V (1998) Precopulatory mate guarding in crustaceans: male competitive strategy and intersexual conflict. Q Rev Biol 73:275–304
Jormalainen V, Merilaita S, Tuomi J (1994) Male choice and male–male competition in Idotea baltica (Crustacea, Isopoda). Ethology 96:46–57
Lin J, Zhang D (2001) Reproduction in a simultaneous hermaphroditic shrimp, Lysmata wurdemanni: any two will do? Mar Biol 139:919–922
Raimondi PT, Martin JE (1991) Evidence that mating group size affects allocation of reproductive resources in a simultaneous hermaphrodite. Am Nat 138:1206–1217
Ridley M (ed) (1983) The explanation of organic diversity: the comparative method and adaptations for mating. Clarendon, Oxford
Ridley M, Thompson DJ (1985) Sexual selection of population dynamics in aquatic Crustacea. In: Sibly RM, Smith RH (eds) Behavioral ecology: Ecological consequences of adaptive behavior. Blackwell, Oxford, pp 409–422
Sirot LK, Brockmann HJ (2001) Costs of sexual interactions to females in Rambur’s forktail damselfly, Ischnura ramburi (Zygoptera: Coenagrionidae). Anim Behav 61:415–424
Thornhill R, Alcock S (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Visser JAGM De, Maat AT, Zonneveld C (1994) Energy budget and reproductive allocation in the simultaneous hermaphrodite pond snail, Lymnaea stagnalis (L.): a trade-off between male and female function. Am Nat 144:861–867
Watson PJ, Arnqvist G, Stallmann RR (1998) Sexual conflict and the energetic costs of mating and mate choice in water striders. Am Nat 151:46–58
Wickler W, Seibt U (1981) Monogamy in Crustacea and man. Z Tierpsychol 57:215–234
Zhang D, Lin J (2004) Mating without anterior pleopods in a simultaneous hermaphroditic shrimp, Lysmata wurdemanni (Decapoda, Caridea). Crustaceana 77:1203–1212
Zhang D, Lin J (2005) Development of sexual morphs in two simultaneous hermaphroditic shrimp, Lysmata rathbunae and Lysmata wurdemanni. Invert Reprod Dev (in press)
Zhang D, Lin J, Creswell RL (1998) Effects of the food and temperature on survival and development in the peppermint shrimp Lysmata wurdemanni. J World Aquacult 29:471–476
Acknowledgements
The study was partially supported by a contract from Shrimp Culture Technologies, Inc. Dr. Martin Thiel and anonymous reviewers provided valuable comments on a draft of this manuscript. The experiments comply with current laws of the United States.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle
Rights and permissions
About this article
Cite this article
Zhang, D., Lin, J. Comparative mating success of smaller male-phase and larger male-role euhermaphrodite-phase shrimp, Lysmata wurdemanni (Caridea: Hippolytidae). Marine Biology 147, 1387–1392 (2005). https://doi.org/10.1007/s00227-005-0029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0029-y