Abstract
Settlement into the benthic habitat may be an important process in regulating sea urchin abundance, which potentially modifies the structure of benthic communities. Strong settlement events may increase sea urchin abundance beyond a certain threshold, leading to the formation of coralline barrens (overgrazed communities with a dominance of encrusting coralline algae). To understand the role of settlement in regulating sea urchin populations we first need to determine settlement variability. Temporal variation in settlement of the sea urchin Paracentrotus lividus was monitored at three sites in the Medes Islands, NW Mediterranean, during three settlement seasons (March 1998 through October 2000). Spatial variation in settlement was studied in 1999 at 50 sites along a gradient of exposures to waves and currents, inside and outside the archipelago, and separated by distances from tens to thousands of meters. Bathymetric distribution of settlement was also studied in 2000 at six sites at 5, 10, 15 and 20 m depths. Settlement of P. lividus occurred in a single annual peak within 3 weeks in May–June. Differences in settlement between years were more than two orders of magnitude. Spatial variability was found at all scales investigated, showing strong patchiness at the smallest spatial scales (tens of meters). Sea urchins settled preferentially at depths between 5 and 10 m. Substratum type, level of protection, and adult population densities were not significant in determining settlement. However, settlement was found to be related to the degree of exposure to waves and currents, indicating that physical processes are very important at the spatial scales investigated. This greatly variable settlement is a necessary, although not sufficient, condition to create gradients of adult P. lividus abundance. Further studies should be designed to investigate the interaction between settlement strength and post-settlement mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea urchins have a key role in structuring infralittoral benthic communities. At high densities they can turn complex algal communities into coralline barrens with low complexity and diversity (Lawrence 1975; Harrold and Pearse 1987; VanBlaricom and Estes 1988). It has been suggested that abundant predators can regulate sea urchin abundance and inhibit the creation of coralline barrens (Duggins 1980; Tegner and Dayton 1981; Tegner and Levin 1983; Estes et al. 1998; Sala et al. 1998). However, mosaics of complex algal communities and coralline barrens have been observed at some sites where predator abundance is high (Foster 1990; Sala et al. 1998). This can occur if the effects of sea urchin settlement patterns overcome in the short term the longer term controlling effects of post-settlement mortality (e.g. Menge 2000). A necessary, although not sufficient, condition for these coralline barrens to develop is, hence, that the strong settlement episodes take place, and that sea urchin settlement exhibits great variability in time and space. This paper is part of a larger study designed to investigate the interaction between predatory fishes, sea urchins and algae in the Mediterranean sublittoral. It has been shown that the abundance of a sea urchin (Paracentrotus lividus) and the processes controlling it, such as settlement and predation, play key roles in determining the structure of the whole community structure in this system (Sala et al. 1998). Here, we present the results of a study aimed at describing the spatio-temporal variability of sea urchin settlement.
Settlement is an important process in determining adult population structure and dynamics (Underwood and Denley 1984; Connell 1985; Gaines and Roughgarden 1985; Roughgarden et al. 1985), and it can be extremely important in situations with high larval supply and low post-settlement mortality (Paine and Vadas 1969; Talbot et al. 1978; Vance 1979; Verlaque 1984; Andrew 1993), but it can also be important in shaping communities when supply is limited (e.g. Roughgarden et al. 1985). Sea urchin settlement can be highly variable at both temporal and spatial scales (Ebert 1983; Keesing et al. 1993; Balch and Scheibling 2000; Lamare and Barker 2001).
In the Mediterranean infralittoral, the most important grazer is the sea urchin P. lividus (Lamarck) (Verlaque 1987). Episodes of strong P. lividus proliferation leading to severe regression of algal assemblages have been reported repeatedly (Kempf 1962; Nédelec 1982; Vukovic 1982; Verlaque 1984, 1987). Spatial and temporal variability in settlement of P. lividus might be a key factor in the fate of infralittoral Mediterranean communities, which has not yet been adequately quantified. Both the unpredictability of the number of settlement events (one or two peaks a year) and the duration of the larval stage of P. lividus (20–40 days; Fenaux et al. 1985; Pedrotti 1993) suggest the potential for highly variable settlement episodes (Lozano et al. 1995; Sala and Zabala 1996; Lopez et al. 1998). The objectives of the present study were to quantify the variability of P. lividus settlement at different spatial scales (along the depth gradient and from tens to thousands of meters in a geographical sense) and at different temporal scales (intra- and inter-annual).
Materials and methods
Study site
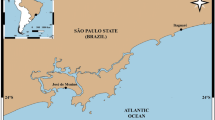
The study was carried out in the Medes Islands Marine Reserve and on the nearby coast (NW Mediterranean Sea) (Ros et al. 1984). The reserve, where fishing has been prohibited (93.2 ha) since 1983, is located 1 km offshore from the town of l’Estartit (42°16′N; 03°13′E) and encompasses a group of small calcareous islands (total surface <20 ha). The unprotected nearby coast is also calcareous and harbors the same types of habitats. The study was carried out on rocky bottoms between 5 and 20 m in depth, which were colonized by a rich algal assemblage dominated by erect algae, articulated calcareous algae and small filamentous algae (Sala and Boudouresque 1997).
Settlement collectors
Settlement of Paracentrotus lividus was determined using artificial collectors. Artificial collectors are a non-destructive method, easily installed and replaced, and the samples are easily and quickly analyzed. Artificial collectors, by providing both standardized surface and rugosity, reduce factors affecting settlement and appear to be sufficient for obtaining a reliable relative index of settlement (Harrold et al. 1991). Nevertheless, the possibility that other processes affect settlement in collectors (e.g. predation on settlers, natural mortality) cannot be excluded. Other studies of settlement of echinoderms have used different collectors: oyster shells (Loosanoff 1964), rigid plastic (Tegner 1989), plastic light diffusers (Bak 1985), plastic matrix and articulated coralline algae in a PVC pipe (Harrold et al. 1991), artificial grass (Ebert et al. 1991) and scrub brushes with nylon bristles (Ebert et al. 1994). After an initial experiment with oyster shells, plastic matrix, artificial grass and different types of scrub brushes in 1997 (Table 1), scrub brushes with vegetal bristles were selected as the most effective and easily treatable collectors for P. lividus (ANOVA, F 4,15=10.84, P<0.01). Brushes had an 18×6 cm wooden base with 2.5-cm-long vegetal bristles. The advantage of this type of collector is that no special manufacture is required, it is cheap, and easily available for extensive replication (Ebert et al. 1994). Each collector was composed of groups of four brushes attached to nylon lines. The lines were anchored at the bottom and suspended in the water column with a small subsurface float. On each line, the first pair of brushes was 30 cm above the bottom and the second pair 10 cm above the lower pair.
Brushes were collected in situ, placed in individual plastic bags in an ice chest, and transported to the laboratory for sorting. To remove recruits from the collectors individual brushes were cleaned in a high-pressure freshwater shower, rubbing bristles by hand. A prior experiment comparing this method with that of Ebert et al. (1994) using a sonic cleaner showed no significant differences in sea urchin recovery rate between treatments (t-test, t=1.32, P=0.21). Following cleaning, the water was filtered through a 250 µm sieve, and sea urchins and other organisms were collected and preserved in 70% ethanol. Identification and measurement procedures of settled sea urchins and other echinoderm species were carried out under the microscope.
Temporal variability of settlement
Three sites with similar topography (boulders >10 m3), orientation (approximately S), wave exposure, depth (5–10 m) and algal assemblages [a transition between Padino–Cladostephetum hirsutae Feldmann (1938) and Rhodimenio–Codietum vermilarae Ballesteros (1989)] were randomly chosen for the study of temporal variability among all possible sites sharing these habitat characteristics (Fig. 1). Water temperature was recorded weekly with a CTD from a hydrographic station located ~2 miles offshore and north of the Medes Islands (J. Pascual, unpublished data).
Patterns of settlement were monitored from March 1998 through October 2000. At each site, collectors were sampled and new collectors were installed weekly during the highest settlement period (spring–late summer) and every other week during the rest of the year. Some gaps occurred in the temporal series as a result of losses caused by storms that prevented retrieval of collectors. Gaps occurred mostly during winter, but never during the potential period of peak settlement (Fig. 2).
Spatial variability of settlement
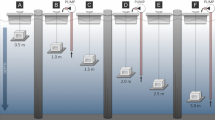
To monitor spatial variability in P. lividus settlement an experiment was carried out during the 1999 settlement peak. Fifty collectors (200 brushes in total) were distributed at 50 randomly selected sites at least tens of meters from the nearest collector (Fig. 1). We placed 32 collectors in the marine reserve and 18 in the adjacent unprotected area, randomly distributed in different orientations and at different wave exposures, from depths of 6 to 9 m. All collectors were deployed on 17 May and collected on 27 May 1999. To determine the variability in settlement with depth, collectors were deployed at four depths (5, 10, 15 and 20 m) at three sites in the Medes Islands and three sites along the nearby coast (Fig. 1) on 11 May and collected on 21 May 2000. Five sites in the spatial-variability study and one site in the depth-gradient study were not used in the analysis because of the loss of collectors due to rough seas.
Variability of settlement among benthic substrata
We carried out an experiment to determine the variability of settlement on different substrata. The experiment was carried out in spring because this is the period of largest abundance of P. lividus larvae in plankton samples (Lopez et al. 1998). Three different substrata, simulating a gradient of habitat complexity corresponding to erect algal assemblages, coralline barrens, and a habitat with no structural complexity (no algae), were placed on ceramic 30×30 cm plates. A total of 9 thalli per plate of the brown alga Halopteris scoparia were used to simulate an erect algal assemblage, and 12 empty oyster shells (covered with encrusting corallines) per plate were used to simulate a coralline barren. Brown algae are known to be poorer inducers of sea urchin metamorphosis than articulated calcareous algae (Harrold et al. 1991), which were also abundant in the algal assemblage. Our choice was justified because Corallina elongata thalli were easily lost and also because the brown alga H. scoparia was the dominant architectural builder in the assemblage. Thalli of H. scoparia were tied at the base using monofilament and then attached to a plastic grid placed on the plate. Oyster shells (individual valves) were tied and fixed to the plastic grid with their external side, covered with encrusting coralline algae, exposed to the open water. Settlement plates were placed on the bottom, and, prior to collection, the underside was cleaned to eliminate settlers sheltered therein. Five collectors per treatment were placed at each sampling site (Cova de la Reina, Sant Estiu and Racó del Portitxol) for a period of 10 days (15–25 May 1998), and then collected.
The settlement–abundance relationship of adult sea urchins
To determine whether the variability in sea urchin settlement was associated with the variability in adult sea urchin abundance, we conducted visual censuses of adults. Numbers and sizes of adult P. lividus were quantified by SCUBA diving along randomly located 50×1 m transects at 11 sites inside and outside the marine reserve, where settlement collectors had been placed. Sampling (three transects per site and date) was carried out in the settlement season. We hypothesized that settlement is decoupled from the local abundance of reproductive adults (>3 cm in diameter, Lopez et al. 1998).
Data analysis
To test for differences in sea urchin settlement between site (random factor) and years (fixed factor), two-way ANOVAs were performed. Due to strong seasonality, only samples conducted during the settlement period were used in the analysis. To test for differences between sampling sites in the experiment of spatial distribution, one-way ANOVA was performed.
To understand the heterogeneity of settlement, we determined the spatial autocorrelation structure for P. lividus settlers using the Spatial Autocorrelation Analysis Package (SAAP version 4.3 by D. Watemberg). Moran’s I autocorrelation coefficient was calculated for 19 distance classes. Moran’s I ranges from −1 to 1, 0 indicating no correlation, negative values indicating negative autocorrelations, and positive values indicating positive autocorrelation. A positive autocorrelation indicates that settlement at each sampling site has greater similarity to that in other sites than could be expected by chance (Legendre and Legendre 1998). To test for differences in sea urchin settlement between sites (random factor) and depth (fixed factor), a two-way ANOVA was performed.
To test for differences in settlement between substrata, a two-way ANOVA was performed, with site (random factor) and substratum type (fixed factor) as independent variables. To test for differences in the number of settled P. lividus between level of protection (reserve vs. unprotected area), orientation (N, E, S, W) and exposure, an ANOVA was performed. We determined orientations between 315°N and 45°N, between 45°E and 135°E, between 135°S and 225°S and between 225°W and 315°W.
For all analyses, Cochran’s tests were conducted prior to ANOVA to test the assumption of homogeneity of variance. When necessary, data were log-transformed to satisfy this assumption. When differences were significant, post hoc comparisons were made using the Student–Newman–Keuls’ (SNK) test.
Results
Temporal variability in settlement
Settlement of Paracentrotus lividus exhibited a clear seasonal pattern, with a consistent single settlement peak from May to early July (Fig. 2), when surface water temperature starts to increase (settlement peak occurred between 14°C and 18°C).
The number of settled sea urchins showed significant differences through time (F 2,4=22.31, P<0.01) and between sites (F 2,79=4.22, P<0.05), but the interaction was not significant (F 2,79=0,673, P=0,637). Settlement of P. lividus in the Medes Islands showed strong interannual variation, settlers being scarce in 1998 (mean±SD: 3.00±2.76 collector−1) and 12 and 47 times more abundant in 1999 (38.30±26.72) and 2000 (141.37±77.07), respectively (Fig. 2). Sea urchin larvae collected with artificial collectors in all experiments ranged between 230 and 583 µm (mean±SD: 374±48). We could not determine the existence of distinct pulses of larval arrival, because samples were collected weekly and it was impossible to know the size of the urchins immediately after settlement.
Spatial variability in settlement
P. lividus settlement varied significantly from site to site, the number of settled urchins ranging from (mean±SD) 6.3±3.8 to 109.0±12.1 urchins collector−1 (ANOVA, F 44,128=9.11, P<0.01) (Fig. 1). These results reinforce those above on a different scale, since sites were separated by distances from tens to thousands of meters (Fig. 1). There was no autocorrelation in the shortest distances (Fig. 3); thus, we can assume a highly heterogeneous distribution of settlement, even at a scale of tens of meters.
Paracentrotus lividus. Correlogram showing the spatial correlation of sea urchin settlers at the different sampling sites. Moran’s autocorrelation coefficient I is shown in relation to 19 distance classes (class with variable). Moran’s I coefficient is based on a minimum of ten centroid pairs. Filled squares represent significant autocorrelation coefficients at the P=0.05 level, after applying the Bonferroni correction to test for the overall significance of the correlogram
P. lividus settled at greater abundance at 5 and 10 m than at deeper habitats at all sites (Fig. 4) (ANOVA, F 3,12=70.23, P<0.001). The analysis also showed significant differences between sites (F 4,56=13.28, P<0.001), although there was no interaction between depth and site (F 12,56=1.05, P=0.414).
Settlement showed no significant differences between the Medes Islands and the nearby coast (ANOVA, F 1,171=3.33, P=0.07). However, settlement was significantly different between orientations of sampling sites (ANOVA, F 3,169=6.77, P<0.01). Settlement in sites oriented N was significantly lower in comparison to those oriented S, E and W, the latter showing the strongest settlement (SNK test, P<0.01) (Fig. 5). Settlement at exposed sites (mean±SD: 43.57±32.08 settlers collector−1) was significantly higher than in bays and at protected sites (31.00±28.08 settlers collector−1) (ANOVA, F 1,171=11.46, P<0.01).
Substratum selection
Settlement of P. lividus did not show significant differences between experimental substrata (F 2,4=0.16, P=0.86), nor interaction between substrata and site (F 4,36=1.32, P=0.28), although there were significant differences among sites (F 2,36=13.33, P<0.001) (Fig. 6). These results suggest that P. lividus larvae do not have substrate preferences for settlement. Thus, spatial variability in settlement must be due to factors other than larval substratum selection behavior.
The settlement–adult population size relationship
The number of settled P. lividus showed no significant correlation with local abundance of adult sea urchins (Pearson’s F=4.53; df=1,9; P=0.062), although the probability was very close to the 5% significance level (Fig. 7). The percentage of variance explained by the model was very low (r 2=0.33), thus suggesting that adult densities and settlement are decoupled at the spatial scales investigated in this study.
Discussion
The intensity of Paracentrotus lividus settlement in the Medes Islands Marine Reserve and nearby unprotected area was highly variable in time (more than two orders of magnitude in number of settlers per collector), within and between years. However, >90% of the yearly settlement occurred in a single peak within 3 weeks in May–June. These results confirm recent reports of a single settlement peak during early summer (Lozano et al. 1995; Sala and Zabala 1996; Lopez et al. 1998), in contrast to previous studies conducted in other Mediterranean regions suggesting a longer settlement period or two distinct peaks in spring and fall (Fenaux 1968; Crapp and Willis 1975; Verlaque 1984; Guetaff et al. 2000). Discrepancies among studies could be explained by the variability in the time and intensity of smaller secondary settlement episodes (Lopez et al. 1998), which were not detected in the present study. However, they may also be an artifact of data collection methods, since studies reporting two settlement peaks did not monitor settlement, but the gonadic cycle of reproductive adults. Seasonality of sea urchins settlement has also been shown for other species for which temporal settlement patterns are predictable at sites located hundreds of kilometers away (Ebert et al. 1994). Settlement in the study region shows a clear synchronicity with planktonic echinoid larval peaks during spring (Lopez et al. 1998), although we did not find the second settlement phase to correspond to the second peak of planktonic larval abundance described by Pedrotti (1993).
Spatial variability in settlement of P. lividus was striking at all scales investigated, from tens to thousands of meters. The lack of significant autocorrelations between sampling sites located tens of meters apart indicates settlement heterogeneity of a very small patch size (tens of meters). Our results contrast with those of Keesing et al. (1993), who found that echinoid settlement showed spatial variability at a scale of thousands of meters, but not at smaller scales. Spatial autocorrelations are notoriously affected by singularities in the data (i.e. a very localized clump of individuals) and where they happen to occur. Thus, we do not give the 300–400 m correlation any special significance. The most significant result of this analysis is the absence of correlation at even the smallest spatial scales.
The striking spatial heterogeneity of P. lividus settlement is probably a result of the heterogeneity in the distribution of sea urchin larvae in the water column, which could be explained by several influences such as the aggregative feeding behavior of larvae (Metaxas and Young 1998; Starr et al. 1990), heterogeneous mortality in plankton by predation (Tegner and Dayton 1981), food limitation (Ebert 1983; Olson and Olson 1989; Lopez et al. 1998) and purely physical factors such as circulation (Pedrotti and Fenaux 1992; Balch et al. 1999). Our results also suggest that coastal topography, in particular orientation and exposure to waves, may be important in determining settlement, since the ranking of abundance of settlers among the three permanent sites was maintained throughout the study period.
According to these results, it is not surprising that there was no significant correlation between settlement and local abundance of reproductive P. lividus at the spatial scales considered (tens to thousands of meters). The planktonic life span of P. lividus larvae (20–40 days, Fenaux et al. 1985; Pedrotti 1993) can allow significant dispersal distances. Although P. lividus larvae could be retained locally but settle heterogeneously because of local processes, the available evidence suggests that reproduction and settlement of P. lividus are decoupled at spatial scales smaller than thousands of meters.
P. lividus settlement had a clear peak between 5 and 10 m, which indicates that P. lividus larval behavior may exert some control on settlement, although our results also suggest that substrate is not a determinant cue for P. lividus settlement.
Differences in numbers of settlers between natural substrata and brushes may be due to the different textures and the locations with regard to the bottom. While brushes were suspended in the water column, plates were disposed on the bottom and were thus accessible to possible micropredators and sedimentation. Nevertheless, these two methods were designed to test different hypotheses, and not to obtain absolute settlement densities.
What are the implications of this heterogeneity in settlement for the distribution and abundance of P. lividus populations? The depth-related settlement pattern is similar to patterns of adult P. lividus abundance, with the highest counts in shallow habitats and decreasing abundance below 10 m (Kempf 1962; Gamble 1965; Harmelin et al. 1980; Chelazzi et al. 1997). Since the mobility of P. lividus is low (on the scale of a few meters; Dance 1987; Sala 1996), settlement appears to be a major factor in determining the distribution and relative abundance of P. lividus. However, our results do not allow us to define the role of settlement in determining absolute abundance of P. lividus. Although both strong and variable settlement may be necessary conditions for the sudden outbursts of P. lividus, they are not necessarily sufficient stimulants, because the transition from a large abundance of P. lividus recruits to adult numbers will also be affected by processes not considered in this study, such as post-settlement predation. Prior studies have pointed out the important role of post-settlement events (mostly mortality) in shaping adult sea urchin populations (Cameron and Schroeter 1980; Rowley 1989). Mortality of post-settled sea urchins can be very high (Rowley 1990). In the Mediterranean, mortality of recruits has been estimated to be at least 75% over the first 6 months (Sala and Zabala 1996) and >99% over the first year after settlement (Lopez et al. 1998). Further demographic and experimental studies are needed to determine whether the patchy, strong settlement episodes reported here have long-term demographic and community-wide consequences.
References
Andrew NL (1993) Spatial heterogeneity, sea urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74:292–302
Bak RP (1985) Recruitment patterns and mass mortality in the sea urchin Diadema antillarum. In: Gabrié C, et al (eds) Proc 5th Int Coral Reef Congr, vol 5. Antenne Museum–EPHE, Moorea, French Polynesia, pp 267–272
Balch T, Scheibling RE (2000) Temporal and spatial variability in settlement and recruitment of echinoderms in kelp beds and barrens in Nova Scotia. Mar Ecol Prog Ser 205:139–154
Balch T, Hatcher BG, Scheibling RE (1999) A major settlement event associated with minor metereologic and oceanographic fluctuations. Can J Zool 77:1657–1662
Ballesteros E (1989) Estructura y dinámica de la comunidad infralitoral de Codium vermilara (Olivi) Della Chiaje de la Costa Brava (Mediterráneo occidental). An Biol 15:191–208
Cameron RA, Schroeter SC (1980) Sea urchin recruitment: effect of substrate selection on juvenile distribution. Mar Ecol Prog Ser 2:243–247
Chelazzi G, Serra G, Bucciarelli G (1997) Zonal recovery after experimental displacement into two sea urchins co-occurring in the Mediterranean. Mar Ecol Prog Ser 212:1–7
Connell JH (1985) The consequences of variation in initial settlement vs. post-settlement mortality in rocky intertidal commmunities. J Exp Mar Biol Ecol 93:11–45
Crapp GB, Willis ME (1975) Age determinantion in the sea urchins Paracentrotus lividus (Lamarck), with notes on the reproductive cycle. J Exp Mar Biol Ecol 20:157–178
Dance C (1987) Pattern of the activity of the sea urchin Paracentrotus lividus in the bay of Port Cros (Var, France, Méditerranean). Mar Ecol 8:131–142
Duggins DO (1980) Kelp beds and sea otters: an experimental approach. Ecology 61:447–453
Ebert TA (1983) Recuitment in echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol I Balkema, Rotterdam, pp 169–203
Ebert TA, Schroeter SC, Dixon JD (1991) Studies of the feasibility of sea urchin enhancement in California. In: Final Tech Rep FG9310. California Department of Fish and Game, Sacramento, Calif., pp 1–21
Ebert TA, Schroeter SC, Dixon JD, Kalvass P (1994) Settlement patterns of red and purple sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in California, USA. Mar Ecol Prog Ser 111:41–52
Estes JA, Tinker MT, Williams TM, Doak DF (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Feldmann J (1938) Recherches sur la vegetation marine de la Méditerranée. La Côte des Albères. Rev Algol 10:1–340
Fenaux L (1968) Maturation des gonades et cycle saisonnier des larves chez A. lixula, P. lividus et P. microtuberculatus (Echinides) à Villfranche-Sur-Mer. Vie Milieu 19:1–52
Fenaux L, Cellario C, Etienne M (1985) Variations in the ingestion rate of algal cells with morphological development of larvae of Paracentrotus lividus (Echinodermata: Echinoidea). Mar Ecol Prog Ser 24:161–165
Foster (1990) Organization of macroalgal assemblages in the Northeast Pacific: the assumption of homogeneity and the illusion of generality. Hydrobiologia 192:21–33
Gaines S, Roughgarden J (1985) Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711
Gamble JC (1965) Some observations of the behaviour of two regular echinoids. Symp Underwater Assoc Malta 1965:47–50
Guetaff M, San Martin GA, Francour P (2000) Interpopulation variability of the reproductive cycle of Paracentrotus lividus (Echinodermata: Echinoidea) in the south-western Mediterranean. J Mar Biol Assoc UK 80:899–907
Harmelin JG, Bouchon C, Duval C, Hong JS (1980) Les echinoderms de substrats durs de de l’ille de Port-Cros, Parc National (Méditerranée nord-occidental). Élement pour un inventaire quantitative. Trav Sci Parc Natl Port-Cros 6:25–38
Harrold C, Pearse JS (1987) The ecological role of echinoderms in kelp forests. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 2. Balkema, Rotterdam, pp 137–233
Harrold C, Lisin S, Light KH, Tudor S (1991) Isolating settlement from recruitment of sea urchins. J Exp Mar Biol Ecol 147:81–94
Keesing JK, Cartwright CM, Hall KC (1993) Measuring settlement intensity of echinoderms in coral reefs. Mar Biol 117:399–407
Kempf M (1962) Recherches d’écologie comparée sur Paracentrotus lividus (Lmk) et Arbacia lixula (L). Recl Trav Stn Mar Endoume Fac Sci Mars 25:47–115
Lamare MD, Barker MF (2001) Settlement and recruitment of the New Zealand sea urchin Evechinus chloroticus. Mar Ecol Prog Ser 218:153–166
Lawrence JM (1975) On the relationship between marine plants and sea urchin. Oceanogr Mar Biol Annu Rev 13:213–286
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Loosanoff VL (1964) Variation in time and intensity of setting of the starfish, Asterias forbesi, in Long Island Sound during a twenty-five year period. Biol Bull (Woods Hole) 126:423–439
Lopez S, Turon X, Monterio E, Palacin C, Duarte CM, Tarjuelo I (1998) Larval abundance, recruitment and early mortality in Paracentrotus lividus (Echinoidea). Interannual variability and plankton–benthos coupling. Mar Ecol Prog Ser 172:239–251
Lozano J, Galera J, López S, Turon X, Palacín C, Morera G (1995) Biological cycles and recruitment of Paracentrotus lividus (Lamarck) (Echinodermata: Echinoidea) in two contrasting habitats. Mar Ecol Prog Ser 122:179–191
Menge BA (2000) Recruitment vs. postrecruitment processes as determinants of barnacle population abundance. Ecol Monogr 70:265–288
Metaxas A, Young CM (1998) Responses of echinoid larvae to food patches of different algal densities. Mar Biol 130:433–445
Nédelec H (1982) Etologie alimentaire de Paracentrotus lividus dans le baie de Galeria (Corse) et son impact sur les peuplements phytobenthoniques. PhD thesis, Univ Pierre et Marie Curie and Univ Aix-Marseille II, Marseille
Olson RR, Olson MH (1989) Food limitation of planktotrophic marine invertebrate larvae: does it control recruitment success? Annu Rev Ecol Syst 20:225–247
Paine RT, Vadas RL (1969) The effects of grazing by sea urchins Strongylocentrotus ssp. on benthic algal populations. Limnol Oceanogr 14:710–719
Pedrotti ML (1993) Spatial and temporal distribution and recruitment of echinoderm larvae in the Ligurian Sea. J Mar Biol Assoc UK 73:513–530
Pedrotti ML, Fenaux L (1992) Dispersal of echinoderm larvae in a geographical area marked by upwelling (Ligurian Sea, NW Mediterranean). Mar Ecol Prog Ser 86:217–227
Ros J, Olivella I, Gili JM (1984) Els sistemes naturals de les Illes Medes. Institut d’Estudis Catalans, Barcelona
Roughgarden J, Iwasa I, Baxter C (1985) Demographic theory for an open marine population with space-limited recruitment. Ecology 66:54–67
Rowley RJ (1989) Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea-urchin barren ground and a kelp bed: are populations regulated by settlement or post-settlement processes? Mar Biol 100:485–494
Rowley RJ (1990) Newly settled sea urchins in a kelp bed and urchin barren ground: a comparison of growth and mortality. Mar Ecol Prog Ser 62:229–240
Sala E (1996) The role of fishes in the organization of a Mediterranean subtidal community. PhD thesis, Univ Aix-Marseille II, Marseille
Sala E, Boudouresque CF (1997) The role of fishes in the organization of a Mediterranean sublittoral community. I. Algal communities. J Exp Mar Biol Ecol 212:25–44
Sala E, Zabala M (1996) Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar Ecol Prog Ser 140:71–81
Sala E, Boudouresque CF, Harmelin-Vivien M (1998) Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82:425–439
Starr M, Himmelman JH, Therriault JC (1990) Direct coupling of marine invertebrate spawning with phytoplankton blooms. Science 247:1071–1074
Talbot FH, Russell BC, Anderson GRV (1978) Coral reef fish communities: unstable or high-diversity systems? Ecol Monogr 48:425–440
Tegner MJ (1989) The feasibility of enhancing red sea urchin Strongylocentrotus franciscanus, stocks in California: an analysis of the options. Mar Fish Rev 51:1–22
Tegner MJ, Dayton PK (1981) Population structure, recruitment and mortality of two sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in a kelp forest. Mar Ecol Prog Ser 5:255–268
Tegner M, Levin LA (1983) Spiny lobsters and sea urchins: analysis of a predator–prey interaction. J Exp Mar Biol Ecol 73:125–150
Underwood AJ, Denley EJ (1984) Paradigms, explanations and generalizations in models for the structure of intertidal communities on rocky shores. In: Strong DR, Simberloff D, Abele LG, Thliste AB (eds) Ecological communities: conceptual issues and the evidence. Princeton University Press, Princeton, pp 151–180
VanBlaricom GR, Estes JA (1988). The community ecology of sea otters. Springer, Berlin Heidelberg New York
Vance RR (1979) Effects of grazing by the sea urchins Centrostephanus coronatus on prey community composition. Ecology 60:537–546
Verlaque M (1984) Biologie des juvéniles de l’oursin herbivore Paracentrotus lividus (Lamarck): séléctivité du broutage et impact de l’espèce sur les communautés de substrat rocheux en Corse (Méditeranée, France). Bot Mar 27:401–424
Verlaque M (1987) Relations entre Paracentrotus lividus (Lamrk) et le phytobenthos de Méditeranée occidentale. In: Boudouresque CF (ed) Colloque international sur Paracentrotus lividus et les oursins comestibles. GIS Posidonie, Marseille, pp 5–36
Vukovic A (1982) Florofaunistic changes in the infralittoral zone after Paracentrotus lividus (L.) population explosion. Acta Adriat 23:237–241
Acknowledgements
We are grateful to O. Guadayol, J. Pujol, N. Teixido, D. Diaz, F. Tomas and M. Marí for field and laboratory assistance. Thanks to E. Ballesteros and three anonymous referees for comments and discussions. This study was funded by grant MAR1999-0526 of the Ministry for Science and Technology of Spain, and by the Department of the Environment of the Catalan Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Hereu, B., Zabala, M., Linares, C. et al. Temporal and spatial variability in settlement of the sea urchin Paracentrotus lividus in the NW Mediterranean. Marine Biology 144, 1011–1018 (2004). https://doi.org/10.1007/s00227-003-1266-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1266-6