Abstract
Walls of egg capsules of the gastropod Crepidula fecunda Gallardo, 1979 were examined at different developmental stages during the period of intracapsular development of embryos. The weight, biochemical composition, and structural features (using scanning and transmission electron microscopy) of the capsule walls were examined at several intervals during development of the embryos to the hatching stage. Biochemical analyses were also carried out on the intracapsular fluid to identify the possible transfer of organic material from the capsule wall to the intracapsular fluid. The capsule wall is composed principally of organic matter, primarily protein (91%), plus minor lipid and carbohydrate components. The capsule wall consists of a thin, fibrous external layer, which overlies a thicker, spongy inner layer. The spongy layer has almost disappeared by the end of the developmental period, losing about 90% of its thickness. The 40% loss in weight of the capsule walls over the developmental period is due to loss of organic matter as protein. This suggests that the inner layer of the capsules dissolves and/or disintegrates as larval development advances. The levels of dissolved and/or particulate proteins in the intracapsular fluid are much higher than those typical of the seawater surrounding the capsules. This suggests that, as embryonic development proceeds, the inner capsule walls could potentially provide extra nutrients to the embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many marine invertebrates whose eggs undergo partial or complete development in the benthos deposit them in enclosing structures, which range from multilaminated capsules to fragile gelatinous masses and belts (Fretter and Graham 1994). The encapsulation allows benthic development of the embryos and is particularly common in polychaetes and gastropod molluscs (Pechenick 1979). Capsules protect embryos against desiccation and predation (Pechenik 1979), osmotic stress (Pechenik 1982, 1983), microorganisms (Pechenik et al. 1984; Lord 1986), and UV irradiation (Rawlings 1996). In some gastropod species, females may deposit nutritive eggs along with the embryos, which the embryos use as an energy source during development (Gallardo and Garrido 1987; Chaparro and Paschke 1990). Most species that deposit eggs rely on energy reserves within the oocytes, and in some cases also on organic substances dissolved in the capsular fluid bathing the embryos. The importance of intracapsular fluid as a nutrient reserve has been recognized in pulmonate gastropods (Taylor 1973), but little is known concerning its importance for prosobranch gastropods (Pechenik et al. 1984; Moran 1999). It is known, however, that the fluid bathing the embryos may contain amino acids, proteins, and polysaccharides, whether nutritive eggs are present or not (Bayne 1968; De Maheiu et al. 1974; Paschke 1992; Miloslavich 1999). High nutritive value of the fluid for encapsulated embryos of Nucella lapillus was observed by Stöckmann-Bosbach and Althoff (1989) and for Urosalpinx cinerea by Rivest (1986). The proteins present in the intracapsular fluids of the egg capsules of Adomelon brasiliana arise in part from the dissolution of the internal membrane of the capsule, suggesting that the capsule wall contributes to the organic content of the intracapsular fluid, becoming a potential source of nutrition (De Mahieu et al. 1974). Dissolved organic material may be used as a nutrient source by some larval stages of marine invertebrates (Manahan and Crisp 1983; Jaeckle and Manahan 1989; Welborn and Manahan 1990; Moran 1999).
The egg capsules are acellular, and their morphology and structure are related to their functional roles. Knowledge of the composition of the capsule walls is important in interpreting the origin, function, and evolutionary relationships among the different structural patterns observed (Garrido and Gallardo 1993).
All species of the genus Crepidula are characterized by embryonic capsule development and parental brooding of the deposited capsules as part of their reproductive strategy (Hoagland 1977). Crepidula fecunda is abundant in Chile, occurring in stacks in intertidal and subtidal habitats (Gallardo 1977). C. fecunda is a protandric hermaphrodite (Chaparro et al. 2001), with internal fertilization typical of other prosobranchs in the family Calyptraeidae (Hoagland 1986). It shows mixed larval development, with an initial brooding of encapsulated embryos by the females for about 4 weeks in the natural environment (Saldivia 2000). After liberation of veliger larvae, the planktonic phase lasts about 15 days (Soto, personal communication). The egg capsules are flattened, triangular sacs, each attached to the substrate by a peduncle. The average number of capsules per spawning is 46. The total height of the capsule (including stalk) varies between 5 and 10 mm (Chaparro and Flores 2002). There are 400–1600 eggs capsule−1, the eggs varying from 204 to 238 µm in diameter (Gallardo 1979). The height of the capsule and the number of eggs per capsule are strongly influenced by the size of the female (Chaparro and Flores 2002). Embryos within the capsules are immersed in intracapsular fluid, and there are no nutritive eggs (Gallardo 1979). Thus, these embryos depend for their nutrition on their maternal yolk and possibly on the organic material in the intracapsular fluid.
In this study we examined gravimetric, morphological, and biochemical changes in the capsule walls during embryonic development of C. fecunda.
Materials and methods
Stacks of individuals of Crepidula fecunda Gallardo, 1979 were collected from the intertidal zone at Yaldad Bay, Chiloé Island, southern Chile (43°08′S; 73°44′W) during the austral spring–summer 1999–2000 and maintained in the laboratory under conditions similar to those in the environment (temperature: 18±1°C; salinity: 30±1‰) until processing. About 250 incubating females of 37–43 mm shell length (greatest anterior–posterior axis) were removed from substrates for study, and the length of each individual was measured to the nearest 0.1 mm with a micrometer caliper. Only egg capsule batches from this female size range were collected, ensuring that all stages of intracapsular development of embryos were represented. The stage of development and lengths of embryos from three to five capsules from each clutch were recorded from images obtained from an inverted microscope fitted with a Pullnix camera. Images were processed with the SCION PC image processing program.
Since there was no information on the duration of each of the intracapsular development stages of C. fecunda, it was necessary to establish a time-independent reference variable for standardizing comparisons between the different egg (embryo) masses. For this, the variable employed was the shell length (μm) of shelled stages (veliger larvae, >200 μm shell length). Larval stages prior to shell formation were scored as zygotes, trochophores, or those showing initiation of velar development (early veliger).
Analysis of capsule walls
Gravimetric analysis
About 30 batches of capsules were analyzed, representing all stages of intracapsular embryonic development. Embryos from any given batch were all at the same developmental stage. Embryos and intracapsular fluids were removed from capsules of each batch. At least 30 capsules from each clutch were collected on previously washed, ashed, and tared 24-mm glass fiber filters, constituting a representative sample of each clutch. Each sample filter was briefly rinsed with distilled water under a gentle vacuum to remove soluble salts, and enclosed in dry, tared aluminum foil. They were then dried at 60°C for 48 h to constant weight, cooled in a desiccator, and weighed (±10 μg). The filters were then ignited in a muffle furnace at 450°C for 3 h, cooled in a desiccator, and reweighed to determine ash content for calculation of ash-free dry weight (AFDW, i.e. organic matter).
Histochemical analysis
For histochemical detection of protein and carbohydrate, three to five capsules from each batch, representing the initial (zygote), intermediate (veliger: 220–270 μm shell length), and advanced stages (veliger: 320–370 μm shell length, prehatching) were fixed in 10% formalin saturated with CaCO3. Samples were embedded in paraffin, and blocks sectioned at 4 μm using an E-Leit Minot microtome to obtain horizontal sections through the mid-plane of the capsules. Histochemistry of lipids was carried out on sections of frozen samples. Lipids were detected with Sudan III staining. The Schiff (PAS) reaction was used to detect neutral mucopolysaccharides, and Alcian blue to detect acid mucopolysaccharides. Proteins were detected with hematoxylin-eosin staining. This indirectly indicates the presence of proteins, because hematoxylin attaches to acid proteins and eosin to basic proteins.
Biochemical analysis
At least 30 capsule walls from each batch were pooled for each analysis. Each sample was rinsed briefly with distilled water and lyophilized. The lyophilized samples were analyzed for total lipids, carbohydrates, and proteins. For total lipids, the colorimetric method of Marsh and Weinstein (1966) was employed, using tripalmitin in chloroform as a standard. Total carbohydrates were determined by the colorimetric phenol-sulfuric acid method (Dubois et al. 1956) using glucose as a standard. The capsules were pretreated to extract the total carbohydrates from the capsule walls by boiling the sample in a 5% trichloroacetic acid solution containing 0.1% silver sulfate (Barnes and Heath 1966). Samples for protein analysis were combusted in a Perkin-Elmer CHN analyzer using acetanalide as a standard. The value for total protein was calculated from the nitrogen content using a conversion factor of 5.8 (Gnaiger and Bitterlich 1984).
Scanning electron microscopy (SEM)
Twenty batches were selected, including the initial, intermediate, and advanced stages of intracapsular development. Samples of capsule walls were placed in Eppendorf tubes and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer on ice for at least 2 h. The fixative was then removed using three 25-min rinses with ice-cold 0.1 M phosphate buffer. The samples were dehydrated in an ethanol gradient series from 50% to 90% ethanol in 10% increments, terminating with two final rinses in 100% ethanol. Complete dehydration was achieved in a Hitachi critical point dryer with CO2 as a transitional fluid. Samples were affixed to SEM stubs and coated with gold-palladium for observation in a LEO 420 SEM with a PC image output. Images representative of each capsular stage were stored for later analysis.
Transmission electron microscopy (TEM)
Capsules in the four developmental stages described above (zygote, initial, intermediate, and terminal veligers) were selected for study. A total of 20 samples was fixed for 2 h in ice-cold 2.5% glutaraldehyde in seawater, and then washed three times with 0.2 M phosphate buffer on ice, followed by post-fixation for 2 h in 2% osmium tetroxide. Dehydration was carried out in successive baths of 50%, 70%, and 96% ethanol, followed by three 10-min washes in 100% ethanol, and two 5-min washes in 100% acetone. Dehydrated samples were pre-embedded in a 1:1 mixture of acetone and epol-araldite, with final embedding in an epol-araldite mix (24 h under vacuum). Specimens were then placed in an oven at 60°C for 48 h to ensure complete polymerization. Sections of 600 Å thickness were cut with a Sorvall MT-1 ultramicrotome. The ultrafine sections were placed on grids and stained with uranyl acetate and lead citrate for subsequent observation with a Hitachi model H700 TEM. Selected representative fields were photographed.
Biochemical analysis of intracapsular fluids
About 60 batches of capsules with veligers from 220 to 370 μm in shell length were used to quantify total carbohydrates, lipids, and proteins in the intracapsular fluid. No determinations were made for initial developmental stages (without shell) owing to the fragility of the embryos, which disintegrated during manipulation, contaminating the intracapsular fluids. Capsules were immediately placed in 0.45-μm-filtered seawater, thoroughly dried with absorbent paper, and then placed in a petri dish. They were then perforated with a needle to allow escape of intracapsular fluid without damaging the embryos. Each sample, consisting of the pooled fluid from all the capsules in a batch, was placed in an Eppendorf tube of which the bottom had been cut off and replaced with a 100-μm-mesh Nitex screen to retain any embryos or debris present while allowing passage of intracapsular fluid. Each Eppendorf tube was placed in a 10-ml centrifuge tube and centrifuged at 3000 rpm for 20 min to transfer the intracapsular fluid to the centrifuge tube through the Nitex screen. Known volumes of the resulting centrifuged intracapsular fluid were taken with microcapillary pipets, transferred to Eppendorf tubes, and frozen for later biochemical analysis.
Total lipids and carbohydrates were determined on samples of centrifuged intracapsular fluid using the methods described above for capsule walls. Total proteins were determined using the BCA method (Pierce Laboratories), which employs bicinconinic acid (BCA) for colorimetric quantification of total proteins. Samples of filtered seawater (0.45 μm) from aquaria containing capsules were analyzed for content of proteins, lipids, and carbohydrates for comparison with values from the capsular fluid.
Results and discussion
Analysis of capsule walls
Gravimetric analysis
The dry weight of the capsule walls of Crepidula fecunda decreased significantly with the progress of embryonic developmental of veliger larvae (Fig. 1; P<0.01). Thus, in the zygote stage, mean (±SD) weight of the capsule wall was 0.12±0.01 mg, whereas, in a veliger 344 μm in shell length, the estimated weight was 0.0443 mg.
Crepidula fecunda. Variation in dry weight of the capsule wall during capsular development. Filled symbols represent mean values and error bars represent their respective standard deviations. Total samples=39 [12 samples of intermediate or late veligers (open symbols) and the rest belonging to zygote, trochophore, or early veliger stage]. Each sample had 30 capsule walls
The organic content of the capsule walls also decreased significantly (P<0.01) during development (Fig. 2). Recently deposited capsules of C. fecunda were composed principally of organic matter (68%), but as intracapsular development of embryos progressed, a loss in capsule mass was observed, represented by a 40% drop in organic matter from a mean (±SD) initial value of 0.0726±0.0049 mg organic matter in the zygote to a mean of 0.0290 mg per capsule wall when veligers reached 344 μm in shell length. A high percentage of organic matter was also found in capsules of Concholepas concholepas by Paschke (1992). In contrast, the inorganic content of capsule walls in C. fecunda showed no significant variation over the capsular development period (P>0.05).
Crepidula fecunda. Organic material content of capsule walls during capsular development. Symbols as in Fig. 1; n=42
Histochemical analysis
The Sudan III did not stain lipids in the capsule walls. Conversely, positive reactions with Alcian blue (blue stain) and PAS (fuchsia stain) indicated the presence of acid and neutral mucopolysaccharides in the capsule walls. Staining with hematoxylin-eosin produced a pronounced red color, indicating the predominance of basic proteins. Bayne (1968) similarly demonstrated the presence of proteins and carbohydrates in the capsule walls of eight gastropod species. Histochemical studies on the neogastropods Ilyanassa obsoleta (Sullivan and Maugel 1984) and Ocenebra erinacea (Hawkins and Hutchinson 1988) also showed the capsule walls to be formed of proteins and carbohydrates, with no lipids detectable. On the other hand, our biochemical determinations, which were carried out with at least 30 capsules per sample, showed that the capsule walls of C. fecunda did contain lipids which were not revealed by histochemical examination of individual capsules.
Biochemical analysis
The protein content declined significantly (P<0.01) with intracapsular embryonic development (Fig. 3), especially during the period in which the veliger larvae grew from 207 µm (approx. 40 μg protein capsule−1) to 365 μm (approx. 25 μg protein capsule−1) in shell length. Neither total carbohydrate nor total lipid showed any relationship with intracapsular development (P>0.05). Protein was the major constituent of capsule walls, with lipid (4.092±2.462 μg capsule−1; mean±SD, n=47) and carbohydrate (0.273±0.168 μg capsule−1; n=48) forming their minor constituents.
Crepidula fecunda. Variation in protein content of capsule walls during intracapsular development. Symbols as in Fig. 1; n=26
The very high protein content (>90% of the organic component) of the capsule walls of C. fecunda is consistent with data from the whelk Buccinum undatum, in which >77% of the capsule is made up of amino acids (Hunt 1966). The complex protein structure of capsule walls has been demonstrated in ultrastructural studies of the protein fibers in egg capsules of B. undatum (Flower et al. 1969) and Cominella maculosa (Flower 1973). At least two structural proteins are found in capsules of the latter species, and the importance of these proteins accounts for the high protein content of the capsule wall, as suggested by Paschke (1992) for Concholepas concholepas. In C. fecunda protein always forms the major component of the capsules, although it declines by about 40% in mass during embryonic development.
Scanning and transmission electron microscopy
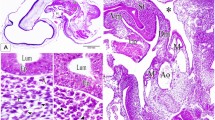
Two layers formed the capsular wall, a thin, fibrous exterior layer and a thicker internal layer with a loose, spongy consistency (Fig. 4A, B). The latter was in direct contact with the intracapsular fluid containing the embryos.
TEM provided supporting data on the structure of the two capsular layers. The fibrous external layer did not change in thickness over the developmental period, while the internal spongy layer became thinner (Fig. 4A, B, C). The greatest rate of decrease occurred between the zygote and the early veliger (<200 μm in shell length; Fig. 5). The thickness (mean±SD) of the capsular wall at the zygote stage was about 11.44±4.96 μm, decreasing to 3.28±0.31 μm by the end of development. The external layer was 2.60±1.21 μm thick and remained nearly constant over the entire development period. The inner layer began with a thickness of 7.57±1.99 μm, which decreased to 0.83±0.18 μm in the veliger phase (220–270 μm shell length). This decrease was about 70% of the initial value, representing about 90% of the inner wall of the capsule. By the end of the developmental period the inner wall had nearly disappeared (Fig. 6).
The capsule wall in C. fecunda is a laminated structure, which appears to be a common feature in gastropod capsule organization. Optical and electron microscopy of capsule walls in neogastropods (primarily muricaceans and buccinaceans) has revealed a multilaminated structure, usually consisting of more than four layers (Tamarin and Carriker 1967; Sullivan and Maugel 1984; D’Asaro 1988; Hawkins and Hutchinson 1988; Garrido and Gallardo 1993). This configuration provides resistance to mechanical damage and biological deterioration (Hawkins and Hutchinson 1988). At the beginning of intracapsular development in C. fecunda, two layers were observed forming the capsule wall. Although the thin, fibrous external layer showed regions with variable electron densities and patterns of fiber orientation, sub-layers within the external layer were not identified. The internal spongy layer in contact with the embryos and intracapsular fluid in C. fecunda may be comparable to the fourth layer or “albumin sac”, a term used by some authors for the inner capsular layer in contact with intracapsular fluid in other gastropods; these layers have been described as very thin (60 nm–2.8 μm in thickness), and as having the function of retaining the intracapsular fluid (Tamarin and Carriker 1967; Sullivan and Maugel 1984; D’Asaro 1988; Hawkins and Hutchinson 1988; Garrido and Gallardo 1993).
Biochemical analysis of intracapsular fluid
The intracapsular fluid in the veliger (220–363 μm shell length) contained protein, but no lipid or carbohydrate was detected.
The highest values for protein in the intracapsular fluid were 905 μg protein ml−1 fluid at a veliger shell length of 272 μm, and 141 μg protein ml−1 fluid at a shell length of 363 μm. There was only 12±3.02 μg protein ml−1 (±SD) in the surrounding seawater (Fig. 7).
Loss in weight in the capsule wall suggests that material from the inner capsule wall is transferred to the capsular fluid in dissolved or particulate form. This represents a transfer of almost 15 µg capsule−1 (0.015 J capsule−1; Gnaiger 1983) during the early veliger to pre-hatching veliger period in the egg mass of a typical female of shell length 40 mm. Mechanical (Vaughn 1953) or chemical (Pechenik 1975; Sullivan and Bonar 1984; Sullivan and Maugel 1984; Hawkins and Hutchinson 1988) action of embryos against the inner capsular wall or specific portions of it have been proposed as possible mechanisms to explain the thinning of the wall. In C. fecunda, reduction in thickness of the capsule wall may be due to unknown mechanical or chemical effects produced by the contained larvae. Another possibility is that the flow of the intracapsular fluid and the friction between shelled larvae and the inner capsule wall could cause inner surface erosion.
Although higher levels of protein were found in the intracapsular fluid than in the surrounding seawater, this study did not establish any trophic function for this protein. Some authors have suggested that bivalve larvae have the capacity to absorb dissolved nutrients such as amino acids from seawater through the epidermis (Manahan and Crisp 1983). Protein uptake has also been recorded for encapsulated larvae of gastropods, e.g. Nerita picea (Rivest and Strathmann 1994) and Littorina spp. (L. saxatilis, L. sitkana, L. subrotundata; Moran 1999). Lecithotrophic larvae of the gastropod Haliotis rufescens acquire energy by absorbing dissolved amino acids and sugars directly from seawater (Jaeckle and Manahan 1989; Welborn and Manahan 1990). Some organic components of the intracapsular fluid may be incorporated by pinocytosis into integumental cells of the cephalic region and a small portion of the fluid internalized by ectodermic larval kidneys in Nucella lapillus (Fioroni et al. 1985) and Searlesia dira (≅Lirabuccinum dirum) (Rivest 1980). The use of dissolved organic material has also been described for gastropod embryos, including Adelomelon brasiliana (De Mahieu et al. 1974) and N. lapillus (Stöckmann-Bosbach and Althoff 1989). The protein content of the intracapsular fluid in N. lapillus decreases during capsular development, in part due to uptake by the embryos. The presence of proteins in the intracapsular fluid of A. brasiliana, and the decline due in part to uptake by enclosed embryos, has been demonstrated by De Mahieu et al. (1974). These observations suggest that embryos may use intracapsular fluid proteins as a source of dissolved and/or particulate extraembryonic nutrition, arising entirely or in part from the inner capsular layer. However, the possibility that protein, at least in part, could be lost to the outside medium must also be considered. Although C. fecunda embryos and developing larvae may be unable to absorb dissolved proteins from the intracapsular fluid, encapsulated veligers of this species are certainly able to ingest extraembryonic particles (Chaparro et al. 2002a, 2002b). The spongy, fragile nature of the inner capsular layer as revealed by SEM/TEM in this study suggests that disaggregated particles of this layer may be consumed by larval filtration within the capsules. The present study provides evidence that organic material (dissolved or particulate proteins) present in the intracapsular fluids of C. fecunda originates from the inner capsular layer. Future study is required to examine the possibility that this material provides an extraembryonic source of nutrition for the developing larva.
References
Barnes H, Heath J (1966) The extraction of glycogen from marine invertebrate tissues. Helgol Wiss Meeresunters 13:115–117
Bayne CJ (1968) Histochemical studies on the egg capsules of eight gastropod molluscs. Proc Malacol Soc Lond 38:199
Chaparro OR, Flores ML (2002) Reproductive output of Crepidula fecunda females: distribution of energy in the production of gametes and capsular walls. NZ J Mar Freshw Res 36:661–673
Chaparro OR, Paschke KA (1990) Nurse egg feeding and energy balance in embryos of Crepidula dilatata (Gastropoda: Calyptraeidae) during intracapsular development. Mar Ecol Prog Ser 65:183–191
Chaparro OR, Pereda SV, Bahamondes-Rojas I (2001) Effects of protandric sex change on radula, pedal morphology, and mobility in Crepidula fecunda (Gastropoda: Calyptraeidae). NZ J Mar Freshw Res 35:881–890
Chaparro OR, Soto AE, Bertran CE (2002a) Velar characteristics and feeding capacity of encapsulated and pelagic larvae of Crepidula fecunda Gallardo, 1979 (Gastropoda, Calyptraeidae). J Shellfish Res 21:233–237
Chaparro OR, Charpentier JL, Collin R (2002b) Embryonic velar structure and function of two sibling species of Crepidula with different modes of development. Biol Bull (Woods Hole) 203:80–86
D’Asaro CN (1988) Micromorphology of neogastropod egg capsules. Nautilus 102:134–148
De Mahieu GC, Penchaszadeh PE, Casal AB (1974) Algunos aspectos de las variaciones de proteínas y aminoácidos libres totales del líquido intracapsular en relación al desarrollo embrionario en Adelomelon brasiliana (Lamarck, 1811) (Gastropoda, Prosobranchia, Volutidae). Cah Biol Mar 15:215–227
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fioroni P, Scheidegger DP, Sundermann G (1985) Die Ultrastruktur der Larvalnieren bei intrakapsulären Larven von Nucella lapillus (Gastropoda, Prosobranchia, Stenoglossa). Zool Jahrb Abt Anat Ontolog Tiere 113:145–164
Flower NE (1973) The storage and structure of proteins used in the production of egg capsules by the mollusc Cominella maculosa. J Ultrastruct Res 44:134–145
Flower NE, Geddes AJ, Rudall KM (1969) Ultrastructure of the fibrous protein from the egg capsules of the whelk Buccinum undatum. J Ultrastruct Res 26:262–273
Fretter V, Graham A (1994) British prosobranch molluscs. Their functional anatomy and ecology. Ray Society, London
Gallardo CS (1977) Two modes of development in the morphospecies Crepidula dilatata (Gastropoda: Calyptraeidae) from southern Chile. Mar Biol 39:241–251
Gallardo CS (1979) Especies gemelas de género Crepidula (Gastropoda, Calyptraeidae) en la costa de Chile; una redescripción de C. dilatata Lamarck y descripción de C. fecunda n. sp. Stud Neotrop Fauna Environ 14:215–226
Gallardo CS, Garrido O (1987) Nutritive egg formation in the marine snails Crepidula dilatata and Nucella crassilabrum. Int J Invertebr Reprod Dev 11:239–254
Garrido O, Gallardo CS (1993) Ultraestructura de la cápsula ovífera de Concholepas concholepas (Bruguiere, 1789) (Gastropoda: Muricidae). Rev Biol Mar 28:191–201
Gnaiger E (1983) Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In: Gnaiger E, Forstner H (eds) Polarographic oxygen sensors. Springer, Berlin Heidelberg New York
Gnaiger E, Bitterlich G (1984) Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62:289–298
Hawkins LE, Hutchinson S (1988) Egg capsule structure and hatching mechanism of Ocenebra erinacea (L.) (Prosobranchia: Muricidae). J Exp Mar Biol Ecol 119:269–283
Hoagland KE (1977) Systematic review of fossil and recent Crepidula and discussion of evolution of the Calyptraeidae. Malacologia 17:365–381
Hoagland KE (1986) Patterns of encapsulation and brooding in the Calyptraeidae (Prosobranchia: Mesogastropoda). Am Malacol Bull 4:173–183
Hunt S (1966) Carbohidrate and amino-acid composition of the egg capsule of the whelk Buccinum undatum L. Nature 210:436–437
Jaeckle WB, Manahan DT (1989) Feeding by a “nonfeeding” larva: uptake of dissolved amino acids from seawater by lecithotrophic larvae of the gastropod Haliotis rufescens. Mar Biol 103:87–94
Lord A (1986) Are the contents of egg capsules of the marine gastropod Nucella lapillus (L.) axenic? Am Malacol Bull 4:201–203
Manahan DT, Crisp DJ (1983) Autoradiographic studies on the uptake of dissolved amino acids from sea water by bivalve larvae. J Mar Biol Assoc UK 63:673–682
Marsh JB, Weinstein DB (1966) Simple charring method for determination of lipids. J Lipid Res 7:574–576
Miloslavich P (1999) Nutritional value of the intracapsular liquid of Engoniophos unicinctus Say, 1825 (Caenogastropoda: Buccinidae). J Moll Stud 65:502–503
Moran AL (1999) Intracapsular feeding by embryos of the gastropod genus Littorina. Biol Bull (Woods Hole) 196:229–244
Paschke KA (1992) Fisiología, energética y composición bioquímica de Concholepas concholepas (Bruguiere, 1789) (Gastropoda: Muricidae) durante el desarrollo intracapsular. Tesis, Esc. de Biología Marina, Fac. de Ciencias, Univ. Austral de Chile
Pechenik JA (1975) The escape of veligers from the egg capsules of Nassarius obsoletus and Nassarius trivittatus (Gastropoda, Prosobranchia). Biol Bull (Woods Hole) 149:580–589
Pechenik JA (1979) Role of encapsulation in invertebrate life histories. Am Nat 114:859–870
Pechenik JA (1982) Ability of some gastropod egg capsules to protect against low-salinity stress. J Exp Mar Biol Ecol 63:195–208
Pechenik JA (1983) Egg capsules of Nucella lapillus (L.) protect against low-salinity stress. J Exp Mar Biol Ecol 71:165–179
Pechenik JA, Chang SC, Lord A (1984) Encapsulated development of the marine prosobranch gastropod Nucella lapillus. Mar Biol 78:223–229
Rawlings TA (1996) Shields against ultraviolet radiation: an additional protective role for the egg capsules of benthic marine gastropods. Mar Ecol Prog Ser 136:81–95
Rivest BR (1980) Larval kidneys in marine prosobranch embryos: specialized structures for the uptake of egg capsule albumen. Am Zool 20:905
Rivest BR (1986) Extra-embryonic nutrition in the prosobranch gastropod Urosalpinx cinerea (Say, 1822). Bull Mar Sci 39:498–505
Rivest BR, Strathmann RR (1994) Uptake of protein by an independently evolved transitory cell complex in encapsulated embryos of neritoidean gastropods. In: Wilson WH, Stricker SA, Shinn GL (eds) Reproduction and development of marine invertebrates. Johns Hopkins University Press, Baltimore, pp 166–176
Saldivia CL (2000) Ovoposición y aspectos regulatorios de la capacidad de incubación en Crepidula fecunda Gallardo, 1979 (Gastropoda, Calyptraeidae). Tesis, Esc. de Biología Marina, Fac. de Ciencias, Univ. Austral de Chile
Stöckmann-Bosbach R, Althoff J (1989) A correlated morphological and biochemical study of capsular fluid of Nucella lapillus (Gastropoda: Prosobranchia: Muricidae). Mar Biol 102:283–289
Sullivan ChH, Bonar DB (1984) Biochemical characterization of the hatching process of Ilyanassa obsoleta. J Exp Zool 229:223–234
Sullivan Ch, Maugel T (1984) Formation, organization, and composition of the egg capsule of the marine gastropod, Ilyanassa obsoleta. Biol Bull (Woods Hole) 167:378–389
Tamarin A, Carriker MR (1967) The egg capsule of the muricid gastropod Urosalpinx cinerea: an integrated study of the wall by ordinary light, polarized light, and electron microscopy. J Ultrastruct Res 21:26–40
Taylor HH (1973) The ionic properties of the capsular fluid bathing embryos of Lymnaea stagnalis and Biomphalaria sudanica (Mollusca: Pulmonata). J Exp Biol 59:543–564
Vaughn CM (1953) Effects of temperature on hatching and growth of Lymnaea stagnalis appressa Say. Am Midl Nat 49:214–228
Welborn JR, Manahan DT (1990) Direct measurements of sugar uptake from seawater into molluscan larvae. Mar Ecol Prog Ser 65:233–239
Acknowledgements
We thank Mr. R. Silva for his help with the SEM samples and Dr. R.J. Thompson for commenting on this manuscript. This research was funded by Fondecyt–Chile (grants 1980984 and 1020171).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Ojeda, J.A., Chaparro, O.R. Morphological, gravimetric, and biochemical changes in Crepidula fecunda (Gastropoda: Calyptraeidae) egg capsule walls during embryonic development. Marine Biology 144, 263–269 (2004). https://doi.org/10.1007/s00227-003-1194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1194-5