Abstract
Dynamic mechanical analysis and small-angle X-ray scattering (SAXS) measurements of hinoki wood swollen with water and/or ethanol in the temperature range of 20–78/95 °C were performed to clarify the relationship between swelling and microstructure in different swelling states. For the sample swollen in a water–ethanol mixture with an ethanol mole fraction of 0.2, a peak in tanδ, i.e., the ratio of the dynamic elastic modulus (E′) to the dynamic loss modulus (E″), was observed at around 50 °C. No clear peak was observed in the temperature range of the sample swollen with water or ethanol, but thermal softening behavior due to micro-Brownian motion of lignin was observed. The scattering behavior of the samples swollen with water and/or ethanol differed significantly from one solution to another. The SAXS intensity of samples swollen with water or mixture of water and ethanol increased with increasing temperature, while the SAXS intensity of samples swollen with ethanol changed little with increasing temperature. This suggested that the adsorption sites of ethanol were different. The position of the peak for the sample swollen with the water–ethanol mixture, observed in the Kratky plot, was shifted to the low-q side compared to the pure liquid. It was suggested that the aggregation state of the sample swollen with the mixture of water and ethanol was very different from those of the wood swollen with the pure liquid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood swells when it contains water, and when it swells, its physical properties change significantly.
It is also known that wood is swollen by liquids other than water. It has long been reported that the degree of swelling varies greatly depending on the properties of the liquid (Gordy 1941; Stamm and Loughborough 1942; Nayer and Hossfeld 1949; Ishimaru and Maruta 1996a; Ishimaru and Adachi 1988; Ishimaru and Sakai 1988; Mantanis et al. 1994a, 1994b). Similar effects have been observed in lignocellulosic materials and wood-polymer composites (Prusov et al. 2014). Ishimaru and Adachi (1988) showed that the swelling of wood in liquids with only proton acceptors is greater than that in liquids with both proton acceptors and donors, and that the greater the molar volume of the liquid, the smaller the swelling of wood when compared at the same proton accepting power. On the other hand, Mantanis et al. (1994a, b) precisely measured the rate of swelling of wood in various liquids and concluded that the apparent activation energy of the swelling process is consistent with the activation energy of the scission of internal hydrogen bonds among wood constituents. Ishimaru et al. (1991) reported on the swelling of wood with 11 different organic liquids in terms of cross-sectional swelling and its anisotropy. The amount of cross-sectional swelling of wood is closely related to the proton-acceptor capacity of the liquid, and they reported that liquids with both proton accepting and donating properties have significantly lower swelling capacity for wood than liquids with only proton accepting properties, even among liquids with similar proton accepting properties. The swelling of wood by liquids requires that the liquid molecules must have the proton-acceptor capacity in order to break hydrogen bonds and to displace and adsorb between wood components, but it is also important to break away from the self-association state in the bulk liquid and to approach the adsorption point relatively freely (Ishimaru et al. 1991). It has also been inferred that low cohesive properties are advantageous for swelling, since liquids with only proton accepting properties show higher swelling capacity than liquids with both proton accepting and proton donating properties (Ishimaru et al. 1991).
There have been several reports on the swelling behavior of wood using mixed organic liquids (Ishimaru and Sakai 1988; Sakai and Ishimaru 1989; Meier et al. 2005, 2006a, 2006b; Chang et al. 2009, 2012; Bossu et al. 2018; Aguilera-Segura et al. 2019; Yadav et al. 2024). For example, it is known that water–ethanol mixtures can significantly change the amount of wood swelling depending on the mole fraction of ethanol. The phenomenon that the swelling of wood by the mixed liquid is higher than that of each single liquid is explained by the activity coefficient of the mixed liquid (Ishimaru and Sakai 1988; Sakai and Ishimaru 1989). On the other hand, it is possible that each component of the liquid mixture has a separate adsorption site with high affinity. For example, some researchers reported differences in adsorption on cellulose, hemicellulose, and lignin for 6 organic liquids (Nakatani et al. 2008; Ishimaru et al. 1996b). In dry samples, ethanol and dimethyl sulfoxide (DMSO) show higher affinity for lignin than cellulose and hemicellulose. In other words, different microstructural changes are thought to occur with different swelling liquids. In recent years, macro- and molecular-scale studies have been conducted by Bossu et al. (2018) and Aguilera-Segura et al. (2019) on wood swollen with water–ethanol mixture liquids. On the macro scale, cellular observations of wood swollen in each liquid suggest that compound middle lamellae (CML) deagglomeration occurs in systems containing water–ethanol (Bossu et al. 2018). On the molecular scale, dynamic molecular modeling has been used to show the increase or decrease in hydrogen bonds between wood constituents and water or ethanol at various ethanol mole fractions (Aguilera-Segura et al. 2019).
Many studies have been conducted using dynamic viscoelasticity measurements as a method to determine the microstructural state of wood in a swollen state (Ishimaru et al. 1996b; Furuta et al. 1995a, 1995b, 2008; Kojiro et al. 2010; Miyoshi et al. 2018, 2020; Seki et al. 2022; Horiyama et al. 2022). Dynamic viscoelasticity measurements of water-saturated wood in the range of 0–100 °C at a measurement frequency of 0.05 Hz show that the tanδ peaks exist around 80–90 °C for softwoods and 60–70 °C for hardwoods (Furuta et al. 2008). This peak is attributed to micro-Brownian motion of lignin. The difference in peak temperature is also thought to be due to differences in the lignin structure. There is also a study on the dynamic viscosity inertia of wood swollen with DMSO or formamide, which have higher swelling capacity than water (Ishimaru et al. 1996b). It is believed that relaxation due to micro-Brownian motion of lignin occurs in these swollen solutions at much lower temperatures than in water-saturated wood (Ishimaru et al. 1996b). Recently, it has also been reported that the peak of tan δ is found at lower temperatures in wood swollen in mixed solutions with different ethanol mole fractions at mole ratios of 0.2–0.3 (Tanaka et al. 2019). The influence of each component has been discussed based on the results of dynamic viscoelasticity measurements of chemically treated and de-componentized lumber, but the microstructural changes of wood in untreated lumber have not been directly explained (Miyoshi et al. 2018, 2020; Seki et al. 2022). In addition, in recent years, the small-angle X-ray scattering (SAXS) and the small-angle neutron scattering (SANS) measurements have been widely used to understand the crystal structure in cell walls (Penttilä et al. 2019, 2020, 2021; Paajanen et al. 2022). Penttilä et al. (2019, 2020) used SAXS to study in detail the change in microstructure with increasing or decreasing moisture content. In a previous report, a combined analysis of microstructure using dynamic mechanical analysis (DMA) and SAXS was performed (Horiyama et al. 2022), showing that changes in the microstructure due to the thermal softening behavior of lignin obtained by DMA can be observed more directly by SAXS measurements.
The main goal of this study was to understand the microstructure of wood in different swelling states in more detail. Therefore, we focused on wood swollen in a water–ethanol mixture, for which various studies have been conducted, and attempted a combined analysis using DMA and SAXS measurements.
Materials and methods
Materials
The samples used for measurements were Japanese cypress (Chamaecyparis obtusa Endl.). For swelling measurements, cross-sectional wood pieces of 30 mm in the radial direction (R), 30 mm in the tangential direction (T), and 7 mm in the longitudinal direction (L) were successively cut from a wood stick with a cross section of 30 (R) × 30 (T) mm. After cutting the samples, they were boiled for 2 h and then slowly cooled to room temperature. After curing at 25 °C and 65%RH for at least one week to air-dry, they were dried in a desiccator using phosphorus pentoxide to a completely dry state to unify the thermal and drying histories. Then, they were immersed in each adjusted solution and decompressed for 30 min under a pressure of 100 hPa. After releasing the pressure to atmospheric pressure, the solvents were allowed to stand still in the solvents for one day and night. The temperature was then raised to near the boiling point of each solvent in each solvent and then slowly cooled to room temperature. The dimensions of the swollen state were then measured. The number of specimens used in this study was five.
The solutions used were distilled water, ethanol and mixtures of water–ethanol. The water–ethanol mole fraction was 0.8:0.2. The reason for that mixing ratio was that the activity of the mixed liquid showed a significant positive deviation compared to the ideal behaviour based on the mixing ratio. (Ishimaru and Sakai 1988). The swelling of wood was shown to be higher when soaked in a mixture of water–ethanol with a 0.8:0.2 ratio compared to that obtained after soaking in single liquid (Sakai and Ishimaru 1989).
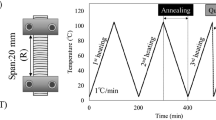
The samples used for dynamic mechanical analysis (DMA) and small angle X-ray scattering (SAXS) measurements were prepared by cutting them out of the samples after the swelling test had been completed. The sample sizes were 1.2 (T) × 30 (R) × 1.0(L) mm for the measurement of dynamic viscoelastic properties, and 4.5 (R) × 1.0 (T) × 7.0(L) mm for the measurement of SAXS. A schematic diagram of the samples is shown in Fig. 1.
Dynamic mechanical analysis (DMA)
Temperature dependence of dynamic elastic modulus (E′), loss modulus (E″), and tanδ was measured by the tensile forced oscillation method using a dynamic mechanical analyzer (DMS6100, Seiko Instruments, Chiba, Japan). The samples swollen by the distilled water were measured in the distilled water at a temperature range of 20–95 °C. The samples swollen by ethanol or mixture of water–ethanol were measured in ethanol or mixture of water–ethanol at a temperature range of 20–78 °C. The heating and cooling rate was 1 °C/min. Frequencies for the measurement were 0.5, 1.0, 2.0, 5.0, and 10 Hz. The span was 10 mm, and the displacement amplitude was 5 μm. The tensile direction was radial. A schematic diagram of the sample attached to the dynamic mechanical analyzer is shown in Fig. 2. Results were obtained in the second heating process to uniform the heating and cooling histories (Furuta et al. 2008). The number of specimens used in this study was one. One measurement was taken on each sample (see Fig. 1 for sample details). The reproducibility in the trend of the results of the DMA measurements has been confirmed through several preliminary experiments to confirm the trend of the results.
Small-angle X-ray scattering (SAXS)
SAXS measurements were conducted at a synchrotron facility, BL40B2 beamline in SPring-8 (Hyogo, Japan). The wavelength of X-rays and sample-to-detector distance were set to 1 Å−1 and 1.0 m, respectively, which allowed a q range from 0.005 to 0.7 Å−1 to be measured (scattering vector q = 4πsin(θ)/λ with 2θ being the scattering angle). The scattering patterns were recorded with a hybrid pixel counting detector, Pilatus2M 3S (Dectris Inc., Switzerland). Incident and transmitted X-ray flux were counted with ionizing chambers located upstream and downstream of the sample holder, respectively. These two values were used for estimating the absorbance of X-ray by the sample, which was used for the absorbance correction of the scattering data.
The samples had their radial plane perpendicular to the X-ray beam. The longitudinal direction of the samples was always vertical and perpendicular to the X-ray beam. The sample swollen by each liquid were placed in a liquid cell made of stainless steel filled with the respective liquid, which has a beam path of 3 mm and 0.02 mm thickness quartz windows to allow the beam to pass through but hold the liquid. The liquid cell carrying the sample inside was placed in the sample holder whose temperature was controlled by mK2000 (INSTEC Inc., USA). A schematic diagram of SAXS measurement is shown in Fig. 2. The number of specimens used in this study was two. Three measurements were taken on each sample (see Fig. 1 for sample details). The reproducibility in the trend of the results of the SAXS measurements has been confirmed through several preliminary experiments to confirm the trend of the results.
SAXS data analysis
All the data reduction, including camera length calibration by silver behenate, was done using pyFAI (Ashiotis et al. 2015), a python package for SAXS data reduction. Solvent-subtraction and absorbance correction were made for the two-dimensional scattering pattern at first. Then, the radial integration was performed to obtain the scattering intensity distribution along the azimuth angle (χ-I plot) with the q range of 0.05–0.4 Å−1, and the azimuth angles where the scattering intensity is the highest was searched and found on the χ-I plot. The scattering intensity at the azimuth, which was defined as the equatorial, was analyzed in this study The azimuthal integration was then performed of the azimuths with the maximal scattering intensity, with an azimuthal range of 15°. The obtained one-dimensional scattering data at the equatorial was averaged per pixel, and for oriented samples, the scattering intensity was corrected by multiplying by q. The corrected scattering intensity was defined as the scattering intensity I, and q-I plot was plotted. The q2 was then multiplied by the scattering intensity I to produce a q- q2I plot (Kratky plot).
Results
Swelling
The relative representation for the wood blocks swollen by water–ethanol mixtures with different ratios at 25 °C is shown in Fig. 3, in which the dimension of the wood block swollen by water (the mole fraction of ethanol fEtOH = 0) is set as 100%. As demonstrated in the previous report (Ishimaru and Sakai 1988), the swelling at fEtOH = 0.2 and 1.0 was higher and lower than at fEtOH = 0, respectively. The swelling at fEtOH = 1.0 is about 80%, when the swelling at fEtOH = 0 is considered to be 100%, which is similar to a previous report (Ishimaru and Sakai 1988). Ishimaru and Sakai (1988) studied the activity of each component of the mixtures and the adsorption of each component from dilute solutions in the water/ethanol and water/acetone systems. The reason for the lower mixing ratio of ethanol or acetone to water exceeding the swelling based on that mixing ratio is that the positive deviation in activity due to the interaction between the liquids at that mixing ratio affects the increased adsorption of each component on the adsorption sites. (Ishimaru and Sakai 1988).
Dynamic mechanical analysis (DMA)
The viscoelastic property of the swollen wood blocks was then analyzed by DMA as summarized in Fig. 4. Although the peak values differed depending on the frequency, similar trends were obtained and were omitted from this paper. Clear temperature dependency was observed for E′, E″, and tanδ in the radial direction of the samples. The wood swollen by pure water (fEtOH = 0) and pure ethanol (fEtOH = 1) exhibited the same behavior as in a previous report (Ishimaru et al. 1996b). A monotonical continuous decrease along the increasing temperature was observed for E′, while tanδ monotonically increased, which is reflected as the increase of E″ with increasing temperature. However, it is notable that E″ showed a clear peak for the wood swollen by water, indicating the thermal softening of lignin in water as already reported and discussed by Furuta et al (2008). The peak temperature of E″ for the wood swollen by water was 81.3 °C. For the wood swollen by pure ethanol, we could not obtain the data at a temperature high enough to invoke the thermal softening, owing to the lower boiling point of ethanol than water.
It was notable that the temperature dependency for the wood swollen at fEtOH = 0.2 appeared to be characteristic in comparison to the other conditions, namely swelling by pure water and pure ethanol. First of all, E′ for fEtOH = 0.2 was lower than the other samples (fEtOH = 0 and 1) in the measured temperature range. Furthermore, E′ decreased monotonically with increasing temperature in the measured temperature range as well as pure water and pure ethanol, but the decreasing trend of E′ was more striking in lower temperatures (~ 60 °C) than in the case of fEtOH = 0 and 1. The behavior of E″ and tanδ for the wood swollen at fEtOH = 0.2 was very different from the other two cases. A continuous decrease was observed for E″ in the measured temperature range, and the decreasing trend of E″ along the increasing temperature was slower up to around 50 °C and became rapid in higher temperatures. More significantly, the peak tanδ temperature for wood swollen at fEtOH = 0.2 was 54.2 °C. This peak, which appears in the range of 0–100 °C, is attributed to the micro-Brownian motion of lignin (Furuta et al 2008). It was thus found that there were differences in thermal softening behavior for different mole fractions of water–ethanol mixtures.
In Fig. 5, the relationship between the relative swelling and the viscoelastic property represented by E′ and E″ at 25 °C is shown. In general, the swelling of wood in liquid is considered to be caused by breaking hydrogen bonds between the wood component macromolecules by adsorbed liquid molecules, which subsequently interfere with the cohesion between the macromolecules. Given the more significant hydrogen bonding ability of water than ethanol, lower E′ and E″ for the water-swollen sample than ethanol is reasonable, as shown in Fig. 5; this observation is supposed to reflect the more significant changes of the microstructure in the cell wall of water-swollen wood.
In contrast to the relatively simpler comparison between water- and ethanol-swollen wood, the sample swollen at fEtOH = 0.2 gave a lower E′ value than even the water-swollen sample, which is consistent with the large swelling state as shown in Fig. 3. It is insightful that E″ of the sample swollen at fEtOH = 0.2 was, at 25 °C, larger than the sample swollen with water (fEtOH = 0). Ishimaru et al. (1996b) explain in terms of a mechanical model that when wood swells, E′ and E″ basically decreases because the overall cohesive structure is loosened, but E″ increases due to the packing effect of moderate adsorption of the swelling liquid on the relatively loose hydrogen bonds between the component molecules of wood. The packing effect does not contribute much to E′, but it does contribute significantly to the increase in E″. As a result, E″ of wood swollen by organic liquids is believed to be higher than that of wood swollen to the same degree by water. This packing effect was found to be similar for water–ethanol mixtures.
Small-angle X-ray scattering (SAXS)
SAXS pattern from wood is generally anisotropic as shown in Fig. 6, which is derived from the preferential orientation of cellulose microfibrils (CMFs) in the secondary wall. The azimuth where the scattering intensity is the highest is called the “equator”, and the scattering at this azimuth reflects the density distribution perpendicular to the fiber axis of the wood sample, and in this study, we analyzed the scattering intensity at the equator that majorly represents the distribution of the CMFs in the matrix of the middle layer of the secondary wall (S2) of wood, which is important for wood mechanical properties (Fig. 7). The Kratky plot of scattering intensity I multiplied by q2 is shown in Fig. 8. At 25 °C, the equatorial scattering intensity was higher for the ethanol-swollen sample than for the others in the measured q-range. This indicates a smaller gap between the cellulose and the matrix. Although ethanol has a significant effect on swelling and viscoelastic properties as described above, the ethanol molecules are slightly adsorbed in the matrix portion of the S2 layer, possibly resulting in a smaller decrease in apparent density of the matrix portion in the wood cell wall than in water. The scattering intensity of the swollen wood at fEtOH = 0.2 was almost the same as the water-swollen wood in q > 0.1 Å−1, but it becomes larger at the lower q range under 0.1 Å−1, indicating significant structural changes at the level larger than cellulose microfibrils which represent at around q of 0.1 Å−1. Peak tops of swollen wood at fEtOH = 0 and 1.0 were found at q = 0.166 Å−1 and 0.172 Å−1, respectively. On the other hand, the peak top of the swollen wood at fEtOH = 0.2 was shifted to the low q side at q = 0.153 Å−1.
The q-I plots at different temperatures are shown in Fig. 9. For the samples at fEtOH = 0 and 0.2, the scattering increases in parallel to the increase in temperature especially at the lower q-region, as previously observed in a previous study (Horiyama et al. 2022). The temperature-dependent increase in the scattering was more striking for the sample swollen at fEtOH = 0.2 than fEtOH = 0; the sample swollen at fEtOH = 1.0 showed a very weak temperature-dependent increase in equatorial scattering intensity, especially from 50 to 70 °C. This indicates that the structural change with increasing temperature in pure ethanol is small. As discussed in the previous studies, the equatorial scattering is majorly derived from the difference between cellulose microfibril and matrix components (hemicellulose and lignin), and then the increased scattering at higher temperatures is putatively due to the thermal softening of lignin (Horiyama et al. 2022). The scattering intensities of the samples with fEtOH = 0.2 and 1.0 slightly increase with increasing temperature, even at the wide-angle side.
Discussion
Nakatani et al. (2006) reported the existence of adsorption sites in lignin with only fine voids or loose hydrogen bonds, which do not exist in cellulose amorphous regions or hemicellulose. These adsorption sites have been shown to be accessible to liquid molecules as large as ethanol (Nakatani et al. 2008). In addition, Bossu et al. (2018) reported that the dynamic viscoelasticity measurements of ethanol-swollen wood showed smaller changes in E′ and tanδ than those of water-swollen wood, suggesting the presence of a different adsorption site. In addition, they reported that cell wall thickness observed in the thin sections of poplar swollen with water and ethanol is approximately 3 μm and 2 μm, respectively (Bossu et al. 2018). Moreover, cell observations suggested that ethanol, when co-absorbed with water, causes cell decohesion and releases the binding forces between the wood cell wall layers (Bossu et al. 2018). This is believed to play an important role in allowing cell wall over-swelling upon absorption of the mixed solvent (Bossu et al. 2018). As shown in Fig. 7, the scattering intensity of the water-swollen sample and ethanol-swollen sample was also significantly different. The ethanol-swollen sample also began thermal softening in the same way as the water-swollen sample as shown in Fig. 4. However, the increase in the scattering intensity of the sample swollen with ethanol with increasing temperature was small as shown in Fig. 9. The increase in the scattering intensity of the water-swollen sample with increasing temperature is thought to be due to the increased electron density difference between the CMFs and matrix components accompanying the thermal softening of lignin (Horiyama et al. 2022). In addition, the scattering in the equatorial direction is thought to reflect scattering in the S2 layer. These results suggest that ethanol adsorption contributing to lignin softening is small near the CMF of the S2 layer. Chen et al. and Bossu et al. also reported that ethanol may be selectively adsorbed in the compound middle lamellae (CML) where lignin concentration is high (Chang et al. 2012; Bossu et al. 2018). These results suggested that ethanol is selectively adsorbed to lignin-rich regions in ethanol-swollen samples, more frequently in the CML than in the surrounding CMFs in the cell wall. In this study, the analysis focused on the equatorial direction, but it is important to clarify which layer of the cell wall is responsible for the scattering intensity in each direction of the SAXS pattern from the wood in order to understand the microstructural changes associated with swelling in detail.
As shown in Fig. 4, a peak in tanδ was observed around 50 °C for the sample swollen in a water–ethanol mixture. On the other hand, in the sample swollen with water, no peak of tanδ was observed in the temperature range of the measurement, but the peak of E″ was observed around 80 °C. This indicates that the sample is in a greatly softened state even in the low-temperature range. Kratky plots are often used to analyze molecular aggregation processes such as protein folding and cellulose molecule assembly (Semisotnov et al. 1996; Tajima et al. 2019). In general, the spatial scale d is expressed as d [Å] = 2π/q. In other words, the smaller q is, the larger the spatial scale. As shown in Fig. 8, the position of the peak for the sample swollen with the water–ethanol mixture, observed in the Kratky plot, was shifted to the low-q side compared to the pure liquid. This suggested that samples swollen with a mixture of water and ethanol have a greater molecular cohesive structure than wood swollen with pure liquid. Paajanen et al. (2022) reported that microfibril packing changes when the moisture content is above 10–15%, whereas deformations in cellulose crystallites take place closer to the dry state. Aguilera-Segura et al. (2019) reported results from molecular dynamics simulations of the interaction between water and/or ethanol solutions and models of celluloses and lignin. It was clear that as the ethanol mass fraction increases, cellulose-water hydrogen bonds (HBs) decrease and cellulose-ethanol HBs increase (Aguilera-Segura et al. 2019). At the same time, it has been reported that hydrogen bonds between cellulose microfibrils increase with increasing ethanol mass fraction (Aguilera-Segura et al. 2019). These results are consistent with the difference in cohesion between samples swollen with the water and ethanol obtained in the SAXS results. It is shown that the solvation of lignin reaches a maximum between a 50% aqueous ethanol solution and pure ethanol. In addition, Aguilera-Segura et al. (2019) reported that the lignin-cellulose interaction decreases in the mixed solvents. In solutions where the activity for each liquid deviates positively from ideal behavior, such as water–ethanol mixtures, the number of molecules that can be released from the cohesive force of each component of the mixture increases above the number expected at a given concentration. Ishimaru et al. (1991) reported that a positive deviation from the ideal behavior of the activity of a constituent gives the constituent a greater chance to attack the adsorption sites responsible for the swelling of wood. This should lead to greater swelling than that expected in the ideal mixture (Ishimaru et al 1991; Sakai and Ishimaru 1989). From the above, it is considered that the ease of adsorption varies from biopolymer to biopolymer depending on the ethanol mole fraction, i.e., there is a distribution in adsorption. A more careful discussion is needed by measuring various molar ratios of swollen wood. However, the fact that dynamic viscoelasticity and small-angle X-ray scattering differ significantly between wood swollen at fEtOH = 0.2 and wood swollen in pure liquid indicates that the microstructures are very different.
Conclusion
DMA and SAXS measurements were performed on hinoki wood swollen with water and/or ethanol to clarify the relationship between swelling and microstructure in different swelling states. The conclusions obtained were as follows.
-
1)
Thermal softening behavior caused by the micro-Brownian motion of lignin was observed in samples swollen with water and/or ethanol. The peak of tanδ was found at around 50 °C for the sample swollen with water–ethanol mixture, but no clear peak was observed within the measurement temperature for the sample swollen with water or ethanol. The peak value of E″ of the sample swollen with water was observed around 80 °C, suggesting that the peak was shifted to a lower temperature for the sample swollen with the mixture of water and ethanol.
-
2)
The scattering behaviors of samples swollen with water and/or ethanol were differed greatly depending on the respective solutions. The SAXS intensity of the samples swollen in water or water–ethanol mixtures increased with increasing temperature. On the other hand, the SAXS intensity of the ethanol-swollen sample changed little with increasing temperature. The position of the peak for the sample swollen with the water–ethanol mixture, observed in the Kratky plot, was shifted to the low-q side compared to the pure liquid.
The combined analysis of DMA and SAXS suggested that ethanol adsorption sites are more common in lignin-rich CML. In addition, the fact that dynamic viscoelasticity and small-angle X-ray scattering differ significantly between wood swollen with a mixture of water and ethanol and wood swollen with pure liquid indicates that the microstructures are very different. In particular, the aggregation state of molecules is very likely to be different. More careful discussion is needed by measuring various mole fractions of swollen wood.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilera-Segura SM, Bossu J, Corn S, Trens P, Mineva T, Le Moigne N, Di Renzo F (2019) Synergistic sorption of mixed solvents in wood cell walls: experimental and theoretical approach. Macromol Symp 386(1):1900022
Ashiotis G, Deschildre A, Nawaz Z, Wright JP, Karkoulis D, Picca FE, Kieffer J (2015) The fast azimuthal integration Python library: pyFAI. J Appl Crystallogr 48(2):510–519
Bossu J, Moigne NL, Corn S, Trens P, Renzo FD (2018) Sorption of water-ethanol mixtures by poplar wood: swelling and viscoelastic behaviour. Wood Sci Technol 52:987–1008
Chang SS, Clair B, Gril J, Yamamoto H, Quignard F (2009) Deformation induced by ethanol substitution in normal and tension wood of chestnut (Castanea sativa Mill.) and simarouba (Simarouba amara Aubl.). Wood Sci Technol 43:703–712
Chang SS, Quignard F, Renzo FD, Clair B (2012) Solvent polarity and internal stresses control the swelling behaviour of green wood during dehydration in organic solution. BioResources 7(2):2418–2430
Furuta Y, Yano H, Kajita H (1995a) Thermal-softening properties of water-swollen wood:I. The effect of drying history. J Jpn Wood Res Soc (Jpn) 41(8):718–721 (in Japanese)
Furuta Y, Norimoto M, Yano H (1995b) Thermal-softening properties of water-swollen wood: V. The effect of drying and heating history. J Jpn Wood Res Soc (Jpn) 44(2):82–88 (in Japanese)
Furuta Y, Nakajima M, Nakatani T, Kojiro K, Ishimaru Y (2008) Effects of lignin on the thermal- softening properties of water-swollen wood. J Soc Mater Sci Jpn 57(4):344–349 (in Japanese)
Gordy WJ (1941) Spectroscopic comparison of the proton-attracting properties of liquids. J Phys Chem 9:204–215
Horiyama H, Kojiro K, Okahisa Y, Imai T, Itoh T, Furuta Y (2022) Combined analysis of microstructures within an annual ring of Douglas fir (Pseudotsuga menziesii) by dynamic mechanical analysis and small angle X-ray scattering. J Wood Sci 68:52
Ishimaru Y, Adachi A (1988) Swelling anisotropy of wood in organic liquids I: external swelling and its anisotropy. J Jpn Wood Res Soc 34(3):200–206 (in Japanese)
Ishimaru Y, Maruta T (1996a) Wood swelling and its transverse anisotropy in organic liquids having two or more functional groups in a molecule. J Jpn Wood Res Soc 42(3):234–242
Ishimaru Y, Sakai H (1988) Swelling of wood in liquid mixtures, 1: water-ethanol and water-acetone. J Jpn Wood Res Soc 34(11):889–895 (in Japanese)
Ishimaru Y, Sakai H, Adachi A (1991) Transverse swelling anisotropy of wood in various states of swelling. J Jpn Wood Res Soc 37(3):187–193 (in Japanese)
Ishimaru Y, Yamada Y, Iida I, Urakami H (1996b) Dynamic viscoelastic properties of wood in various stages of swelling. J Jpn Wood Res Soc 42(3):250–257 (in Japanese)
Kojiro K, Miki T, Sugimoto H, Nakajima M, Kanayama K (2010) Micropores and mesopores in the cell wall of dry wood. J Wood Sci 56(2):107–111
Mantanis GI, Young RA, Rowell RM (1994a) Swelling of wood: Part I: swelling in water. Wood Sci Technol 28:119–134
Mantanis GI, Young RA, Rowell RM (1994b) Swelling of wood: part II. Swelling in organic liquids. Holzforschung 48:480–490
Meier P, Kaps T, Kallavus U (2005) Swelling of pinewood (pinus sylvestris) in binary aqueous solutions of organic substances. Mater Sci Medzg 11:140–145
Meier P, Kaps T, Kallavus U (2006a) Multiple swelling of pinewood (pinus sylvestris) in binary and ternary mixtures of ethanol, acetone and water. Mater Sci Medzg 12(1):25–30
Meier P, Stöör E, Kaps T, Kallavus U (2006b) Mechanical properties of pinewood (Pinus Sylvestris) swollen in organic liquids. Est J Eng 12:125–133
Miyoshi Y, Sakae A, Arimura N, Kojiro K, Furuta Y (2018) Temperature dependences of the dynamic viscoelastic properties of wood and acetylated wood swollen by water or organic liquids. J Wood Sci 64(2):157–163
Miyoshi Y, Sakae A, Arimura N, Kojiro K, Furuta Y (2020) Dynamic viscoelastic properties od wood and acetylated wood in nonequilibrium states swollen by water or organic liquids. J Wood Sci 60:6
Nakatani T, Ishimaru Y, Iida I, Furuta Y (2006) Adsorption of some organic liquids onto the main constituents of wood. J Jpn Wood Res Soc 52(5):285–292 (in Japanese)
Nakatani T, Ishimaru Y, Iida I, Furuta Y (2008) Contribution of lignin to adsorption of organic liquids onto wood. J Jpn Wood Res Soc 54(1):17–23 (in Japanese)
Nayer AN, Hossfeld RL (1949) Hydrogen bonding and the swelling of wood in various organic liquids. J Am Chem Soc 71:2852–2855
Paajanen A, Zitting A, Rautkari L, Ketoja JA, Penttilä PA (2022) Nanoscale mechanism of moisture-induced swelling in wood microfibril bundles. Nano Lett 22(13):5143–5150
Penttilä PA, Zitting A, Lourencon T, Altgen M, Schweins R, Rautkari L (2021) Water-accessibility of interfibrillar spaces in spruce wood cell walls. Cellulose 28:11231–11245
Penttilä PA, Rautkari L, Österberg M, Schweins R (2019) Small-angle scattering model for efficient characterization of wood nanostructure and moisture behaviour. J Appl Crystallogr 52(2):369–377
Penttilä PA, Altgen M, Carl N, van der Linden P, Morfin I, Österberg M, Schweins R, Rautkari L (2020) Moisture-related changes in the nanostructure of woods studied with X-ray and neutron scattering. Cellulose 27(1):71–87
Prusov AN, Prusova SM, Zakharov AG (2014) Interaction of cellulose and lignocellulosic polymers with water and aqueous systems. Russ Chem Bull 63:1926–1945. https://doi.org/10.1007/s11172-014-0683-7
Sakai H, Ishimaru Y (1989) Swelling of wood in liquid mixtures, 2: relationships between swelling and activity of each constituent in several binary liquid mixtures. J Jpn Wood Res Soc 35(6):465–472 (in Japanese)
Seki M, Yashima Y, Abe M, Miki T, Nishida M (2022) Influence of delignification on plastic flow deformation of wood. Cellulose 29(7):4153–4165
Semisotnov GV, Kihara H, Kotova NV, Kimura K, Amemiya Y, Wakabayashi K, Serdyuk IN, Timchenko AA, Chiba K, Nikaido K, Ikura T, Kuwajima K (1996) Protein globularization during folding: a study by synchrotron small-angle X-ray scattering. J Mol Biol 262(4):559–574
Stamm AJ, Loughborough WK (1942) Variation in shrinking and swelling of wood. Trans Am Soc Mech Eng 64:379–386
Tajima H, Penttilä PA, Imai T, Yamamoto K, Yuguchi Y (2019) Observation of in vitro cellulose synthesis by bacterial cellulose synthase with time-resolved small angle X-ray scattering. Int J Biol Macromol 130:765–777
Tanaka K, Kojiro K, Furuta Y (2019) Thermal softening properties of wood swollen by a binary liquid mixture of water and ethanol. Proceedings of the sixty-ninth annual meeting of the Japan wood research society, 14–16 March, 2019: C15-P-06. Japan Wood Research Society, Hakodate
Yadav P, Bossu J, Le Moigne N, Corn S, Di Renzo F, Trens P (2024) Sorption of water and ethanol pure vapours and vapour mixtures by four hardwoods. Wood Sci Technol 58(1):177–194
Acknowledgements
The synchrotron radiation experiments were performed at the BL40B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2019B1176, 2020A1592, 2021A1384) The research was financially supported in part by the Mission-2 research in RISH, Kyoto University.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Hiroaki Horiyama designed the study, collected and analyzed data and wrote the initial draft of the manuscript. Keisuke Kojiro and Yuzo Furuta contributed to dynamic viscoelastic analysis and interpretation of data, and assisted in the preparation of the manuscript. Yoko Okahisa and Tomoya Imai contributed to SAXS measurement and SAXS data analysis, and critically reviewed the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horiyama, H., Kojiro, K., Okahisa, Y. et al. Combined analysis of the microstructure of wood swollen by water and/or ethanol through dynamic mechanical analysis and small-angle X-ray scattering. Wood Sci Technol (2024). https://doi.org/10.1007/s00226-024-01599-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00226-024-01599-2