Abstract
Genetic parameters for various wood density traits were estimated in 29-year-old trees of 18 full-sib families of hybrid larch (Larix gmelinii var. japonica × Larix kaempferi) F1. Intra-ring density variation (IDV) was also evaluated using a model that expresses the pattern curve from earlywood to latewood as a power function. A high IDV indicates an abrupt change in wood density from earlywood to latewood. The ring width and wood density traits of individual rings were determined by X-ray densitometry. Overall wood density (RD) was shown to increase with increasing ring number, ranging from 0.42–0.59 g/cm3, whereas IDV of individual rings decreased gradually from pith outwards. Estimates of individual tree narrow-sense heritability of RD and IDV were 0.66 and 0.67, respectively. IDV showed negative genetic and phenotypic correlations with RD (r g = −0.99, r p = −0.72). The predicted genetic gains in latewood proportion and IDV were higher than that of RD. These results suggest that the intra-ring density variation is under moderate genetic control equivalent to wood density. The trend of increasing wood density from earlywood to latewood was associated with changes in both tracheid diameter and cell wall thickness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood density is one of the most useful indicators in forest product manufacturing because it is strongly correlated with many other traits, such as wood strength properties and the quality of pulp and paper (Zobel and van Buijtenen 1989). Wood density can be altered by silviculture and breeding (Williams and Hamilton 1961; Zobel 1961). However, wood density is a complex trait that is a result of various combinations of the proportions of earlywood and latewood, and their relative densities. Therefore, knowledge of the genetic control of these density components and their interrelationships would improve understanding of the genetics of overall wood density (Vargas-Hernandez and Adams 1991; Zhang and Morgenstern 1995; Greaves et al. 1997; Hylen 1997; Kumar and Lee 2002).
Like most wood characteristics, wood density is a highly variable trait within and among trees. Larson (1969) considered this general lack of uniformity of wood properties as one of the greatest problems facing the wood industry. In particular, the variability and distribution pattern of wood density within the annual growth rings have a major effect upon the suitability of wood materials for end products (Kanowski 1985; Wright and Burley 1990). For example, an abrupt change in wood density from earlywood to latewood causes increased variation of veneer thickness and more veneer fissures (Vargas-Hernandez and Adams 1991). Although several attempts have been made to quantify the within ring density variation (Echols 1973; Olson and Arganbright 1977; Walker and Dodd 1988), there are few reports that have addressed the genetic control of this trait.
The hybrid between Kurile larch (Larix gmelinii var. japonica) and Japanese larch (Larix kaempferi) shows rapid juvenile growth and high resistance to various pests and diseases compared with Japanese larch and other hybrid larches (Takahashi and Nishiguchi 1966; Hamaya and Kurahashi 1981). Furthermore, this hybrid provides wood with desirable qualities, such as a straight stem, and high wood density and strength (Oshima and Nishikoori 1994; Iizuka et al. 2000). Therefore, the hybrid larch appears to be suitable for use in intensively managed forests. However, the above findings were demonstrated through interspecies comparisons with other Larix species and their hybrids, and little is known about variation within the hybrid species. The wood properties of this hybrid larch vary widely (Oshima and Kuromaru 1995). Development of a breeding program for wood quality requires information about the genetic variation in wood properties and genetic relationships among these traits.
In the present study, we evaluated the intra-ring density variation (IDV) using a model that expresses the pattern curve from earlywood to latewood as a power function. Genetic variations of IDV and various density traits and correlations among these traits were also estimated. Furthermore, wood anatomical characteristics were observed and their relationships to wood density and its pattern variation are discussed.
Material and methods
Plant material
Wood samples were collected from a 29-year-old Larix gmelinii × Larix kaempferi progeny test stand in Bibai, Hokkaido, northern Japan. This stand was the same one described in detail in previous articles (Fujimoto et al. 2006a, b). The progeny test consisted of 18 full-sib families derived from an incomplete factorial mating design involving four female and six male parent trees (Table 1). The test was established in 1974 with 2-year-old seedlings. Families were planted in 21-tree plots at 2 × 2 m spacing according to a randomized complete block experimental design with two replicates. In 2002, 4–6 sample trees were harvested from each family (Table 1), resulting in a total of 95 sample trees. A 1.8 m butt log was obtained from each tree and a diametric flitch, 40 mm thick, was sawn from each log (Fig. 1).
Wood density measurements
A 2 mm long (longitudinal direction) section including the pith was cut from the top end of each flitch free from decay and knots (Fig. 1). Resin was then extracted from these sections with a solution of benzene–ethanol (2:1) for 1 week. Thereafter, the sections were dried to an equilibrium moisture content of approximately 12%.
Intra-ring density and width information was obtained using an X-ray densitometry technique (Ohta 1970; Polge and Nicholls 1972). Each sample section was scanned from the pith toward the bark in two radial directions and density was expressed as the mean of both values. The first and last annual rings were excluded from analyses because they were generally incomplete. For each of the remaining annual rings in the samples, various intra-ring wood density and width characteristics were obtained based on the densitometric profiles (see Table 2). Demarcation between earlywood and latewood in each ring was defined as the point where the wood density in each ring was 0.55 g/cm3 (Ohta 1970). This point was based on a detailed comparison of results obtained by X-ray analyses and the definition of latewood given by Mork (1928).
Modelling of wood density distribution pattern within growth rings
In order to compare the intra-ring density variation pattern obtained from all annual rings among families, we used the model suggested by Yahata et al. (1987) that expresses the pattern curve from earlywood to latewood as a power function (Eq. 1) (see Fig. 2).
where g (MIN) is the minimum wood density, r (RW) is the annual ring width, and a and b are the parameters that determine the form of the function.
Assuming that the integral of Eq. 1 over the annual ring equals the product of g (RD) and r (RW) (Fig. 2), coefficients of a and b were determined.
where g (RD) is the overall ring density and g (MAX) is the maximum wood density. Combining Eqs. 2 and 3 gives the following equations:
The coefficient of b is considered as a useful indicator to compare the intra-ring density variation among families, because it is independent of the ring-width variation (Eq. 5). Furthermore, the coefficient of b is not influenced by the differences of definition for the demarcation between earlywood and latewood. Thus, we adopt the b values for the following analyses as the intra-ring density variation (IDV) in this article. A high IDV indicates an abrupt change in wood density from earlywood to latewood.
Statistical analysis
Analyses of variance for all observed values for each growth ring were performed according to the model in Eq. 6 using the SAS Proc GLM SS3 (SAS Institute 1990) as follows:
where Y ijkl is the observed value of the lth tree of the jth female parent and kth male parent in the ith replicate, μ is the general mean, R i is the fixed effect of replicate i, F j is the random effect of female j with variance σ2 f, M k is the random effect of male k with variance σ2 m, FM jk is the random interaction effect between female parent j and male parent k with variance σ2 fm, RFM ijk is the between-plot error, random effect, with variance σ2 rfm, and ɛ ijkl is the within-plot error, random effect, with variance σ2 e. Variance components for each trait were estimated using the restricted maximum likelihood (REML) method of the SAS VARCOMP procedure, and estimates of the covariance between different traits were obtained from the MANOVA statement (SAS Institute 1990).
For analysis of the factorial mating design, the variance components for male and female plants were assumed to be equal to one quarter of the additive genetic variance (σ2 A = 4σ2 f = 4σ2 m); the variance component σ2 fm estimated to be one quarter of the dominance variance (σ2 D = 4σ2 fm), assuming that epistatic and maternal effects were negligible and that the coefficient of inbreeding among parents was zero (Cotterill et al. 1987). The narrow-sense heritability is given by the following formula on an individual tree basis (Zobel and Talbert 1984):
The genetic correlation was calculated as follows (Becker 1984):
where r g is the genetic correlation, cov(x,y) is the covariance between traits x and y, σ2 (x) and σ2 (y) are variance components of the two traits, and subscripts f and m indicate female and male parents, respectively. Standard errors of the heritability and additive genetic correlations were calculated respectively as in Becker (1984) and Falconer and Mackay (1996).
The predicted genetic gain (ΔG) from direct selection of a single trait and the predicted correlated genetic responses of indirect selection were calculated as follows (Falconer and Mackay 1996):
where i is the standardized selection intensity, h 2 F is family mean heritability, and σF is the standard deviation of the family mean, CR is the correlated response of trait y due to the selection of trait x, h (x) and h (y) are the square roots of family mean heritability for traits x and y, r g is the genetic correlation between traits x and y, and σ(y) is the standard deviation of the family mean for trait y. Family mean heritability was calculated following Barnes and Schweppenhauser (1978).
Measurements of anatomical characteristics
Tracheid diameter and cell wall thickness were determined in order to explore the relationship between wood density and anatomical characteristics. For the high and low IDV families, transverse sections, 15 μm thick, were cut from a characteristic growth ring using a sliding microtome. The sections were stained in aqueous safranin and mounted permanently. The images of the microscope slides were captured through a CCD video camera attached to a light microscope and analyzed by a Luzex-F (NIRECO Co.) image processing device. Consecutive images (512 × 512 pixel) with ×100 magnification were collected starting at the earlywood until the latewood was reached for three radial rows. The measured parameters were tracheid diameter and double cell wall thickness in the radial direction. The data shown were means of 50–100 cells measured at each frame. The microscope was calibrated with a stage micrometer and the resolution of digital images was 0.88 μm/pixel.
Result and discussion
Population means
Table 3 shows the mean, range and coefficient of variation for the measured traits. The value of these traits for each individual tree was the average of all annual rings. The mean values and ranges were consistent with a previous report of hybrid larch (Fujimoto et al. 2003). The values of coefficient of variation for growth traits, that are RW, EW and LW, were generally larger than those of density traits. The same results are found in many earlier studies (Zhang and Morgenstern 1995; Hylen 1997; Zhang 1998). Comparing other density traits, the coefficient of variation for IDV was large (CV = 14.9%), nearly identical with that of LWP.
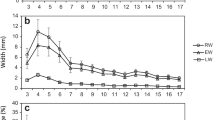
The radial trends from pith to bark for each ring width and density traits are shown in Fig. 3. The trends for RW and EW were similar and these values declined dramatically with increasing ring number, whereas LW was stable over the rings. These radial trends resulted in the steady increase of LWP. Large variations were not found in the MAX, LD, ED and MIN (rates of change from pith to bark were approximately 10%). In contrast, RD was shown to increase with increasing ring number, ranging from 0.42–0.58 g/cm3. These results indicate that radial variation of overall wood density (RD) is mainly influenced by the change of latewood proportion. Warren (1979) also reported that the proportion of latewood was the major contributor to overall RD. IDV of individual rings decreased gradually over the rings, which was the inverse of the trend in LWP. Thus, IDV is a feasible indicator to evaluate the proportion of latewood, namely, wood uniformity within growth rings.
Variance components and heritability
Table 4 shows additive and dominance genetic variance components and narrow-sense heritability estimates of various traits. The additive genetic variance components associated with both female and male parents differed significantly from zero for all measured traits, with some exceptions. The magnitude of the additive genetic variance component for the female or male parent effects varied according to each trait. No dominance effect was shown with any of the traits as almost all dominance genetic variance components were zero. These results indicate that the genetic effects of the variables studied here are largely additive, while the specific combining ability is only a minor variance component in hybrid larch. However, a mating design with few full-sib families per half-sib family, as used here, is not optimal for estimating dominance variance (Namkoong and Roberds 1974). Further studies using more families are therefore, required to better understand the genetic structure of the RD traits of hybrid larch.
Narrow-sense heritability estimates of the density traits were moderate, ranging from 0.26 to 0.67. Standard errors of the heritability estimates were large, ranging from 0.19 to 0.23, due to the small sample size. It should be noted that all the estimates of heritability in this study may be upwardly biased because measurements were restricted to a single test site where family-site interactions were not taken into account. None of the individual wood density components had estimates of heritability greater than that found for RD (h 2 = 0.66). This was consistent with the studies of Douglas-fir (Vargas-Hernandez and Adams 1991) and Eucalyptus (Greaves et al. 1997). However, there are many diverse reports in various species that other density components appear to be under stronger genetic control than overall density (Zhang and Morgenstern 1995; Hylen 1997). Many previous reports have shown that the heritability estimates of overall RD and its components are higher than those of growth characteristics (Zobel and van Buijtenen 1989; Zhang 1998). However, this tendency was not found in this study. Hylen (1997) noted that growth traits are more affected by variation in growth conditions at the planting site than are density traits. The small number of families from a single stand may also have influenced the result.
Intra-ring density variation showed the highest heritability estimate at 0.67 which is almost equal to those of RD and LWP. This finding suggests that the intra-ring density variation is under moderate genetic control equivalent to overall RD. In other words, wood uniformity within a growth ring is expected to respond to selection in a tree improvement program. As compared to the growth and RD characteristics, little is known about the genetic control of the intra-ring density variation. Vargas-Hernandez and Adams (1991) evaluated the genetic control of intra-ring density variation, which was defined by using the squared differences between overall density and earlywood density and latewood density, and latewood proportion. They estimated the individual-tree and family heritabilities of this trait as 0.25 and 0.39, respectively. Ivković and Rozenberg (2004) investigated the frequency distribution of within-ring density that was described using both moment-based and non-parametric statistics and its sample quantiles were modeled using the generalized lambda distribution. Significant clone variability was found in within-ring frequency distribution parameters.
Genetic and phenotypic correlations among traits and their implications for breeding
Estimates of phenotypic and genetic correlations among measured traits are shown in Table 5. The predicted genetic gains from direct selection of these traits and correlated responses in target traits (RD and IDV) from indirect selection are listed in Table 6. The predicted genetic gain was arbitrarily calculated for the top 5% of the 18 families, and selection intensity was given to be 1.820 according to Becker (1984), in this case.
Wood density had strong positive phenotypic (0.77–0.86) and genetic (0.87–1.00) correlations with all of the wood density components. Predicted genetic gain in RD from direct selection for this trait was higher than that from selection for its components owing to the magnitude of the heritability estimates. Furthermore, wood density components were strongly correlated among themselves, indicating that wood density components have limited value in improving the efficiency of selection for overall density (Zhang and Morgenstern 1995). In fact, the predicted correlated responses in RD, while selecting for wood density components, were lower than direct selection of RD.
Latewood proportion and IDV showed strong phenotypic and genetic correlation with RD. This suggests that selection for overall density in hybrid larch would result in a correlated increase in wood uniformity. A high predicted genetic gain was found in LWP and IDV, greater than that of RD. This is because of the high phenotypic variation (see Table 3) and heritability estimates for these traits. The indirect responses in RD using LWP and IDV were nearly identical with the direct selection of RD. It might be possible to improve the latewood proportion and intra-ring density variation pattern without reducing the overall density.
Annual ring width had negative correlations with RD and LWP, and had a positive correlation with IDV. The negative response for RD (−47.2%) and positive response for IDV (44.5%) were found from indirect selection of RW. These results indicate that selection based solely on growth rate will simultaneously result in a reduction in the overall density and wood uniformity within growth rings. The wood density components showed slightly weaker correlations with RW (r p = −0.12 to 0.04; r g = −0.27 to −0.60) than RD and IDV. Therefore, to achieve optimal genetic gains, index selection of multiple traits seems essential (Vargas-Hernandez and Adams 1991).
Intra-ring density variation pattern and its relation to tracheid morphology
Examples of density variations from earlywood to latewood for high and low IDV families at 9th and 17th rings are shown in Fig. 4. These plot data were derived from an individual tree from each family. The trends in density variation from earlywood to latewood were apparently different in these families. Wood density within the high IDV family increased abruptly from earlywood to latewood. On the other hand, a gradual increase was observed in the low IDV family at both growth rings. These results imply that the model used in this study could successfully express the density variation pattern within growth rings, and show the transition from earlywood to latewood.
Another important feature is that the low IDV family showed high RD in the earlywood (Fig. 4). This fact was not contradictory to the results that ED showed strong phenotypic and genetic correlation with IDV (see Table 5). Hence the selection of the family having high earlywood density might be effective in improving the wood uniformity within growth rings, although the genetic control of ED was not so strong in this study (see Table 4).
Since extractive content is low and the density of the cell wall material itself varies very little, the whole wood density is a reflection mainly of the proportion of cell wall substance to lumen. Thus, RD depends primarily on tracheid diameter relative to cell wall thickness, including their absolute values in earlywood and latewood and their pattern of change across each ring (Mitchell and Denne 1997). Within-ring variation in tracheid diameter and double cell wall thickness in the radial direction for high and low IDV families at the 17th ring are shown in Fig. 5. The gradual increase of RD observed in the low IDV family (Fig. 4) was associated with the changes in both tracheid diameter and cell wall thickness. Tracheid diameters of the low IDV family were smaller than those of the high IDV family over the growth ring. In addition, the reduction of tracheid diameter was remarkable in the low IDV family. The low IDV family formed thicker cell walls from the early phase and this continued into the latewood area. The small tracheid diameters in earlywood were also consistent with the high earlywood density in the low IDV family (see Fig. 4). A number of studies have reported that it could be possible to improve anatomical characteristics via tree breeding (Zobel and van Buijtenen 1989; King et al. 1998; Ivkovich et al. 2002). However, further research, including tree physiology research, would be required to better understand the relationship between wood density and tracheid morphology of hybrid larch.
References
Barnes RD, Schweppenhauser MA (1978) Pinus patula Schiede and Deppe progeny tests in Rhodesia genetic control of nursery traits. Silvae Genet 27:200–204
Becker WA (1984) Manual of quantitative genetics, 4th edn. Academic Enterprises, Pullman
Cotterill PP, Dean CA, van Wyk G (1987) Additive and dominance genetic effects in Pinus pinaster, P. radiata and P. elliottii and some implications for breeding strategy. Silvae Genet 36:221–232
Echols RM (1973) Uniformity of wood density assesses from X-rays of increment cores. Wood Sci Technol 7:34–44
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman, Essex
Fujimoto T, Akutsu H, Kita K (2003) Age trends in genetic parameters of wood density in hybrid larch (Larix gmelinii var. japonica × L. leptolepis) F1. J Hokkaido For Prod Res Inst 17(6):7–13
Fujimoto T, Akutsu H, Kita K, Uchiyama K, Kuromaru M, Oda K (2006a) Age trends of genetic parameters of spiral grain in hybrid larch F1 and implications for efficiency of early selection. J Wood Sci 52:101–106
Fujimoto T, Kita K, Uchiyama K, Kuromaru M, Akutsu H, Oda K (2006b) Age trends in the genetic parameters of wood density and the relationship with growth rates in hybrid larch (Larix gmelinii var. japonica × L. kaempferi) F1. J For Res 11:157–163
Greaves BL, Borralho NMG, Raymond CA, Evans R, Whiteman Ph (1997) Age-age correlations in, and relationships between basic density and growth in Eucalyptus nitens. Silvae Genet 46:264–270
Hamaya T, Kurahashi A (1981) Breeding of larch by species hybridization in Japan. In: Krugman SL, Katsutapp M (eds) Proceedings of the XVII IUFRO World Congress, Kyoto, 6–7 September, Division 2, pp 157–168
Hylen G (1997) Genetic variation of wood density and its relationship with growth traits in young Norway spruce. Silvae Genet 46:55–60
Iizuka K, Kohno K, Fujimoto T (2000) Variations of growth and wood quality in young interspecies between Larix gmelinii var. japonica and L. leptolepis. J Jpn For Soc 82:295–300
Ivkovich M, Namkoong G, Koshy M (2002) Genetic variation in wood properties of interior spruce. II. Tracheid characteristic. Can J For Res 32:2128–2139
Ivković M, Rozenberg P (2004) A method for describing and modelling of within-ring wood density distribution in clones of three coniferous species. Ann For Sci 61:759–769
Kanowski P (1985) Densitometric analysis of a large number of wood samples. J Inst Wood Sci 10(4):145–151
King JN, Cartwright C, Hatton J, Yanchuk AD (1998) The potential of improving western hemlock pulp and paper quality. I. Genetic control and interrelationships of wood and fibre traits. Can J For Res 28:863–870
Kumar S, Lee J (2002) Age-age correlations and early selection for end-of-rotation wood densitiy in radiata pine. For Genet 9:323–330
Larson PR (1969) Wood formation and the concept of wood quality. Yale Univ Sch For Bull No.74
Mitchell MD, Denne MP (1997) Variation in density of Picea sitchensis in relation to within-tree trends in tracheid diameter and wall thickness. Forestry 70:47–60
Mork E (1928) Die Qualität des Fichtenholzes unter besonderer Rücksichtnahme auf Schleif- und Papierholz. Papier-Fabrikant 26:741–747
Namkoong G, Roberds JH (1974) Choosing mating designs to efficiently estimate genetic variance components for trees. Silvae Genet 23:43–53
Ohta S (1970) Measurement of the wood density by the soft X-ray and densitometric technique. Mokuzai Kogyo 25:27–29
Olson JR, Arganbright DG (1977) The uniformity factor—a proposed method for expressing variations in specific gravity. Wood Fiber Sci 9:202–210
Oshima T, Kuromaru M (1995) Variation among families of wood properties in hybrid larch (Larix gmelinii var. japonica × L. leptolepis). Trans 106th Mtg Jpn For Soc 297–298
Oshima T, Nishikoori M (1994) Variation of stem crook in hybrid larch (Larix gmelinii var. japonica × L. leptolepis). Trans Mtg Hokkaido Br Jpn For Soc 42:37–39
Polge H, Nicholls JWP (1972) Quantitative radiography and the densitometric analysis of wood. Wood Sci 5:51–59
SAS Institute (1990) SAS/STAT user’s guide, version 6, vol 2, 4th edn. SAS, Cary
Takahashi N, Nishiguchi C (1966) Studies on the resistance of forest trees to the red-backed vole, Clethrionomys rufocanus bedfordiae (Thomas). Part 2. Relative feeding preference of the vole for seedlings of larch F1 hybrids under laboratory condition. Bull Tokyo Univ For 62:173–188
Vargas-Hernandez J, Adams WT (1991) Genetic variation of wood density in young coastal Douglas-fir: implications for tree breeding. Can J For Res 21:1801–1807
Walker NK, Dodd RS (1988) Calculation of wood density variation from X-ray densitometer data. Wood Fiber Sci 20:35–43
Warren WG (1979) The contribution of earlywood and latewood specific gravities to overall wood specific gravity. Wood Fiber Sci 11:127–135
Williams RF, Hamilton JR (1961) The effect of fertilization on four wood properties of slash pine. J For 59:662–665
Wright JA, Burley J (1990) The correlation of wood uniformity with the papermaking traits of tropical pines. Tappi 73(3):231–235
Yahata H, Miyajima H, Sairinji T, Huruie H, Kodama T, Yuruki T, Yamamoto H, Kubota S, Watanabe K, Nogami K, Kuroki H (1987) Wood quality variations of native cultivars and the clones of plus trees in the experimental areas of cultivars of Cryptomeria japonica in Kyusyu. Bull Kyushu Univ For 57:149–173
Zhang SY (1998) Effect of age on the variation, correlations and inheritance of selected wood characteristics in black spruce (Picea mariana). Wood Sci Technol 32:197–204
Zhang SY, Morgenstern EK (1995) Genetic variation and inheritance of wood density in black spruce (Picea mariana) and its relationship with growth: implications for tree breeding. Wood Sci Technol 30:63–75
Zobel BJ (1961) Inheritance of wood properties in conifers. Silvae Genet 10:65–70
Zobel B, Talbert J (1984) Applied forest tree improvement. Wiley, New York
Zobel BJ, van Buijtenen JP (1989) Wood variation. Its causes and control. Springer, Berlin
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujimoto, T., Kita, K. & Kuromaru, M. Genetic control of intra-ring wood density variation in hybrid larch (Larix gmelinii var. japonica × L. kaempferi) F1 . Wood Sci Technol 42, 227–240 (2008). https://doi.org/10.1007/s00226-007-0171-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-007-0171-4