Abstract
Movement of polyethylene glycol (PEG) 1000 from wood cell lumens into adjacent cell walls was studied for different cell wall moisture contents (MCs) and PEG solvents; relative amounts of PEG that penetrated into the cell walls were estimated by Raman microscopy. It was shown that samples treated with PEG from chloroform solution adsorb less PEG than samples treated with PEG-water solution even in the case of samples with open cell wall structure near fibre saturation point MC. Reconditioning of PEG-chloroform treated samples at high relative humidities enhanced PEG bulking of cell walls, but not to the maximum amounts observed with PEG-water treatments. Raman microscopy was a useful tool for estimating relative PEG amounts in the cell walls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyethylene glycol (PEG) is often used for studying penetration of a polymer into wood cell walls by evaluating the extent of bulking, i.e. ability of a polymer to retain wood in its swollen state upon drying. It has been shown that PEG has a very good bulking ability when introduced to pre-saturated wood from water solutions even in the case of high molecular weights (Cooper et al. 1991; Stamm 1964a), but dry wood samples could not be bulked with liquid PEG, even at very low molecular weights (Schneider von 1969; Sadoh 1968). Non-polar toluene solution of high molecular weight PEG effectively bulked solvent-exchange dried, pre-swollen wood samples (Ishimaru et al. 1986). These findings indicated the importance of creation of microvoids, either prior to or during the treatment in order to achieve good bulking. It is unclear, though, how samples of different moisture content (MC) below fiber saturation point (FSP) are bulked with PEG from solvents of different swelling abilities and different capacities for PEG, such as water and chloroform. The investigation of this matter is important in order to understand the mode of penetration of large molecules into cell walls during such processes as adhesive bonding, coating penetration, wood preservation and wood chemical modification where relatively large molecules are expected to penetrate the cell walls. PEG was chosen in the present study due to its good solubility in both polar and non-polar solvents.
Earlier procedures of estimation of PEG amounts in cell walls involved both indirect and direct PEG measurements. The indirect methods included (1) measurements of concentration changes of supernatant before and after treatment in order to calculate the amount of PEG penetrating the cell walls (Ishimaru 1976; Kitani et al. 1970; Tarkow et al. 1966) and (2) gravimetric determination of PEG amounts in the cell walls after extraction with benzene (Sadoh 1968). The latter method assumed that no PEG would be extracted from the cell walls after a 10-day extraction in benzene, but the comparisons of unextracted and extracted samples showed approximately 40% less bulking of extracted samples. These approaches gave great variability in results; for example, estimated amounts of PEG 600 adsorbed into open microvoids ranged from less than 0.02 (Kitani et al. 1970) to over 0.25 g PEG per gram of wood (Sadoh 1968). Similar discrepancies were observed for estimated PEG amounts of other molecular weights, as well as for relative amounts of different molecular weights of PEG. Direct estimates of KMnO4 labelled PEG amounts were made using the Scanning Electron Microscopy coupled with Energy Dispersive X-ray Analyzer (SEM-EDXA) (Wallstrom and Lindberg 1999). This technique was also found to have limitations of low detection limits for K-labelled PEG. Consequently, a new approach of estimating relative PEG cell wall amounts by Raman spectroscopy has been undertaken in this study.
The Raman method does not require any other sample preparation but a clean, smooth surface, which makes it applicable for uses in conservation as well (Faria de et al. 2004; Castillejo et al. 2003). Raman microscopy has already been proven as a useful tool for estimating propiconazole amounts in wood (Kurti et al. 2005).
Materials and methods
Red pine (Pinus resinosa Ait.) cubes (15 × 25 × 5 mm³; t × r × l) were vacuum- dried in a freeze-drier at −50°C to constant MC (less than 0.2% MC) and their mass and volume (measured by buoyancy force in mercury) were recorded. The samples were then conditioned above saturated water solutions of KOH, NaCl, or CuSO4 selected to develop relative humidities (RH) of 9, 76 and 98%, respectively, until samples reached their equilibrium moisture contents (EMC). Six samples at each EMC were treated with 30% PEG-water or 30% PEG-chloroform solutions and three control samples of each EMC were treated with distilled water or chloroform (HPLC grade, Caledon Laboratories). Analytical grade PEG 1000 (Mr 950–1050) was purchased from Fluka. Treatment was performed by placing samples in a treating jar, applying vacuum for 1 min, then introducing solution into the jar and maintaining the submerged samples under vacuum until disappearance of air-bubbles. PEG-chloroform and chloroform treated samples were kept in non-drying conditions for 2 days in order to enhance PEG penetration into wood cell walls and then vacuum dried as above. Sample volumes were measured immediately after treatment, and after the 2-day equilibration (chloroform treated samples only), and both mass and volume were recorded after vacuum-drying. Swelling due to solution penetration was calculated immediately after treatment and after 2-day equilibration for chloroform treatments, and bulking was expressed in terms of retained swelling upon drying using Eq. 1:
RSTR – retained swelling upon treatment, %; \( V^{{\text{D}}}_{{{\text{TR}}}} \) – dry volume of samples upon treatment, cm3; \( V^{{\text{D}}}_{{{\text{UNTR}}}} \) – initial volume of dry samples, cm3. Polyethylene glycol uptake into bulk wood was calculated by Eq. 2.
where PEG uptake is expressed as gram PEG per gram dry wood; \( m^{{\text{D}}}_{{{\text{TR}}}} \) – mass of dry treated samples, g; \( m^{{\text{D}}}_{{{\text{UNTR}}}} \) – initial mass of dry samples (prior to EMC conditioning and treatment), g.
Three out of six samples treated with PEG solutions (both water and chloroform) were then re-conditioned above CuSO4 saturated aqueous solution (98% RH), and upon re-drying their volumes were measured and bulking was calculated.
The other three samples were Soxhlet extracted in toluene (HPLC grade, Caledon Laboratories) for 1 h in order to remove excess PEG from the surface and thus to prepare the samples for comparison of PEG amounts in cell walls by Raman microscopy. Retained swelling upon extraction and subsequent drying was also measured to determine if there were losses of PEG from the cell walls due to extractions.
Outer layers of samples’ end grain surfaces were removed by razor-blades up to a depth of 1–2 mm and treated wood spectra were monitored on earlywood cells by Raman Renishaw System 2000 operated in confocal mode. The instrument was equipped with a Pelletier-cooled CCD array that could capture a 600 cm−1 spectral window (spectrograph) with 2.5 cm−1 spectral resolution. Raman spectra (200–2,000 cm−1 Raman shift) were collected in the “continuous” mode, in which the wavelengths are scanned across the CCD array; in this way, a complete spectrum was collected comprising three complete spectral windows. Excitation was achieved with a 785-nm diode laser (25 mW at the head; 1 mW at the sample). Spectra were collected from three cell wall locations (S2 layers in the corners of the cells were scanned in order to avoid cell lumens in case of residual PEG in the lumens) and the three measurements were then averaged. A spectral integration time of 40 s was chosen with the cosmic ray elimination feature activated. The cosmic ray elimination algorithm compares three spectra for unusual features (spikes) that can be ascribed to cosmic ray collision with the CCD. The final spectrum is the average of the three spectra, with the cosmic-ray spikes removed, and the effective total integration time is 120 s.
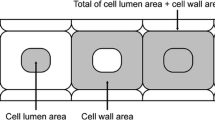
In confocal mode, Raman has a spatial resolution of 1 μm, ensuring that the Raman signals came from the cell walls only (Fig. 1). Since the Raman signal varied with surface characteristics of a sample, a normalization procedure was required. Raman estimation of PEG in cell walls was based on the ratio of the area of Raman spectra characteristic to PEG (750–975 cm−1) to the area of Raman spectra characteristic for lignin (1,550–1,700 cm−1), the wave number regions where there was minimal overlap of wood and PEG spectra (Fig. 2).
Results and discussion
Samples conditioned above the saturated water solutions of KOH, NaCl, or CuSO4 reached EMCs of 2.3 ± 0.4, 13.3 ± 0.5 and 24.5 ± 0.8%, with swelling of 1.1 ± 0.1, 7.5 ± 0.5, and 14.5 ± 0.7%, respectively (means and standard deviations).
Swelling of the samples immediately following treatment and 2-day equilibration for chloroform treated samples is given in Table 1. An average swelling of water control samples to 16.2 ± 0.7% upon treatment indicated that the samples conditioned at 98% RH did not reach FSP. The samples treated with PEG-water solution swelled to significantly lower extents (14.8 ± 1.6%) in comparison to the water controls. The difference in swelling between PEG-water and pure water treatments was statistically significant for 2.3% EMC samples with PEG-water treated samples swelling by 13.8 ± 2.2% while the water controls swelled to 16.5 ± 0.2%. The swelling of samples conditioned to 13.3 and 24.5% EMC did not differ between the samples. This indicates that the water movement into the cell walls of 2.3% EMC conditioned samples was decreased by PEG presence in the solution due to osmotic competition between the concentrated solution and the cell wall.
Samples treated with PEG-chloroform solution or pure chloroform did not change their conditioned volumes immediately upon treatment, but the 2-day equilibration caused significant swelling of 2.3% EMC samples. This indicates that chloroform could penetrate cell wall to some extent, as shown by previous studies (Mantanis et al. 1994, 1995a, b; Stamm 1964b). Similarly to PEG-water treatment, 2.3% EMC PEG-chloroform treated samples swelled less than pure chloroform treated samples.
Gross PEG uptake by the samples significantly differed only among the solvents, PEG-water treated samples adsorbing more PEG than the PEG-chloroform treated samples (Table 1). However, the amount of PEG penetrating the cell walls was dependent on both variables, as measured by retained swelling upon drying (Fig. 3). PEG-chloroform treated samples were bulked to significantly lower extents than the PEG-water treated samples. Bound water played a significant role in facilitating PEG penetration into cell walls from chloroform solutions. Samples with very small amount of transient capillaries (2.3% EMC) allowed only minor (but statistically significant) bulking.
Despite the expected equal bulking values for all PEG-water treated samples due to the similar extent of swelling immediately upon treatment and similar PEG uptakes, 2.3 and 13.3% EMC PEG-water treated samples retained their full swollen volumes after drying and were bulked to a significantly (0.01 level) higher extent than 24.5% EMC PEG-treated water samples. Since the samples were removed from the treating jars immediately upon release of vacuum, the difference in bulking may be explained by higher concentration of PEG in the lumens of the initially dryer samples upon solvent water diffusion into their cell walls, which would result in higher subsequent diffusion of PEG into the cell walls. Earlier studies (Kitani et al. 1970) also indicated the direct relationship between solution concentration and PEG adsorption amounts. Bearing in mind that during drying, PEG concentration in lumens of 24.5% EMC samples would also increase, a more likely explanation is initial mass flow or dynamic transfer of dissolved PEG along with water during hydration of the cell walls of samples with lower initial MCs.
Although 24.5% EMC is assumed to be close to the FSP of wood, PEG-water treated samples had higher retained swelling than PEG-chloroform treated samples (Fig. 3). Since the gross PEG uptake does not statistically differ between these samples, it may be that PEG-water treated samples attained a higher cell wall MC (FSP) allowing more PEG to penetrate the cell walls from the water solution or it may be related to the higher affinity of PEG to chloroform than to water, as noted by higher partitioning of PEG into the chloroform phase of chloroform–water mixtures. We observed that in equal portions of chloroform and water suspension, approximately 75% of PEG 1000 partitions into the chloroform phase.
When PEG-chloroform and PEG-water treated samples of all initial MC’s were conditioned at high relative humidities (RH = 98%) the cell walls hydrated to their maximum swelling as observed for pure water treatments (16.3 ± 0.4 and 16.9 ± 0.2%, respectively). The swelling of PEG-treated samples was higher than that of re-conditioned control samples (13.6 ± 0.3). This is explained by microvoids being partially filled with PEG and higher hygroscopicity of PEG in comparison to wood (Sadoh 1968). The reconditioned PEG-treated samples adsorbed more than twice as much water as control samples.
Hydration of PEG-chloroform treated samples repositioned PEG from the cell lumens into the cell walls increasing their overall bulking to the slightly, but significantly lower level than that of water treated samples (Fig. 3). Water samples did not show significant changes upon reconditioning. Additional PEG movement into the cell walls of chloroform treated samples exposed to high humidity could be explained as follows: re-conditioning results in re-hydration and opening of the cell micropores to a higher level than when initially conditioned. Cell walls in this initial stage presumably contain PEG at a lower concentration than in the cell lumens, and the concentration gradient causes further movement of PEG into the cell walls. Besides the larger volumes available for PEG distribution in the cell walls, its enhanced movement into the cell walls is facilitated by the absence of chloroform in the cell lumens, which appeared to retard PEG movement into the cell walls. The fact that reconditioned PEG-chloroform treated samples did not bulk to the same extent as PEG-water treated samples emphasizes the possibility of dynamic movement of PEG along with liquid water during PEG-water treatments.
Even though the swelling capacities of toluene and chloroform were found to be similarly low (Mantanis et al. 1994, 1995a, b), the current study employs the use of toluene as a PEG extracting agent from the cell lumens for two reasons. First, it is assumed that a solvent with low solubility for PEG (less than 1% in comparison to water) needs a long time to extract PEG from cell walls, and second, a solvent with low swelling capacity for wood will initially dissolve “free” PEG from the surface and lumens, leaving PEG in the cell walls intact. In order to test whether these assumptions were valid, bulking of the samples was compared before and after the toluene extraction.
One hour Soxhlet extraction in toluene caused loss of 45 ± 12% of gross PEG content of treated samples, the values not significantly differing among the samples. PEG-water treated samples of 2.3 and 13.3% initial EMC were the only ones that shrank significantly (approximately 2% upon extraction, Table 2), thus indicating possible loss of PEG from the cell walls.
The unchanged and very little affected volumes due to extraction justified the measurement of PEG amounts in the cell walls by Raman spectroscopy.
Characteristic Raman spectra of pure PEG 1000, untreated red pine samples and PEG treated red pine cell walls are shown in Fig. 2. Values of PEG-to-lignin ratios averaged for three samples were variable (Fig. 4), but clearly resembled the trends of retained swelling of samples, indicating that Raman spectroscopy can be used for comparison of relative PEG amounts in wood cell walls. It is important to emphasize that presented values correspond to the ratio of the Raman intensities of PEG and lignin peaks and not the actual amounts of PEG per gram of dry wood.
A plot of Raman estimated relative PEG amounts against the retained swelling upon extraction (Fig. 5), indicated that similar amounts of PEG from chloroform and water solutions gave similar bulking of the samples. This suggested that the conformation or distribution of PEG in the cell walls of samples treated with different solutions were similar, which could further indicate diffusion of PEG from chloroform into bound water and little, if any, penetration of chloroform into the cell walls during treatment.
Conclusions
Penetration of PEG into wood cell walls greatly depends on solvent nature and bound water amounts. Solvent with a higher capacity for PEG than water is shown to impede the diffusion of PEG into the cell walls. In the case of PEG-chloroform treatment, bound water serves as a medium of PEG diffusion into cell walls, and its amounts determine the level of equilibrium PEG in the lumens and cell walls. In the case of PEG-water treated samples, samples with EMC values below FSP can be bulked better by PEG by preferentially adsorbing water from the solution thus increasing the concentration of the PEG in the cell lumens.
Raman measurements of relative PEG amounts in the cell walls gave meaningful results when compared to bulking trends.
References
Castillejo M, Martin M, Oujja M, Rebollar E, Domingo C, Garcia-Ramos JV, Sanchez-Cortes S (2003) Effect of wavelength on the laser cleaning of polychromes on wood. J Cult Herit 4(3):243–249
Cooper PA, Ung YT, Alexander D, Holzscherer C (1991) Diffusion into and bulking of the wood cell wall with polyethylene glycols (PEG). In: Proceedings of International Research Group on Wood Preservation Document IRG/WP/3660
Faria de DLA, Edwards HGM, Afonso MC, Brody RH, Morais JL (2004) Raman spectroscopic analysis of a tembeta: a resin archaeological artefact in need of conservation. Spectrochim Acta Part A 60(7):1505–1513
Ishimaru Y (1976) Adsorption of polyethylene glycol on swollen wood. I. Molecular weight dependence. Mokuzai Gakkaishi 22(1):22–28
Ishimaru Y, Inoue E, Sadoh T, Nakato K (1986) Dimensional stability of wood with adsorbed polyethylene glycol. I. Effect of molecular weight. Mokuzai Gakkaishi 32(11):888–895
Kitani Y, Ohsawa J, Nakato K (1970) Adsorption of polyethylene glycol on water-swollen wood versus molecular weight. Mokuzai Gakkaishi 16(7):326–333
Kurti E, Heyd DV, Wylie RS (2005) Raman microscopy for the quantitation of propiconazole in white spruce. Wood Sci Technol 39(8):618–629
Mantanis GI, Young RA, Rowell RM (1994) Swelling of wood. Part II. Swelling in organic liquids. Holzforschung 48(6):480–490
Mantanis GI, Young RA, Rowell RM (1995a) Swelling of wood. Part III. Effect of temperature and extractives on rate and maximum swelling. Holzforschung 49(3):239–248
Mantanis GI, Young RA, Rowell RM (1995b) Swelling of wood. Part IV. A statistical model for prediction of maximum swelling of wood in organic liquids. Wood Fiber Sci 27(1):22–24
Sadoh T (1968) Mechanism of dimensional stabilization of wood by polyethylene glycol treatment. Mokuzai Gakkaishi 14(7):353–357
Schneider von A (1969) Basic investigations on the dimensional stabilization of wood with polyethylene glycol. Holz Roh- Werkst 27:209–224
Stamm AJ (1964a) Factors affecting the bulking and dimensional stabilization of wood with polyethylene glycols. For Prod J 14(9):403–408
Stamm AJ (1964b) Swelling in aqueous solutions and nonaqueous liquids. In: Wood and cellulose science. Ronald Press, New York, pp 248–263
Tarkow H, Feist WC, Southerland CF (1966) Interaction of wood with polymeric materials: penetration versus molecular size. For Prod J 16(10):61–65
Wallstrom L, Lindberg KAH (1999) Measurement of cell wall penetration in wood of water-based chemicals using SEM/EDS and STEM/EDS technique. Wood Sci Technol 33(2):111–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeremic, D., Cooper, P. & Heyd, D. PEG bulking of wood cell walls as affected by moisture content and nature of solvent. Wood Sci Technol 41, 597–606 (2007). https://doi.org/10.1007/s00226-006-0120-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-006-0120-7