Abstract
The long-term effects of zoledronate treatment in women with postmenopausal osteoporosis who stop denosumab therapy when they become osteopenic are not known. In a prospective, randomized, controlled clinical trial we previously reported that a single intravenous infusion of zoledronate 5 mg given to such patients 6 months after the last denosumab injection effectively prevents bone loss in the majority of them for up to 3 years. The study was extended for an additional 2 years and included all 19 patients from one Trial Site of the total 27 patients originally randomized in the zoledronate arm. Baseline characteristics of this cohort treated with denosumab for 2.4 ± 0.2 years were not different from those of the whole initial cohort or from the patients who did not participate in this extension. At the end of 5 years 7 patients had become again osteoporotic requiring additional treatment, 9 remained osteopenic while 3 did not complete the study extension. Thus, more than half of the osteoporotic women who became osteopenic with denosumab treatment and stopped it, maintained the BMD gains 5 years after a single zoledronate infusion with no additional treatment. Whether these results are also applicable to patients treated with denosumab for longer periods remains to be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is currently recommended that patients with osteoporosis discontinuing denosumab treatment when achieving osteopenia should be administered bisphosphonates to prevent the rebound of bone turnover and the ensuing bone loss and increase in the risk of vertebral fractures [1]. Most of the evidence in support of this recommendation has been obtained with the use of intravenous zoledronate 5 mg. For example, in the first prospective, randomized, controlled study (AfterDmab) we showed that in treatment-naïve postmenopausal women who had become osteopenic after 2.4 ± 0.2 years of denosumab treatment, a single zoledronate infusion following discontinuation of denosumab maintained bone mineral density (BMD) for 2 years [2]. To address, however, the clinically relevant question of the duration of the effect of zoledronate and, hence, the requirement of a new course of antiosteoporotic treatment, longer term information is essential. In a 3rd year extension of our study we found that only 17.4% of these women became again osteoporotic requiring a new treatment course [3] and we report here BMD results of women followed for an additional 2 years up to a total of 5 years after the zoledronate infusion.
Methods
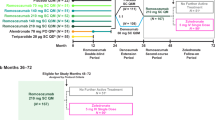
The original cohort comprised 57 treatment-naïve women with postmenopausal osteoporosis treated with denosumab for 1 to 4 years who were randomized to receive a single 5-mg infusion of zoledronate or two additional 60-mg injections of denosumab after achieving osteopenia [2]. Results of the main trial (years 0–2), that had changes in LS-BMD as primary endpoint, and of the 3rd year extension for the 27 women who received zoledronate have been previously published [2, 3]. In 19 of these 27 women, recruited at the Department of Endocrinology of 424 General Military Hospital, follow-up was extended for an additional 2 years to address the question of how many women will remain osteopenic up to 5 years after the zoledronate infusion (Fig. 1). Patients were followed as originally planned and continued receiving cholecalciferol 800 IU/day and calcium carbonate 500 mg b.i.d. However, because of the pandemic, blood samples for the measurement of bone turnover markers were not obtained during this period.
Areal BMD of the Lumbar Spine (LS; L1-L4) and Femoral Neck (FN) of the non-dominant hip were measured at baseline and annually thereafter, by dual energy X-ray absorptiometry (DXA) (Lunar Corporation, Madison, WI, USA). Lateral radiographs of the spine were also performed annually. The study extension was approved by the local ethics committee and informed consent was obtained by the participants.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM) for continuous and as number and/or frequencies for categorical variables. Between group comparisons were performed with independent-sample t-test or Mann–Whitney test (continuous variables) or Chi-square or Fischer’s exact test (categorical variables). Repeated measures ANOVA was used to compare BMD overtime. Logistic regression analysis was used to adjust the need of treatment resume for potential confounders. A two-sided p value of < 0.05 was considered statistically significant in all the above tests. Statistical analysis was performed with SPSS for Macintosh, version 27.0 (IBM Corporation, Armonk, New York, USA).
Results
Baseline characteristics of the women included in the 5-year extension (n = 19) were not different from those of women originally assigned to zoledronate treatment but not included (n = 8) in this extension (Table 1) or from those of the original cohort.
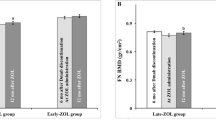
One patient was diagnosed with breast cancer and withdrew from the study, one was lost to follow-up and in one a 5-year BMD measurement could not be obtained (her LS BMD T-score at year 4 was -1.5 equal to the value before the zoledronate infusion). Of the remaining patients, 7 required additional treatment (zoledronate or denosumab) during follow-up because LS-BMD T-score decreased below − 2.5 (1 at 2 years, 3 at 3 years, and 3 at 4 years after the infusion), while 9 patients remained osteopenic at 5 years (LS BMD 0.985 ± 0.036 kg/m2, T-score − 1.7 ± 0.3) not requiring retreatment (Fig. 2). FN BMD, in all patients who did not receive additional treatment remained also osteopenic at 5 years (FN BMD 0.813 ± 0.018 kg/m2, T-score − 1.8 ± 0.2).
Yearly measurements of LS BMD T-scores (mean ± SEM) in osteoporotic women who became osteopenic with denosumab therapy, received a single infusion of zoledronate 5 mg following its discontinuation and were followed for 5 years. Women who remained osteopenic and did not require additional antiosteoporotic treatment (gray bars) and women who became osteoporotic and received new treatment (black bars) are shown separately. The number above each bar (Nr) depicts the number of patients measured at each time point and does not include patients who received a new treatment. Nr number of patients, LS lumbar spine, BMD bone mineral density
Comparison of baseline characteristics of patients who did not require additional treatment with those who received a new treatment course, including those who were lost to follow-up, revealed significantly higher LS BMD T-scores in the former group (− 1.4 ± 0.2 vs. − 2.1 ± 0.1; p = 0.008), but no differences in FN-BMD T-scores (− 1.5 ± 0.2 vs. − 1.6 ± 0.4; p = 0.894), in duration of denosumab treatment, age, age at menopause, and BMI. Interestingly, all, but one of the patients who did not receive additional treatment during the follow-up period had LS BMD T-scores before the zoledronate infusion ≥ − 2 while all, but one, of those who received additional therapy had LS BMD T-scores at baseline < − 2. However, in logistic regression analysis, lower baseline LS BMD T-score was not associated with the need of treatment resumption independently of age, BMI, and years on denosumab. None of the patients sustained a new clinical or morphometric vertebral or peripheral fracture during the 5-year follow-up period.
Discussion
Our results indicate that in more than half of the osteoporotic women who became osteopenic with denosumab treatment, zoledronate 5 mg given at six months after the last denosumab injection could maintain the osteopenia for up to 5 years without the need of additional treatment. This is the first prospective study to report long-term BMD changes in treatment-naïve patients who received a single zoledronate infusion after stopping denosumab therapy. In a retrospective study of women with osteoporosis (48% treatment-naïve) who were treated with denosumab between 2 and 5 years and received a zoledronate infusion after denosumab discontinuation, BMD was measured 18 to 48 months after zoledronate and the authors reported that all of the BMD loss occurred within the first 18 months after zoledronate [4]. In our study, with annual measurements up to year 5, bone loss occurred even later as evidenced by the initiation of additional treatment 3 or 4 years after the zoledronate infusion. Differences in study designs do not allow any conclusions about these, apparently contrasting, findings.
The maintenance of BMD within the osteopenic range for 5 years following the zoledronate infusion in more than half of the patients who completed the study raises the clinically relevant question of the predictability of this response. Notably, the response was primarily observed in women with baseline BMD T-score > − 2 as opposed to loss in nearly all women with BMD ≤ − 2. While this may be a chance finding, it resembles the 5-year response of treatment-naïve osteopenic (BMD T-score between − 1 and − 2) women treated with a single zoledronate infusion [5] and we believe that it should be further considered and investigated. Furthermore, it suggests that treatment with denosumab should not be stopped simply when osteopenia is reached but should be continued until BMD T-scores values higher than − 2 are obtained. This suggestion is consistent with the reported progressive increase in BMD with time and the treat-to-target approach for denosumab [6] and with the post-hoc analysis of the FREEDOM study, which showed that the relationship between hip BMD T-score and incidence of nonvertebral fractures plateaued at a T‐score between − 2.0 and − 1.5 suggesting that a T‐score threshold ≥ − 2.0 would be an appropriate target for therapy to maximize treatment benefits [7].
Apart from the mentioned difference in LS BMD, our results did not identify any factor, including age, BMI and duration of denosumab treatment, that could be associated with the prolonged response to zoledronate in agreement with an earlier report [4]. Contrasting results have also been reported for age and duration of treatment [8, 9]. The latter has emerged as an important determinant of the BMD response to zoledronate [10] but the mechanism is still incompletely understood [11]. The patients included in the present study had received denosumab for a maximum period of 4 years and whether our findings are also applicable to patients treated with denosumab for longer periods remains to be tested. A limitation of this extension of our study is the lack of bone marker measurements, but it should be noted that up to 3 years bone marker changes were not associated with changes in BMD [3].
It is finally reassuring that no new clinical or radiographic vertebral fractures were documented and their overall incidence in the study was much lower than expected in patients stopping denosumab [8], a result consistent also with the low incidence of vertebral fractures in patients treated with bisphosphonates in retrospective studies [12, 13].
In conclusion, we showed that a single zoledronate infusion can consolidate the gains of denosumab treatment in a substantial number of women with postmenopausal osteoporosis. Further studies of potential modulators of this response are essential and, in our view, priority should be given to investigations of the length of denosumab treatment. Independently of these considerations, our study emphasizes and supports recommendations for continuous monitoring of the patients with follow-up BMD measurements.
Data Availability
Data will be available upon reasonable request.
References
Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa756
Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P (2019) Zoledronate for the prevention of bone loss in women discontinuing Denosumab treatment. A prospective 2-year clinical trial. J Bone Miner Res 34(12):2220–2228
Makras P, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Anastasilakis AD (2020) The three-year effect of a single zoledronate infusion on bone mineral density and bone turnover markers following denosumab discontinuation in women with postmenopausal osteoporosis. Bone 138:115478
Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T (2020) A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res 35(7):1207–1215
Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, Reid IR (2012) Five years of anti-resorptive activity after a single dose of zoledronate–results from a randomized double-blind placebo-controlled trial. Bone 50(6):1389–1393
Kendler DL, Cosman F, Stad RK, Ferrari S (2022) Denosumab in the treatment of osteoporosis: 10 years later: a narrative review. Adv Ther 39(1):58–74
Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwinski E, de Gregomicronrio LH, Malouf-Sierra J, Reginster JY, Wang A, Wagman RB, Lewiecki EM (2019) Relationship between bone mineral density t-score and Nonvertebral fracture risk over 10 years of Denosumab treatment. J Bone Miner Res 34(6):1033–1040
Cosman F, Huang S, McDermott M, Cummings SR (2022) Multiple vertebral fractures after Denosumab discontinuation: freedom and freedom extension trials additional post hoc analyses. J Bone Miner Res 37(11):2112–2120
Sølling AS, Harsløf T, Langdahl B (2020) Treatment with Zoledronate subsequent to Denosumab in osteoporosis: a randomized trial. J Bone Miner Res 35(10):1858–1870
Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD (2021) The duration of Denosumab treatment and the efficacy of Zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab 106(10):e4155–e4162
Ferrari S, Langdahl B (2023) Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on bone. Nat Rev Rheumatol 19(5):307–317
Burckhardt P, Faouzi M, Buclin T, Lamy O (2021) The Swiss Denosumab study, fractures after Denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res 36(9):1717–1728
Everts-Graber J, Reichenbach S, Gahl B, Ziswiler HR, Studer U, Lehmann T (2021) Risk factors for vertebral fractures and bone loss after denosumab discontinuation: a real-world observational study. Bone 144:115830
Funding
This work was not supported by any grant, fund, or institution.
Author information
Authors and Affiliations
Contributions
Conception of the hypothesis of the study: ADA. Design of the study: ADA. Acquisition, analysis, interpretation of data: ADA, PM, SAP, SEP. Drafting the manuscript: ADA. Revising the work critically for important intellectual content: PM, SAP, SEP. Final approval of the submitted version: ADA, PM, SAP, SEP. Agreement to be accountable for all aspects of the work: ADA, PM, SAP, SEP.
Corresponding author
Ethics declarations
Conflict of interest
Athanasios D. Anastasilakis reports lecture fees from Amgen, Bianex, Eli-Lilly, Galenica, ITF, Unifarma, and UCB; Polyzois Makras reports fees for lectures/advisory boards and research grants from Amgen and fees for lectures/advisory boards from UCB, Elpen, and Galenica; Stergios A. Polyzos has nothing to declare; Socrates E. Papapoulos reports consulting/speaking fees from Amgen, Entera Bio, Qualix Dot, Radius Health, and UCB.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethics committee.
Human and Animal Rights and Informed Consent
No animals were involved in the study. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anastasilakis, A.D., Makras, P., Polyzos, S.A. et al. The Five-Year Effect of a Single Zoledronate Infusion on Bone Mineral Density Following Denosumab Discontinuation in Women with Postmenopausal Osteoporosis. Calcif Tissue Int 113, 469–473 (2023). https://doi.org/10.1007/s00223-023-01119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01119-7