Abstract
Hypoparathyroidism (HypoPT) and pseudohypoparathyroidism (PHP) are diseases with abnormal calcium and phosphate homeostasis and low and high PTH levels, respectively. It has been hypothesized that this could dispose to vascular calcifications and thereby perhaps also cardiovascular morbidity. The aim of this study was to assess lower leg arterial calcifications (LLAC) in patients with HypoPT or PHP. Using a cross-sectional design, we measured the LLAC using a high-resolution peripheral quantitative computed tomography (HR-pQCT) scanner in 72 patients with HypoPT and 25 patients with PHP and compared them with findings in 61 controls. LLAC were found in only two (3%) of the controls. Compared to the controls, LLAC were significantly more prevalent in patients with HypoPT (N = 12, [17%], p < 0.01) and PHP (N = 4, [16%], p < 0.04). Compared to the patients without calcifications, patients with calcifications had higher plasma calcium levels and a lower eGFR, as well as they were older and more often males. Plasma phosphate levels and the calcium-phosphate product were not associated with LLAC. In conclusion, we found that HypoPT and PHP are associated with an increased prevalence of vascular calcifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoparathyroidism (HypoPT) is a metabolic disease characterized by hypocalcemia with relatively high plasma phosphate levels due to inadequate levels of parathyroid hormone (PTH). The most common cause of HypoPT is post-surgical HypoPT after thyroid or parathyroid surgery. Other causes for hypoparathyroidism include genetic or autoimmune diseases causing the so called non-surgical HypoPT (Ns-HypoPT) [1]. In pseudohypoparathyroidism (PHP) there is a peripheral resistance to PTH which results in similar manifestations as HypoPT, with low plasma calcium levels and relatively high phosphate levels, although plasma PTH levels are elevated [2]. In recent years there has been an increased focus on HypoPT and its effect on the cardiovascular system. It has been hypothesized that disturbances in the calcium and phosphate homeostasis may increase the risk of cardiovascular events [3, 4]. Studies have shown that Ns-HypoPT is associated with an increased risk of cardiovascular disease [5, 6], whereas post-surgical HypoPT does not seem to increase the risk [6, 7], except if plasma calcium levels are too low [8]. A plausible mechanism for the possible increased risk of cardiovascular disease in patients with HypoPT might be vascular calcifications due to disturbances in calcium and phosphate levels. The association between abnormal calcium, phosphate and PTH levels and vascular calcifications and cardiovascular disease have, however, until now mainly been examined in patients with chronic kidney disease (CKD). Similar to patients with HypoPT, patients with CKD have low calcium and high phosphate levels, but unlike patients with HypoPT, CKD patients have high levels of PTH [9]. In CKD patients, the increased risk of vascular calcifications and cardiovascular events has been associated with the abnormal calcium, phosphate and PTH homeostasis [3, 10]. Patients with CKD have increased levels of FGF23 due to the increased phosphate levels. Elevated levels of FGF23 have also been associated with increased risk of vascular calcifications and cardiovascular disease [11]. Vitamin K, on the other hand, has been shown to play a possible role in reducing vascular calcifications [12]. In the presence of vitamin K, the vitamin K-dependent proteins such as matrix gla protein (MGP) is carboxylated. The carboxylated form of this protein (cMGP) inhibits vascular calcifications [12, 13]. Thereby suggesting that vitamin K could offer therapeutic benefits to CKD patients [12]. It is, however, unknown whether such mechanisms are also of importance to patients with HypoPT.
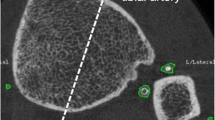
Until now there have only been limited studies examining vascular calcifications in patients with HypoPT, showing that Ns-HypoPT is associated with increased risk of both coronary artery calcifications and increased carotid intima thickness [4, 14]. One way to examine vascular calcifications is by measuring lower leg arterial calcification (LLAC) using a high-resolution peripheral quantitative computed tomography (HR-pQCT) scanner. HR-pQCT scans are primarily used for assessment of bone structure but has also been shown to be useful to identify vascular calcifications. Studies have shown that LLAC assessed by HR-pQCT scans is associated with coronary artery calcifications [15], which is a good marker for atherosclerosis and cardiovascular disease [16].
The aim of this study was to assess vascular calcifications in the lower legs in patient with HypoPT and PHP and compare findings to a control group. Because these patients have disturbances in calcium and phosphate homeostasis, we hypothesized that these patients would have more vascular calcifications.
Subjects and Methods
Patient Selection
We identified patients with HypoPT, both post-surgical and non-surgical, and PHP, as well as people from the general background population who previously have had a HR-pQCT scan performed at our Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Denmark. Bone data from scans of the lower legs have previously been reported [2, 17,18,19]. Patients with HypoPT were diagnosed based on their biochemical characteristic with hypocalcemia due to inappropriately low PTH levels [20], and PHP were diagnosed based on hypocalcemia with inappropriately high PTH levels [21].
Clinical characteristics and biochemistry were retrieved from patient charts. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). The Danish Health and Medicines Authority approved retrieval of data from the scanner database and hospital charts (#31-1521-437). The Danish Data Protection Agency was notified about the study (#2016-051-000001/1845).
Controls
Controls were subjects (N = 61) who were included in our database with HRpQCT scans, as they had served as non-diseased individuals in prior studies [18, 19]. In brief, they have been recruited by direct mailing to a random sample from the general background population and included if they were not suffering from calcium metabolic diseases, thyroid disorders, current malignant diseases or alcohol abuse, as previously detailed [18, 19]. All the controls were asked about these diseases in a questionnaire and had a lab screening performed.
HR-pQCT-Scan
Using a HR-pQCT scanner (XtremeCT; SCANCO Medical AG, Brüttisellen, Switzerland) all cases and controls had their lower leg scanned. Prior to the scan, the lower leg was stabilized in a carbon cast and inserted in the scanner. The right side was scanned unless a history of fracture in the region was present. At each site, 110 computerized tomography slices were obtained. These slices were used to reproduce a 9.02 mm long three-dimensional image. The images were analyzed using software provided by the company (SCANCO Medical AG, Brüttisellen, Switzerland) [15, 22]. Image analysis was performed by first detecting the bone structure, if the outer contours of the tibia and fibula was not correct, it was corrected by hand. These bone structures were then removed from calcification measurement. The computer was set to identify any non-skeletal calcified tissue. Afterwards any calcified area not related to a vessel were removed by hand. This included any area that did not have the typical location of either the anterior or posterior tibial artery or did not have the typical round vascular structure (Fig. 1). The algorithm was set to analyze the identified calcifications. LLAC was calculated in mg hydroxyapatite (HA) as total volume of vascular calcifications (mm3) × mean calcification density (mgHA/cm3)/1000. Like other studies concerning vascular calcifications in HRpQCT scans [15, 22], we used the unit mgHA instead of mgHA/cm3, because the latter would depend on how close to the calcification the outer contours were drawn. In the absence of any visible vascular calcification on the scan, the calcification was recorded as 0 mgHA.
Biochemistry
All blood and 24 h urine samples were collected at the time of the HR-pQCT scan. Blood samples were drawn in the morning in the fasting state and analyzed using standard methods on an automated analyzer (Cobas 6000; Roche Diagnostics GmbH, Mannheim, Germany) at our university Department of Clinical Biochemistry. The laboratory is accredited by the Danish Accreditation Fund (DANAK). If needed, blood samples were stored at − 80 °C until analyzed. Measurement of 24 h renal calcium excretion were only available from the group of patients.
Statistics
For normally distributed data we assessed differences between groups using independent t test. For skewed data, we assessed differences between groups using Mann–Whitney test. For categorical values proportions were compared using chi-square test. Correlation analysis was performed by calculating Pearson’s correlation coefficient. Data are reported as mean ± standard deviation (SD) or 95% confidence interval or median with interquartile (25%; 75% percentile) range (IQR), as appropriate. A two-sided p value < 0.05 was considered statistically different. All calculations were performed using IBM SPSS statistics version 20, (IBM, New York, USA).
Results
In total, we included 97 cases: 22 patients with post-surgical HypoPT, 25 with PHP and 50 with Ns-HypoPT. Findings in patients were compared to findings in 61 healthy controls. On average, the controls had a lower BMI and were a few years younger than the group of patients. However, only patients with HypoPT were on average older than the controls, whereas patients with PHP were slightly younger than the controls (Table 1). The male–female-ratio between patients and controls was almost the same. Compared to the controls, patients had significantly lower plasma calcium levels and higher plasma phosphate levels. Furthermore, patients with HypoPT had higher plasma creatinine levels with a lower eGFR, whereas renal function was not impaired in the group of patients with PHP. Hypercalciuria was more prevalent among patients with HypoPT compared to patients with PHP (Table 1).
Figure 2 shows the results from the HR-pQCT. Prevalence of LLAC was significantly higher among patients compared to the controls (17% vs. 3%, p = 0.01). The percentage of patients with calcifications did not differ between patients with HypoPT and PHP (16.6% vs. 16.0%, p = 0.94) or between patients with post-surgical- and non-surgical HypoPT (18.2% vs. 16.0%, p = 0.82) (data not shown). For individuals with calcifications, the degree of calcifications varied considerably ranging from 0.47 to 40.27 mgHA. It did, however, not differ between patients and controls (13.1 vs. 17.6 mgHA; p = 0.14) or between patients with PHP and HypoPT (15.4 vs. 12.3 mgHA, p = 0.47).
Table 2 compares characteristics of patients with and without calcifications. Compared to patients without calcifications, patients with LLAC were older and more often of the male gender. Furthermore, patients with LLAC had statistically significant higher plasma calcium levels and a lower eGFR. Plasma calcium levels were within the reference range for patients with LLAC, whereas those without calcifications had calcium levels slightly below the reference range. Phosphate, calcium-phosphate product, TSH, 24 h urine calcium, BMI and calcium, alfacalcidol and levothyroxine treatment did not differ significantly between the two groups.
Comparisons of patients with or without calcifications are shown in Tables 3 and 4 for patients with HypoPT and PHP, respectively. Plasma calcium levels were higher among patients with HypoPT and LLAC compared to those without LLAC (Table 3) and a similar (non-significant) tendency was found in the smaller group of patients with PHP (Table 4). Plasma phosphate levels were borderline significantly (p = 0.07) lower in the group of HypoPT patients with LLAC compared to those without LLAC (Table 3). The calcium-phosphate product did not differ between patients with or without LLAC in any of the two groups of patients. HypoPT patients with LLAC had a lower eGFR (p < 0.01) compared to HypoPT without LLAC (Table 3), whereas a similar (non-significant) tendency was found for PHP patients (Table 4). Among patients with PHP the ones with and without calcifications only differed statistically significant concerning levothyroxine and hypercalciuria.
For the 22 patients with post-surgical HypoPT, the average duration of disease was 11 years (n = 18) for patients without calcifications, whereas it was 19 years for the four post-surgical patients with calcifications (p < 0.10) (data not shown).
The two controls with calcifications were similar to the controls without calcifications, except for age (data not shown): an average age of 60 years for those with calcifications and 43 years for those without calcifications.
Bivariate correlation analyses were performed to search for correlation between the degree of mineralization and specific parameters. The bivariate correlation analysis showed that only daily dose of calcium from supplements was significantly associated with LLAC (mgHA) for patients with calcifications (r = 0.581; p < 0.02; slope = 0.007). All the patients with a LLAC higher than 20 mgHA had a daily calcium dose of 1200 mg or more. Neither biochemical findings nor age, BMI or daily dose of alfacalcidol correlated significantly with LLAC (Fig. 3).
Discussion
Our study represents one of the few studies examining vascular calcifications in patients with HypoPT and PHP. To the best of our knowledge, it is also the largest study concerning this topic. A priori we hypothesized that patients with HypoPT or PHP would have more vascular calcifications compared to the general population. This hypothesis is supported by our findings. We found that 17% of the patients with HypoPT or PHP had LLAC compared to only 3% in the control group.
Comparing patients with and without calcifications, those with LLAC had higher plasma calcium levels. They did, however, have calcium levels within the normal range and about the same calcium levels as the control group. In a previous study of Agarwal et al. [4] regarding HypoPT and coronary artery calcifications, an opposite tendency was found, as patients without calcifications had higher plasma calcium levels than those with calcifications [4]. This study only included patients with Ns-HypoPT who presumably were untreated (not reported by the authors), as the entire group of patients had a very low average plasma total calcium level (1.8 ± 0.2 mmol/L), which was significantly lower than levels in a matched group of healthy controls (2.3 ± 0.2 mmol/L). Patients with calcifications had total calcium levels as low as 1.4 ± 0.2 mmol/L [4] which is markedly lower than the levels in our patients. The difference in the calcium levels could be the reason for the different results regarding the association between calcifications and calcium levels. Thus, this discrepancy may indicate that the optimal calcium level to avoid calcifications is just below the normal reference range and that levels below or above that range may result in increased risk of calcifications. Similar to our findings, the study by Agarwal et al. [4] showed no associations between calcifications and serum phosphate level or the calcium-phosphate product, suggesting no major important effect of phosphate on the risk of LLAC in HypoPT.
Calcium supplements are an important part of the standard treatment of HypoPT and PHP. The possible role of calcium supplements in the development of vascular calcifications continuous to be a controversial issue. Use of calcium supplements has been associated with an increased risk of vascular disease in some [23,24,25] but not all [26, 27] studies. These studies have, however, been performed on people not suffering from parathyroid diseases. In our study, there was no statistically significant difference in the use of calcium supplements when comparing the patients with and without calcifications. Nevertheless, we did find a significant correlation between LLAC (mgHA) and daily dose of calcium supplements. The highest LLAC scores were all found among patients with a calcium dose of at least 1200 mg, indicating a threshold value at 1200 mg. Therefore, we cannot exclude a potential harmful effect of calcium supplements in the treatment of HypoPT and PHP.
In this study, we divided the patients into HypoPT and PHP to see whether the number of patients with calcifications differed between groups. The percentage of patients with calcifications were almost the same in the two groups. In the HypoPT group the ones with calcifications were older, the male:female ratio was higher, the calcium levels were higher, and the eGFR was lower compared to the ones without calcifications. These findings are in accordance with known risk factors for LLAC in other groups of patients [28]. In the PHP group, only levothyroxine treatment and hypercalciuria differed statistically significant between patients with or without calcifications. We did, however, find the same tendencies with higher calcium, lower eGFR and higher age among the ones with calcifications, suggesting that similar mechanism may account for the development of calcifications in both HypoPT and PHP.
Several studies have shown an increased prevalence of intracerebral calcifications in patients with HypoPT and PHP [29,30,31,32,33]. PHP is caused by mutations in GNAS gene or mutations upstream of the GNAS complex locus [34]. The GNAS gene encode the Gsα-part of the PTH receptor, resulting in PTH resistance. The defect G protein receptor does not only affect PTH, as Gsα deficiency in mesenchymal stem cells leads to de novo differentiation of osteoblasts in soft tissue, thereby causing ossifications [35]. Accordingly, patients with PHP may have a high occurrence of ossifications which may be misinterpreted as vascular calcifications when evaluating findings on HR-pQCT scans. However, we find it most likely that the identified calcifications were located in the vasculature, as we removed anything without a vessel-like structure and without a location typical of the anterior or posterior tibial artery. Further studies are, nevertheless, needed to determine whether these findings are due to calcifications or ossifications.
Our study has several strengths as well as limitations. A major limitation is the relatively small sample size limiting our abilities to perform adjustments and sub-analyses. The calcifications have probably developed over a long period in the years prior to the scan, but the biochemical characteristics were determined at the time of the scan. We do not have any data on the biochemical characteristics in the years prior to the scan during which the calcifications developed. Furthermore, we were not able to account for other factors known to affect the risk of LLAC such as hyperlipidemia, diabetes mellitus, hypertension, CKD, cardiovascular history and smoking history. The patients with HypoPT were also on average a few years older than the controls, whereas PHP patients were younger. Despite these age differences, a similar prevalence of calcifications was found in both groups of patients which was much higher than in the group of controls. Moreover, the scanner did not scan the entire tibia, but only scanned 9.02 mm of the tibia. It could be of interest in future studies to determine calcification in a larger part of the arteries. It is still, however, to the best of our knowledge the largest study examining vascular calcifications in patients with HypoPT and PHP. A strength of the study was the objective assessment of the size of the calcification, once a calcification had been identified.
In conclusion, our study showed an association between vascular calcifications and PHP and HypoPT, indicating that the general disturbances in calcium homeostasis could dispose to vascular calcifications, and possibly also cardiovascular disease. However, more research is needed on this topic to fully understand the cardiovascular complications of this disease. These findings should be considered in the care and treatment of these patients, since early diagnosis and treatment may reduce cardiovascular mortality. Based on the findings in this study, it is especially older patients with high calcium levels and/or low eGFR who are at risk of vascular calcifications.
References
Cianferotti L, Marcucci G, Brandi ML (2018) Causes and pathophysiology of hypoparathyroidism. Best Pract Res Clin Endocrinol Metab 32(6):909–925. https://doi.org/10.1016/j.beem.2018.07.001
Underbjerg L, Malmstroem S, Sikjaer T, Rejnmark L (2018) bone status among patients with nonsurgical hypoparathyroidism, autosomal dominant hypocalcaemia, and pseudohypoparathyroidism: a cohort study. J Bone Miner Res 33(3):467–477. https://doi.org/10.1002/jbmr.3328
Slinin Y, Foley RN, Collins AJ (2005) Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16(6):1788–1793. https://doi.org/10.1681/asn.2004040275
Agarwal P, Prakash M, Singhal M, Bhadada SK, Gupta Y, Khandelwal N (2015) To assess vascular calcification in the patients of hypoparathyroidism using multidetector computed tomography scan. Indian J Endocrinol Metab 19(6):785–790. https://doi.org/10.4103/2230-8210.167545
Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L (2015) The Epidemiology of Nonsurgical Hypoparathyroidism in Denmark: a Nationwide Case Finding Study. J Bone Miner Res 30(9):1738–1744. https://doi.org/10.1002/jbmr.2501
Vadiveloo T, Donnan PT, Leese CJ, Abraham KJ, Leese GP (2019) Increased mortality and morbidity in patients with chronic hypoparathyroidism: a population-based study. Clin Endocrinol (Oxf) 90(2):285–292. https://doi.org/10.1111/cen.13895
Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L (2013) Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res 28(11):2277–2285. https://doi.org/10.1002/jbmr.1979
Underbjerg L, Sikjaer T, Rejnmark L (2018) Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: a case-control study. J Bone Miner Res 33(5):822–831. https://doi.org/10.1002/jbmr.3368
Hill Gallant KM, Spiegel DM (2017) Calcium balance in chronic kidney disease. Curr Osteoporos Rep 15(3):214–221. https://doi.org/10.1007/s11914-017-0368-x
Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D (2009) Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transpl 24(5):1506–1523. https://doi.org/10.1093/ndt/gfn613
Vogt I, Haffner D, Leifheit-Nestler M (2019) FGF23 and phosphate-cardiovascular toxins in CKD. Toxins (Basel) 11(11):647. https://doi.org/10.3390/toxins11110647
Shea MK, Booth SL (2019) Vitamin K, vascular calcification, and chronic kidney disease: current evidence and unanswered questions. Curr Dev Nutr 3(9):nzz077. https://doi.org/10.1093/cdn/nzz077
Price PA, Faus SA, Williamson MK (1998) Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 18(9):1400–1407. https://doi.org/10.1161/01.atv.18.9.1400
Gupta Y, Bhadada SK, Shah VN, Upreti V, Bhansali A, Jain S, Khurana D, Kalara N (2012) Carotid intima media thickness in patients with sporadic idiopathic hypoparathyroidism: a pilot study. Endocr J 59(7):555–559. https://doi.org/10.1507/endocrj.ej11-0400
Patsch JM, Zulliger MA, Vilayphou N, Samelson EJ, Cejka D, Diarra D, Berzaczy G, Burghardt AJ, Link TM, Weber M, Loewe C (2014) Quantification of lower leg arterial calcifications by high-resolution peripheral quantitative computed tomography. Bone 58:42–47. https://doi.org/10.1016/j.bone.2013.08.006
Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K (2011) Understanding the utility of zero coronary calcium as a prognostic test: a Bayesian approach. Circ Cardiovasc Qual Outcomes 4(2):253–256. https://doi.org/10.1161/circoutcomes.110.958496
Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L (2011) The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res 26(10):2358–2370. https://doi.org/10.1002/jbmr.470
Moser E, Sikjaer T, Mosekilde L, Rejnmark L (2015) Bone indices in thyroidectomized patients on long-term substitution therapy with levothyroxine assessed by DXA and HR-pQCT. J Thyroid Res 2015:796871. https://doi.org/10.1155/2015/796871
Malmstroem S, Grove-Laugesen D, Riis AL, Bruun BJ, Ebbehoj E, Hansen KW, Watt T, Rejnmark L (2019) Muscle performance and postural stability are reduced in patients with newly diagnosed graves’ disease. Thyroid 29(6):783–789. https://doi.org/10.1089/thy.2018.0318
Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, van Biesen W, Dekkers OM (2015) European Society of Endocrinology Clinical Guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol 173(2):G1-20. https://doi.org/10.1530/eje-15-0628
Mantovani G, Bastepe M, Monk D, de Sanctis L, Thiele S, Ahmed SF, Bufo R, Choplin T, De Filippo G, Devernois G, Eggermann T, Elli FM, Garcia Ramirez A, Germain-Lee EL, Groussin L, Hamdy NAT, Hanna P, Hiort O, Jüppner H, Kamenický P, Knight N, Le Norcy E, Lecumberri B, Levine MA, Mäkitie O, Martin R, Martos-Moreno G, Minagawa M, Murray P, Pereda A, Pignolo R, Rejnmark L, Rodado R, Rothenbuhler A, Saraff V, Shoemaker AH, Shore EM, Silve C, Turan S, Woods P, Zillikens MC, Perez de Nanclares G, Linglart A (2020) Recommendations for diagnosis and treatment of pseudohypoparathyroidism and related disorders: an updated practical tool for physicians and patients. Horm Res Paediatr 93(3):182–196. https://doi.org/10.1159/000508985
Paccou J, Edwards MH, Patsch JM, Jameson KA, Ward KA, Moss C, Dennison EM, Cooper C (2016) Lower leg arterial calcification assessed by high-resolution peripheral quantitative computed tomography is associated with bone microstructure abnormalities in women. Osteoporos Int 27(11):3279–3287. https://doi.org/10.1007/s00198-016-3660-1
Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011) Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ (Clinical research ed) 342:d2040–d2040. https://doi.org/10.1136/bmj.d2040
Anderson JJ, Kruszka B, Delaney JA, He K, Burke GL, Alonso A, Bild DE, Budoff M, Michos ED (2016) Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 5(10):e003815. https://doi.org/10.1161/jaha.116.003815
Yang C, Shi X, Xia H, Yang X, Liu H, Pan D, Sun G (2019) The evidence and controversy between dietary calcium intake and calcium supplementation and the risk of cardiovascular disease: a systematic review and meta-analysis of cohort studies and randomized controlled trials. J Am Coll Nutr 39:1–19. https://doi.org/10.1080/07315724.2019.1649219
Samelson EJ, Booth SL, Fox CS, Tucker KL, Wang TJ, Hoffmann U, Cupples LA, O’Donnell CJ, Kiel DP (2012) Calcium intake is not associated with increased coronary artery calcification: the Framingham Study. Am J Clin Nutr 96(6):1274–1280. https://doi.org/10.3945/ajcn.112.044230
Chen F, Du M, Blumberg JB, Chui KKH, Ruan M, Rogers G, Shan Z, Zeng L, Zhang FF (2019) Association among dietary supplement use, nutrient intake, and mortality among U.S. adults: a cohort study. Ann Intern Med 170(9):604–613. https://doi.org/10.7326/m18-2478
Schramm K, Rochon PJ (2018) Gender differences in peripheral vascular disease. Semin Intervent Radiol 35(1):9–16. https://doi.org/10.1055/s-0038-1636515
Donzuso G, Mostile G, Nicoletti A, Zappia M (2019) Basal ganglia calcifications (Fahr’s syndrome): related conditions and clinical features. Neurol Sci 40(11):2251–2263. https://doi.org/10.1007/s10072-019-03998-x
Kim YS, Park J, Park Y, Hwang K, Koo DL, Kim D, Seo DW (2016) Intracranial cortical calcifications in a focal epilepsy patient with pseudohypoparathyroidism. J Epilepsy Res 6(1):31–35. https://doi.org/10.14581/jer.16006
Chen H, Tseng F, Su D, Chen H, Tsai K (2005) Multiple intracranial calcifications and spinal compressions: rare complications of type la pseudohypoparathyroidism. J Endocrinol Invest 28(7):646–650. https://doi.org/10.1007/bf03347265
Harada K, Fujikawa T (2018) Intracranial calcification due to hypoparathyroidism. Am J Med 131(6):e253–e254. https://doi.org/10.1016/j.amjmed.2017.12.030
David K, Moyson C, Vanderschueren D, Decallonne B (2019) Long-term complications in patients with chronic hypoparathyroidism: a cross-sectional study. Eur J Endocrinol 180(1):71–78. https://doi.org/10.1530/eje-18-0580
Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L (2016) Pseudohypoparathyroidism - epidemiology, mortality and risk of complications. Clin Endocrinol (Oxf) 84(6):904–911. https://doi.org/10.1111/cen.12948
Linglart A, Levine MA, Juppner H (2018) Pseudohypoparathyroidism. Endocrinol Metab Clin North Am 47(4):865–888. https://doi.org/10.1016/j.ecl.2018.07.011
Acknowledgement
We would like to thank laboratory technician Ditte Viborg Kofod for her skilled help in using the methods in our laboratory.
Funding
This work has received no grants or other funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Catharina Vind Nielsen, Line Underbjerg, Diana Grove‑Laugesen, Tanja Sikjaer and Lars Rejnmark declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The study was conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As this was a historic study based on previous collected data, allowance to use data from patient charts was granted by the Danish Health and Medicines Authority.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nielsen, C.V., Underbjerg, L., Grove-Laugesen, D. et al. Lower Leg Arterial Calcifications Assessed by High-Resolution Peripheral Quantitative Computed Tomography in Hypoparathyroid and Pseudohypoparathyroid Patients. Calcif Tissue Int 108, 775–784 (2021). https://doi.org/10.1007/s00223-021-00814-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00814-7