Abstract
Distraction osteogenesis (DO) is a clinically effective procedure to regenerate large bone defects. However, the treatment duration is undesirably lengthy, especially in elderly patients. Exosomes derived from mesenchymal stem cells (MSC-Exos) could exert the beneficial effects while avoiding the possible complications of stem cell transplantation. This study aimed to evaluate the effects of MSC-Exos on bone regeneration during DO in older rats. Exosomes were isolated from the supernatants of young bone marrow mesenchymal stem cells (BMSCs) through ultra-centrifugation, and characterized using transmission electron microscopy, western blot, and tunable resistive pulse sensing analysis. The effects of MSC-Exos on the proliferation and differentiation of older BMSCs were evaluated using CCK-8 assay, ALP and ARS staining, and qRT-PCR. Unilateral tibial DO model was established on older Sprague–Dawley rats and MSC-Exos or phosphate buffer saline was locally injected into the distraction gaps after distraction weekly. Bone regeneration were evaluated using X-ray, Micro-CT, mechanical test, and histological staining. The MSC-Exos were round or cup-shaped vesicles ranging from 60 to 130 nm in diameter and expressed markers including CD9, CD63, and TSG101. The in vitro results indicated that MSC-Exos could enhance the proliferation and osteogenic differentiation of older BMSCs. Bone regeneration was markedly accelerated in rats treated with MSC-Exos according to the results of X-ray, micro-CT, and histological analysis. The distracted tibias from the MSC-Exos group also demonstrated better mechanical properties. These results suggest that MSC-Exos promote DO-mediated bone regeneration in older rats through enhancing the proliferation and osteogenic capacity of BMSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distraction osteogenesis (DO) is a clinically effective treatment to regenerate large bone defects [1,2,3]. It involves three sequential stages: the latency stage after application of external fixation and osteotomy, the distraction stage to induce new bone formation by gradual and continuous distraction, and the consolidation stage until the regenerated bone tissue achieves enough mechanical characteristics [4]. A major limitation of this technique is the undesired lengthy treatment duration, leading to increased risks of complications [5, 6]. The treatment duration is further prolonged as the age grows [7]. Therefore, accelerating new bone formation during DO and shortening the external fixation time are of great clinical significance, especially for elderly individuals.
Age-related deficits in DO-mediated bone regeneration have been well established [8, 9]. A possible reason may be the compromised function of older bone marrow mesenchymal stem cells (BMSCs) [10]. The cell proliferation is suppressed and the osteogenic differentiation capacity is diminished in old BMSCs [11]. Increasing studies have confirmed that transplantation of MSCs could stimulate new bone formation during DO in animal experiments [12, 13]. However, direct application of MSCs is generally hindered by the risks of emboli formation, immunogenicity, and malignant transformation [14]. Further, the proliferation and osteogenic differentiation of transplanted BMSCs could be inhibited in the aged bone marrow microenvironment [15, 16].
Recent researches have revealed that transplanted stem cells may exert their beneficial effects by paracrine actions including secreting exosomes [17,18,19]. Exosomes are spherical lipid-bilayered vesicles containing various bioactive proteins and RNAs [20, 21]. These vesicles regulate the functional activity of target cells by transferring RNAs and proteins [22]. Previous studies showed that exosomes secreted by MSCs (MSC-Exos) could promote osteogenesis both in vitro and in vivo [23]. However, it remains unknown how MSC-Exos influences the process of DO.
In the present study, the authors aimed to investigate whether MSC-Exos could promote DO-mediated new bone formation in older rats.
Materials and Methods
Cell Culture
BMSCs were harvested from 2-week and 15-month Sprague–Dawley rats to isolate young and older BMSCs [10]. Briefly, bone marrow cells were collected by flushing the femur cavity. After centrifugation, the cell pellet was cultured in complete α-modified Eagle’s medium (Invitrogen, USA). Non-adherent cells were removed after 24 h and fresh media was changed every other day. Cells were cultured at 37 °C, 5% CO2 in a humidified environment and passaged at 80–90% confluence. BMSCs at passage 3 to 5 were used in further experiments.
Isolation of MSC-Exos
MSC-Exos were isolated from the cultured medium of young BMSCs from 2-week-old rats through differential ultra-centrifugation [24,25,26]. In brief, BMSCs were washed 3 times with phosphate buffer saline (PBS) after reaching about 80% confluence and then cultured in MesenGro MSC medium (StemRD, San Francisco, CA, USA) for an additional 48 h. The medium was then collected, centrifuged (300×g for 15 min and 2000×g for 30 min), and then filtered through a 0.22-μm filter (Millipore, Billerica, MA, USA) to remove remaining cellular debris. The filtered supernatants were then centrifuged at 10,000×g for 1 h and further ultracentrifuged at 100,000×g for 2 h. For purification, the pellet was re-suspended in PBS and ultracentrifugated at 100,000×g for another 2 h. All centrifugation procedures were performed at 4 °C. The isolated exosomes suspended in PBS were used immediately or stored at – 80 °C until further experiments.
Characterization of MSCs-Exos
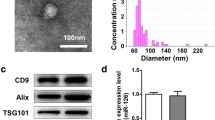
The particle concentration and size distribution of MSC-Exos were measured by tunable resistive pulse sensing analysis using qNano platform (Izon Science, Cambridge, MA, USA) as described previously [27]. Exosome morphology was observed by transmission electron microscopy (TEM; FEI, Eindhoven, Netherlands) after negatively stained with 2% uranyl acetate for 30 s. The characteristic proteins of exosomes including CD9, CD63, and TSG101 (Abcam, Cambridge, UK) were analyzed using western blot.
Cell Proliferation Assay
The effects of MSC-Exos on the proliferation of older BMSCs from 15-month-old rats were evaluated by Cell Counting Kit-8 (CCK-8; Dojindo, Japan) assay. Older BMSCs (5 × 103 per well) were seeded in 96-well plates. After co-culture with serial concentrations of MSC-Exos (0, 1 × 109, 5 × 109, or 1 × 1010 particles/mL) for 1, 3, and 5 days, 100 μL fresh complete medium and 10 μL CCK-8 solution was added to each well and incubated for another 2 h. Then optical density values at 450 nm were measured using a microplate reader (Bio-Rad, USA).
Osteogenic Differentiation
Osteogenic differentiation of older BMSCs was induced using osteogenic induction medium (Cyagen Biosciences, Guangzhou, China) containing serial concentrations of MSC-Exos (0, 1 × 109, 5 × 109, or 1 × 1010 particles/mL). To assess the effects of MSC-Exos on the osteogenic capacity of older BMSCs in vitro, quantitative reverse transcription polymerase chain reaction (qRT-PCR), alkaline phosphatase (ALP) staining, and ALP activity were performed at day 7 after induction, and alizarin red S (ARS) staining at day 21.
qRT-PCR
The effects of MSC-Exos on the expression of osteogenesis-related genes, runt-related transcription factor 2 (Runx2), ALP, and osteocalcin (OCN), were detected using qRT-PCR. Older BMSCs were treated with PBS or MSC-Exos (1 × 1010 particles/mL) for 7 days before subsequent detection. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Invitrogen, USA). Quantitative PCR was performed using FastStart Universal SYBR Green Master (Rox). The mRNA expression levels were analyzed using the 2−ΔΔCt method with GAPDH as the housekeeping gene. The primers (BioTNT, Shanghai, China) used in this study were listed in Table 1.
ALP Staining and Activity
For ALP staining, BMSCs were washed with PBS and fixed with a mixture of sodium citrate, acetone, and 37% formaldehyde (3.1:8.1:1) for 30 s. The cells were then rinsed with deionized water for 50 s. Thereafter, cells were incubated in the mixed reaction solutions (Sigma, USA) for 15 min at room temperature. For ALP activity, cells were lysed and then evaluated using an ALP assay kit (Nanjing Jiancheng Biotechnology, Nanjing, China) according to the manufacturer’s instructions. Absorbance values at 405 nm were measured using a microplate reader and normalized by the total protein content determined using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, USA).
ARS Staining
Calcium mineral deposits were evaluated using ARS staining after osteogenic induction for 21 days. BMSCs were washed carefully with PBS and fixed with 4% paraformaldehyde for 25 min. Then cells were stained with ARS (Cyagen Biosciences) for 15 min at room temperature and washed thoroughly with deionized water. To quantify the mineralization, calcium deposits were eluted using 10% (v/v) cetylpyridinium chloride (Sigma, USA), and the absorbance at 570 nm was measured.
Animal Surgery
A total of 24 older male Sprague–Dawley rats (15 months, 700-750 g) were subjected to tibial DO model and then assigned randomly to the control group (n = 12) or MSC-Exos group (n = 12) [9]. The surgery was performed according to previous researches [28, 29]. After anesthesia, a longitudinal incision was made in the medial lower leg and the tibia was exposed by blunt separation. Four mini half pins were drilled with a guide apparatus. Then, a transverse osteotomy was performed between the second and third pins and a customized monolateral external fixator (Xinzhong Company, Tianjing, China) was mounted to fix the proximal and distal segments of the tibia. The incisions were closed layer by layer.
Distraction Protocol
Distraction began 5 days after surgery (latency stage) and sustained for 10 days (0.25 mm every 12 h; distraction stage). Then, the consolidation stage lasted for 5 weeks. Immediately after distraction, all rats from 2 groups received local injection of PBS (100 μL) or 1 × 1010 MSC-Exos in PBS (100 μL) into the distraction gaps once a week until termination.
Exosome Distribution After Injection
To examine the in vivo distribution of MSC-Exos after injection, two rats treated with PBS or DiR (Life Technologies, Carlsbad, CA) labeled MSC-Exos were imaged 1 week after injection using the IVIS Spectrum Imaging System (PerkinElmer, USA).
Digital Radiographs and Micro-computed Tomography (micro-CT)
X-ray focused on the distraction gap was taken weekly under anesthesia. After consolidation for 5 weeks, the distracted tibia specimens were harvested and subjected to micro-CT scan (SKYSCAN 1176, Bruker, Kontich, Belgium) at the resolution of 18 μm. Three dimensional (3D) reconstructions of the regenerated callus were performed and parameters including bone volume/tissue volume (BV/TV) and BMD (bone mineral density) were analyzed.
Mechanical Test
Mechanical properties of fresh tibia specimens (n = 6 per group) were measured using a three-point bending device (Instron, Norwood, MA, USA). The tibia samples were loaded in the anterior–posterior direction at the loading rate of 1 mm/min until failure. The ultimate load and energy to failure of the lengthened tibias were analyzed.
Histological Staining
After fixation in 4% paraformaldehyde for 48 h, the tibia specimens were decalcified in 10% ethylene diaminetetraacetic acid solution for 4 weeks and embedded in paraffin. Thin sections (5 μm) were cut along the long axis of each specimen in the sagittal plane for subsequent hematoxylin–eosin (HE) and Masson’s trichrome staining.
Statistical Analysis
All quantitative data in this study were presented as mean ± standard deviation (SD) and analyzed using GraphPad Prism 5. Statistical differences were compared with Student’s t test between two groups or one way ANOVA followed by Turkey's post hoc test among groups. P < 0.05 was considered statistically significant.
Results
Characterization of MSC-Exos
MSCs-Exos were isolated from the cultured medium of BMSCs from 2-week-old rats. TPRS analyses showed that the diameters of most MSC-Exos ranged from 60 to 130 nm (Fig. 1a). TEM demonstrated that MSC-Exos were cup or round-shaped vesicles with diameters from 60 to 100 nm (Fig. 1b). Western blot results identified exosome-specific markers CD9, CD63, and TSG101 in the MSC-Exos (Fig. 1c).
MSC-Exos Promote the Proliferation of Older BMSCs
Since the proliferation of MSCs is critical for tissue regeneration, we first investigated the effects of MSC-Exos on the proliferation of older BMSCs using CCK-8 assay. The proliferative capacity of BMSCs treated with MSC-Exos was significantly elevated when compared with the control group at day 3 and day 5 in a dose-dependent manner (P < 0.05) (Fig. 2a).
MSC-Exos promoted the proliferation and upregulated the expression of osteogenic genes in older BMSCs. a The CCK-8 assay demonstrated that MSC-Exos significantly promoted the proliferation of BMSCs in a dose-dependent manner. b–d Expression of Runx2, ALP, and OCN in older BMSCs treated with MSC-Exos or PBS by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.001
MSC-Exos Enhance the Osteogenic Capacity of Older BMSCs
The gene expression of ALP, Runx2, and OCN was detected at day 7. The results of qRT-PCR showed that all these osteogenic genes were significantly upregulated after treatment with MSC-Exos (P < 0.01) (Fig. 2b–d).
The ALP staining became stronger after MSC-Exos treatment (Fig. 3a, upper panel). More calcium deposits were observed in the MSC-Exos groups than that of the control group (Fig. 3a, lower panel). Furthermore, quantitative results of ALP activity and ARS staining showed that MSC-Exos treatment remarkably increased the ALP activity and calcium mineral deposition in a dose-dependent manner (Fig. 3b, c).
MSC-Exos enhanced the osteogenic differentiation of older BMSCs. a Typical ALP staining at day 7 for ALP activity and ARS staining at day 21 for calcium mineral deposition in older BMSCs treated with PBS or MSC-Exos. b, c Quantitative analysis of ALP activity and calcium mineral deposition. *P < 0.05, **P < 0.01, ***P < 0.001
MSC-Exos Promote New Bone Formation During DO in Older Rats
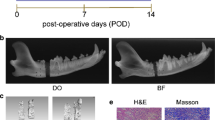
All rats survived the DO procedure without evident complications. Little callus was observed in the distraction gaps immediately after the distraction phase in both the PBS and MSC-Exos groups. New calluses formed from the proximal and distal osteotomy ends to the center of the distraction gaps. In both groups, mineralized callus formation increased with the time. More calluses were observed in the MSC-Exos group when compared with the PBS group at each time point. Typical radiographs from an MSC-Exos-treated rat and a PBS-treated rat at 5 weeks after distraction are shown in Fig. 4. Bone regeneration was further quantified using micro-CT scan after consolidation for 5 weeks. Representative 3D reconstructions of the distracted tibias are shown in Fig. 5a. Both the values of BV/TV and BMD in MSC-Exos group were significantly higher than that of the PBS group (P < 0.01) (Fig. 5b, c).
MSC-Exos accelerated new bone formation during distraction osteogenesis in older rats. a Representative 3D micro-CT images of the distraction regenerates after consolidation for 5 weeks indicated more mineralized callus in the MSC-Exos group. b, c Quantitative analysis of the micro-CT data demonstrated that MSC-Exos significantly increased the values of BV/TV and BMD. **P < 0.01, ***P < 0.001
Mechanical Test
Both the values of ultimate load and energy to failure in the MSC-Exos group showed significant improvement when compared with the PBS group (P < 0.05) (Fig. 6).
Histological Analyses
The results of HE and Masson’s trisome staining of the distraction regenerates demonstrated that the distraction gaps consisted of various amounts of fibrous tissues, cartilaginous tissues, and newly formed calluses. New calluses formed from the proximal and distal osteotomy ends to the center of the distraction gaps. The middle distraction gaps in the PBS groups were collected mainly by fibrous and cartilaginous tissues, while the distraction gaps in the MSC-Exos group were bridged by newly formed calluses (Fig. 7).
Discussion
This study first explored the therapeutic effects of MSC-Exos on new bone formation during DO in older rats. We found that MSCs-Exos could enhance the proliferation and osteogenic capacity of older BMSCs in vitro. Furthermore, local injection of MSC-Exos significantly promoted new bone formation in the distraction gaps in vivo.
DO relies on the recruitment of MSCs to the distraction gap, where they promote new bone formation through proliferating and differentiating into osteoblasts [30]. Increasing evidences demonstrate that transplantation of MSCs notably accelerates the DO process [12]. MSCs from bone marrow, adipose tissue, and even exfoliated deciduous teeth had positive effects on DO bone formation in various animal models. However, little attention has been paid to the enhancement of bone regeneration in older rats. According to a previous study, the engraftment and differentiation of transplanted MSCs may be compromised in aged rats [15].
Recent studies have revealed that the main beneficial effects of stem cells are attributed to their paracrine actions [31, 32]. We therefore evaluated the effects of young MSC-Exos on new bone formation during DO in older rats. The levels of pro-osteogenic and pro-angiogenic factors was maximal at the distraction phase and decreased in the consolidation phase [33]; the expression of growth factor receptors was highest at the beginning of the consolidation phase [34]. For the above reasons, the first injection of MSC-Exos was performed immediately after the distraction phase in this study. The in vivo distribution of DiR-labeled MSC-Exos indicated that most exosomes could remain in the distraction gaps after injection for 1 week (Online Resource 1). Since stromal cell-derived factor-1 (SDF-1) is highly expressed after distraction and MSC-Exos could express CXCR4, the specific receptor of SDF-1 [28], the recruitment of MSC-Exos in the distraction gaps may be mediated through the SDF-1/CXCR4 axis.
The weekly X-ray images indicated accelerated rate of mineralized callus formation in the MSC-Exos group. At consolidation for 5 weeks, both the values of BV/TV and BMD increased significantly after MSC-Exos treatment, suggesting better quality and quantity of bone regeneration in the MSC-Exos group. Furthermore, MSC-Exos treatment also improved the mechanical properties (ultimate load and energy to failure) of the distraction regenerates, which are key parameters for removal of external fixators. All together, the in vivo results demonstrated that MSC-Exos could significantly accelerate new bone formation during DO in older rats. In a prominent research by Aronson [9], exogenous fibroblast growth factor-2 could reverse the endosteal bone formation during DO in old rats. However, clinical application of fibroblast growth factor may be hindered by the rapid clearance and short resident time in tissues [35, 36]. Secretome from human fetal MSCs have been reported to enhance the proliferation and osteogenic differentiation of human adult MSCs in vitro, and improve bone consolidation in a rat DO model [37, 38]. The differences in bioactive contents and beneficial effects between exosomes and secretome from MSCs should be compared in future researches.
Since BMSCs are critical in bone regeneration and the function of BMSCs is compromised in aged rats [10, 11, 39], we further explored the effects of MSC-Exos on the proliferation and differentiation of older BMSCs in vitro. The results showed that MSC-Exos markedly enhanced the proliferation and osteogenic capacity of BMSCs. In addition, MSC-Exos have also been reported to stimulate angiogenesis [40], which is closely coupled with osteogenesis during DO [41, 42].
Advantages of MSC-Exos in promoting DO bone regeneration include the following: (1) MSC-Exos are stable under physiological conditions when compared with biological factors; (2) MSC-Exos can avoid the inherent limitations of stem cell therapies including possible immune response and malignant transformation; and (3) MSC-Exos are promising carriers for drug delivery [43].
There are limitations with this study. First, the precise mechanism of MSC-Exos in promoting bone regeneration remains unclear. MSC-Exos have been reported to exert the function through transferring various bioactive constituents including proteins and RNAs. Hence, the therapeutic effects may be mediated through multiple mechanisms. Second, the effects of MSCs and MSC-Exos are not compared directly.
In summary, this study demonstrate that MSC-Exos promote new bone formation during DO in older rats at least in part by enhancing the proliferation and osteogenic differentiation of older BMSCs. These findings suggest MSC-Exos as a promising therapy to short the treatment duration of DO, especially in older patients.
References
Mauffrey C, Barlow BT, Smith W (2015) Management of segmental bone defects. J Am Acad Orthop Surg 23:143–153. https://doi.org/10.5435/jaaos-d-14-00018
Quinnan SM (2017) Segmental bone loss reconstruction using ring fixation. J Orthop Trauma 31(Suppl 5):S42–S46. https://doi.org/10.1097/bot.0000000000000985
Aktuglu K, Erol K, Vahabi A (2019) Ilizarov bone transport and treatment of critical-sized tibial bone defects: a narrative review. J Orthop Traumatol 20:22. https://doi.org/10.1186/s10195-019-0527-1
Runyan CM, Gabrick KS (2017) Biology of bone formation, fracture healing, and distraction osteogenesis. J Craniofac Surg 28:1380–1389. https://doi.org/10.1097/scs.0000000000003625
Papakostidis C, Bhandari M, Giannoudis PV (2013) Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results: a systematic review and meta-analysis. Bone Joint J 95:1673–1680. https://doi.org/10.1302/0301-620x.95b12.32385
Liantis P, Mavrogenis AF, Stavropoulos NA, Kanellopoulos AD, Papagelopoulos PJ, Soucacos PN, Babis GC (2014) Risk factors for and complications of distraction osteogenesis. Eur J Orthop Surg Traumatol 24:693–698. https://doi.org/10.1007/s00590-013-1261-7
Fischgrund J, Paley D, Suter C (1994) Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res 301:31–37
Aronson J, Gao GG, Shen XC, McLaren SG, Skinner RA, Badger TM, Lumpkin CK Jr (2001) The effect of aging on distraction osteogenesis in the rat. J Orthop Res 19:421–427. https://doi.org/10.1016/s0736-0266(00)90025-1
Aronson J (2004) Modulation of distraction osteogenesis in the aged rat by fibroblast growth factor. Clin Orthop Relat Res 425:264–283. https://doi.org/10.1097/01.blo.0000138186.53426.f9
Asumda FZ, Chase PB (2011) Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol 12:44. https://doi.org/10.1186/1471-2121-12-44
Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA (2017) Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant 26:1520–1529. https://doi.org/10.1177/0963689717721201
Yang Y, Lin S, Wang B, Gu W, Li G (2017) Stem cell therapy for enhancement of bone consolidation in distraction osteogenesis: a contemporary review of experimental studies. Bone Joint Res 6:385–390. https://doi.org/10.1302/2046-3758.66.bjr-2017-0023
Kim IS, Cho TH, Lee ZH, Hwang SJ (2013) Bone regeneration by transplantation of human mesenchymal stromal cells in a rabbit mandibular distraction osteogenesis model. Tissue Eng Part A 19:66–78. https://doi.org/10.1089/ten.TEA.2011.0696
Herberts CA, Kwa MS, Hermsen HP (2011) Risk factors in the development of stem cell therapy. J Transl Med 9:29. https://doi.org/10.1186/1479-5876-9-29
Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, Hill WD, Liu Y, Shi X, Fulzele S, Hamrick MW (2017) MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A 23:1231–1240. https://doi.org/10.1089/ten.TEA.2016.0525
Kotobuki N, Katsube Y, Katou Y, Tadokoro M, Hirose M, Ohgushi H (2008) In vivo survival and osteogenic differentiation of allogeneic rat bone marrow mesenchymal stem cells (MSCs). Cell Transplant 17:705–712. https://doi.org/10.3727/096368908786092793
Sebastiao MJ, Menta R, Serra M, Palacios I, Alves PM, Sanchez B, DelaRosa O, Dalemans W, Lombardo E, Gomes-Alves P (2018) Human cardiac stem cells inhibit lymphocyte proliferation through paracrine mechanisms that correlate with indoleamine 2,3-dioxygenase induction and activity. Stem Cell Res Ther 9:290. https://doi.org/10.1186/s13287-018-1010-2
Guo ZY, Sun X, Xu XL, Zhao Q, Peng J, Wang Y (2015) Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res 10:651–658. https://doi.org/10.4103/1673-5374.155442
Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, Gupta S, Singhal PC (2013) Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol 94:466–473. https://doi.org/10.1016/j.yexmp.2013.03.002
Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, Lim LP, Lim SK, Toh WS (2019) Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater 89:252–264. https://doi.org/10.1016/j.actbio.2019.03.021
Chen B, Li Q, Zhao B, Wang Y (2017) Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem cells Transl Med 6:1753–1758. https://doi.org/10.1002/sctm.16-0477
Jing H, He X, Zheng J (2018) Exosomes and regenerative medicine: state of the art and perspectives. Transl Res 196:1–16. https://doi.org/10.1016/j.trsl.2018.01.005
Qin Y, Sun R, Wu C, Wang L, Zhang C (2016) Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci. https://doi.org/10.3390/ijms17050712
Jia Y, Zhu Y, Qiu S, Xu J, Chai Y (2019) Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res Ther 10:12. https://doi.org/10.1186/s13287-018-1115-7
Zhu Y, Jia Y, Wang Y, Xu J, Chai Y (2019) Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl Med 8:593–605. https://doi.org/10.1002/sctm.18-0199
Chen B, Sun Y, Zhang J, Zhu Q, Yang Y, Niu X, Deng Z, Li Q, Wang Y (2019) Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res Ther 10:142. https://doi.org/10.1186/s13287-019-1253-6
Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L (2017) Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9:4430–4438. https://doi.org/10.1039/c7nr00352h
Xu J, Chen Y, Liu Y, Zhang J, Kang Q, Ho K, Chai Y, Li G (2017) Effect of SDF-1/Cxcr4 signaling antagonist AMD3100 on bone mineralization in distraction osteogenesis. Calcif Tissue Int 100:641–652. https://doi.org/10.1007/s00223-017-0249-4
Wang X, Zhu S, Jiang X, Li Y, Song D, Hu J (2015) Systemic administration of lithium improves distracted bone regeneration in rats. Calcif Tissue Int 96:534–540. https://doi.org/10.1007/s00223-015-0004-7
Morillo CMR, Sloniak MC, Goncalves F, Villar CC (2018) Efficacy of stem cells on bone consolidation of distraction osteogenesis in animal models: a systematic review. Braz Oral Res 32:e83. https://doi.org/10.1590/1807-3107bor-2018.vol32.0083
Li X, Wang Y, An G, Liang D, Zhu Z, Lian X, Niu P, Guo C, Tian L (2017) Bone marrow mesenchymal stem cells attenuate silica-induced pulmonary fibrosis via paracrine mechanisms. Toxicol Lett 270:96–107. https://doi.org/10.1016/j.toxlet.2017.02.016
Chang YH, Lin LM, Lou CW, Chou CK, Ch'ang HJ (2012) Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol 105:371–377. https://doi.org/10.1016/j.radonc.2012.10.005
Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA (2003) Expression of angiogenic factors during distraction osteogenesis. Bone 33:889–898
Siwicka KA, Kitoh H, Kawasumi M, Ishiguro N (2011) Spatial and temporal distribution of growth factors receptors in the callus: implications for improvement of distraction osteogenesis. Nagoya J Med Sci 73:117–127
Makhdom AM, Nayef L, Tabrizian M, Hamdy RC (2015) The potential roles of nanobiomaterials in distraction osteogenesis. Nanomedicine 11:1–18. https://doi.org/10.1016/j.nano.2014.05.009
Makhdom AM, Hamdy RC (2013) The role of growth factors on acceleration of bone regeneration during distraction osteogenesis. Tissue Eng Part B 19:442–453. https://doi.org/10.1089/ten.TEB.2012.0717
Wang B, Lee WY, Huang B, Zhang JF, Wu T, Jiang X, Wang CC, Li G (2016) Secretome of human fetal mesenchymal stem cell ameliorates replicative senescen. Stem Cells Dev 25:1755–1766. https://doi.org/10.1089/scd.2016.0079
Xu J, Wang B, Sun Y, Wu T, Liu Y, Zhang J, Lee WY, Pan X, Chai Y, Li G (2016) Human fetal mesenchymal stem cell secretome enhances bone consolidation in distraction osteogenesis. Stem Cell Res Ther 7:134. https://doi.org/10.1186/s13287-016-0392-2
Kim M, Kim C, Choi YS, Kim M, Park C, Suh Y (2012) Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mech Ageing Dev 133:215–225. https://doi.org/10.1016/j.mad.2012.03.014
Wu P, Zhang B, Shi H, Qian H, Xu W (2018) MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy 20:291–301. https://doi.org/10.1016/j.jcyt.2017.11.002
Rachmiel A, Leiser Y (2014) The molecular and cellular events that take place during craniofacial distraction osteogenesis. Plast Reconstr Surg Glob Open 2:e98. https://doi.org/10.1097/gox.0000000000000043
Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT (2005) Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res 20:1114–1124. https://doi.org/10.1359/jbmr.050301
Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q (2019) Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 86:1–14. https://doi.org/10.1016/j.actbio.2018.12.045
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81572121).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yachao Jia, Shuo Qiu, Jia Xu, Qinglin Kang, and Yimin Chai declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All animal experiments in this study were reviewed and approved by the Institute of Animal Care and Use Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, Y., Qiu, S., Xu, J. et al. Exosomes Secreted by Young Mesenchymal Stem Cells Promote New Bone Formation During Distraction Osteogenesis in Older Rats. Calcif Tissue Int 106, 509–517 (2020). https://doi.org/10.1007/s00223-019-00656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00656-4