Abstract
Gnathodiaphyseal dysplasia (GDD; OMIM#166260) is a rare skeletal disorder which is mainly characterized by cemento-osseous lesions in mandibles, bone fragility, bowing and diaphyseal sclerosis of tubular bones. GDD is caused by point mutations in Anoctamin-5 (ANO5); however, the disease mechanisms remain unclear. Here we generated Ano5-knockout (KO) mice using a CRISPR/Cas 9 approach to study loss of function aspects of GDD mutations. Homozygous Ano5 knockout mice (Ano5−/−) replicate some typical traits of human GDD including massive jawbones, bowing tibia, sclerosis and cortical thickening of femoral and tibial diaphyses. Serum alkaline phosphatase (ALP) levels were elevated in Ano5−/− mice as in GDD patients. Calvaria-derived Ano5−/− osteoblast cultures show increased osteoblastogenesis, which is consistent with our previous in vitro observations. Bone matrix is hypermineralized, and the expression of bone formation-related factors is enhanced in Ano5−/− mice, suggesting that the osteogenic anomaly arises from a genetic disruption of Ano5. We believe this new mouse model will shed more light on the development of skeletal abnormalities in GDD on a cellular and molecular level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gnathodiaphyseal dysplasia (GDD; OMIM:16620) is a generalized bone disorder involving cemento-osseous resorptive lesions of the mandible accompanied by a complex skeletal phenotype of bone fragility, cortical thickening and sclerosis of tubular bone diaphyses. Mandibular lesions can lead to recurrent osteomyelitis of the jaw, and the majority of patients suffer from frequent bone fractures as a result of minor accidents or strain. The term “gnathodiaphyseal sclerosis” was first proposed by Akasaka [1] in a large Japanese kindred of 21 patients. Riminucci et al. [2] redefined the terminology to “gnathodiaphyseal dysplasia (GDD)” because osteosclerosis is not found in all patients while some traits of GDD overlap with other syndromes involving fibro-osseous jaw lesions and skeletal dysfunction, most notably fibrous dysplasia (FD) and McCune-Albright syndrome (MAS) [3]. However, the lack of typical radiological changes in bone such as a cotton wool-like pattern in FD and lack of skin pigmentation or endocrinopathies in MAS caused by mutations in GNAS1 differentiates GDD from FD/MAS.
GDD is inherited as an autosomal dominant trait or occurs sporadically and maps to chromosome 11q14.3–15.1 [4]. Eight missense mutations in the anoctamin 5 (ANO5; GDD1; TMEM16E) gene have been identified in families with GDD and in patients with de novo mutations from various races and ethnicities [5,6,7,8,9,10,11]. ANO5 encodes for a 913 amino acid protein and belongs to a family of transmembrane proteins, which in humans includes 10 members. C356R and C356G mutations in ANO5 decreased cell adhesion and changed the cell morphology to a more rounded cell shape, which is possibly due to the regulatory role of ANO5 in intracellular calcium homeostasis [6]. Although some members of this family such as ANO1 and ANO2 act as calcium-activated chloride channels (CaCCs) [12,13,14], a recent study demonstrated that ANO5 had phospholipid scrambling activity and non-selective ion transport capability [15] and that a T513I mutation [7] in the ANO5 protein caused a gain of function enabling phospholipid scrambling at low cytosolic Ca2+ levels. Murine ANO5 is highly expressed in cardiac muscle, skeletal muscle, growth-plate chondrocytes and osteoblasts [16]. Anoctamin 6 (ANO6), another member of the ANO protein family, is involved in hydroxyapatite deposition [17] as well as in control of bone mineralization by activating the calcium transporter NCX1 [18] and as regulator of C2C12 myoblast proliferation [19]. Recessive mutations in the ANO5 gene cause two types of muscular dystrophies, limb girdle muscular dystrophy type 2L (LGMD2L) and Miyoshi myopathy type 3 (MMD3) [20,21,22]. While ANO5-related muscular dystrophies have been relatively well researched, the function of ANO5 and the molecular pathophysiology of ANO5 mutations leading to GDD are insufficiently explored.
In our previous study, where we knocked down Ano5 mRNA in MC3T3-E1 osteoblast precursors we saw elevated expression of osteoblast-related genes such as Col1a1, osteocalcin, osterix and Runx2 as well as increased mineralization during osteoblast differentiation [5]. Several Ano5 knockout (KO) mouse or rabbit models provide some evidence for reduced capacity to repair sarcolemma, altered lipid metabolism and affecting sperm motility but failed to discuss skeletal phenotypes at all [23,24,25,26]. Here we introduce a new Ano5 knockout mouse model, which replicates clinical features of patients with GDD and exhibits stimulatory effects on osteoblastogenesis.
Materials and Methods
Animals
C57BL/6N mice and Kunming (KM) mice were purchased from Beijing Vital River Laboratory Animal, Co., Ltd. All mice were housed in a specific pathogen-free (SPF) facility. The animal protocol was approved by the Animal Care Committee of Beijing Stomatological Hospital, Capital Medical University, and animal studies were approved by the Institutional Animal Care and Use Committee of the Beijing Stomatological Hospital. Two sgRNAs were designed to target the two sides of exons 11–12 (Fig. 1a). For each targeting site, candidate guide RNAs were designed by the CRISPR design tool (http://crispr.mit.edu). Guide RNAs were screened for on-target activity using the Universal CRISPR Activity Assay (UCATM).. C57BL/6 female mice and KM mouse strains were used as embryo donors and pseudopregnant foster mothers, respectively. Genomic DNA was extracted from mouse tails using an alkaline lysis method. PCR genotyping generates a 759-bp product for wild type and a 660-bp product for mutant Ano5 (Fig. 1b). Mice were backcrossed with C57BL/6N mice and bred for at least four generations to demonstrate hereditary stability.
Generation and genotyping of Ano5−/− mice. (A) Schematic overview of strategy to generate Ano5 KO allele. The guide RNA sequence is underlined, capitalized, and labeled in red. The protospacer-adjacent motif (PAM) sequence is labeled in green. The arrowheads indicate locations of primers 1 and 2 used for PCR. (B) PCR genotyping assay for Ano5+/+, Ano5+/−, and Ano5−/−mice. Wild type allele: 759 bp, mutant allele: 660 bp. (C) Approximately 80% and >99% relative reduction of Ano5 transcript, at the 5’ and 3’ end, respectively, was confirmed by qRT–PCR in in different mouse tissues extracted from the Ano5−/− mouse. Five mice of each group were used; bP < 0.01 indicates statistical significance by one-way ANOVA
Skeletal Imaging Analysis
Mandibles, femurs and tibias of 12-week-old Ano5+/+ male mice (n = 8) and their Ano5−/− littermates (n = 9) were used for X-ray and Micro Computed Tomography (μCT) analysis. This age is equivalent to adulthood in humans, when most of the GDD patients display symptoms. Radiographs of individual bones were taken using a MX20 Radiography System (Faxitron X-ray) with exposure of 0.125 s. μCT was performed with 14 μm resolution in the Central Laboratory of the Capital Medical University (CCMU) (Inveon CT, Siemens, Germany). Mandibular images were obtained by capturing vertical sections at the central fossa of mandibular first molars. For CT scanning, cancellous mandibular bone surrounding the first molar was chosen as the area of interest in Ano5+/+ and Ano5−/− groups. Trabecular measurements of tubular bones were taken at the distal growth plate in 70 consecutive 14 μm slices over a distance of 980 μm. Thirty cross sections in the mid-diaphysis were used to compute cortical bone parameters. Hounsfield unit (HU) and calibrated CT data were applied to calculate bone mineral density (BMD) according to phantom reference data. Bone volume was calculated by an interactive medical image control system (Mimics19.0, Materialise, Belgium) combined with BMD to acquire bone mineral content (BMC).
Mouse Serum Analysis
Serum was prepared from retro-orbital blood sampling of 12-week-old male mice after 1-h incubation at 4 °C and 5 min centrifugation at 3000 rpm. Serum TRACP 5b (Tartrate-Resistant Acid Phosphatase-5b ELISA Kit, MyBioSource, USA) was detected in Ano5+/+, Ano5+/− and Ano5−/− mice. Alkaline phosphatase (ALP) activity was determined according to manufacturer’s specification (Nanjing Jiancheng, China). The number of samples used for serum analysis was a minimum of 7 per group (n ≥ 7).
Bone Tissue Morphology
Mandibles, tibias and femurs of 12-week-old male mice were fixed for 48 h in 4% PFA and then decalcified in 10% EDTA solution and processed for paraffin embedding. Series of 5-µm-thick longitudinal and horizontal sections were cut from paraffin sections of the Ano5+/+, Ano5−/− groups (n ≥ 5) stained with hematoxylin-eosin (H&E). For immunohistochemical (IHC) staining, antigens in paraffin-embedded mandible samples were retrieved by 0.01 M citric acid buffer (pH 6.0). Endogenous peroxide activity was quenched with 3% hydrogen peroxide. Slides were incubated in blocking solution with 10% goat serum for 15 min prior to overnight incubation at 4 °C with primary antibodies against CD31 (Abcam, ab28364,1:200, UK), TNF-α (Abcam, ab6671, 1:500, UK) and IL-6 (Abcam, ab7737, 1:500, UK), respectively. Slides were incubated with a biotin-labeled secondary goat anti-rabbit IgG antibody (ZSGB-BIO, SP-9001, China) for 30 min at 37 °C, visualized with diaminobenzidine and counterstained with hematoxylin. Slides were imaged with an Olympus BX61 microscope (Olympus, Japan), and immuno-positive areas were analyzed by Image-Pro Plus 6.0 (Media cybernetics, USA).
Mouse Calvarial Osteoblast Cultures (mCOBs)
Calvariae from postnatal 24-h-old littermates were isolated and digested with 0.3% collagenase (Type I; Sigma-Aldrich, USA) mixed with equal volume 0.05% trypsin (Invitrogen-Gibco, USA). Calvariae were digested for four cycles at 15 min/cycle at 37 °C. Cells of the first cycle were discarded, and the remaining cells were collected and cultured in DMEM (Gibco, USA). Medium was switched to osteoblast differentiating medium (α-MEM; Invitrogen-Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, USA), 1% dual-antibiotics (Gibco, USA), 4 µg/ml dexamethasone, 50 mg/ml ascorbic acid and 4 mM β-glycerophosphate (Sigma-Aldrich, USA) for osteoblast differentiation. Cells were derived from no less than five mice in each group. Medium was changed three times a week.
Matrix and Mineralization Assays
Alizarin red staining and alkaline phosphatase activity detection were performed to observe the formation of matrix and mineralization. Osteoblasts (passage 2) were seeded at a density of 2 × 105 cells per well in 6-well plates. Alizarin red staining was performed at 7, 14 and 21 days of culture, and mineralized nodules were dissolved by cetylpyridinium chloride for 30 min. Calcium binding to alizarin red was detected by colorimetric detection as indicator for calcification. Absorbance reads were determined at 562 nm. Protein for ALP analysis was extracted and quantified by BCA to assess early osteogenic differentiation at 0, 3, 5 and 7 days. ALP activity was detected by an Alkaline Phosphatase Assay Kit (Nanjing Jiancheng, China) according to manufacturer’s instructions. OD values were measured at 520 nm.
RNA Analysis
We used quantitative real-time PCR (qRT-PCR) to detect expression of Ano5 and osteogenic factors in bone tissue and mCOBs, respectively. PCR primers are listed in Supplementary Table 1. Total RNA derived from tissues or primary cultured cells was extracted with TRIzol (Invitrogen, USA) followed by reverse transcription (SuperRT cDNA Synthesis Kit; CWbio, China). qRT-PCR was carried out using Ultra SYBR Mixture with low ROX (CWbio, China) in a Bio-Rad thermocycler (BioRad, Hercules, USA). Relative quantification of gene expression was measured by the ΔΔCt method and the housekeeping gene Gapdh served as control.
Statistical Analysis
Statistical analysis was performed by paired t test or one-way ANOVA using Prism7 software (GraphPad Software, La Jolla, USA).
Results
Generation and Appearance of Ano5 −/− Mice
We targeted exons 11 and 12 to produce a truncated transcript with a 833 bp deletion. To investigate the expression of Ano5 transcripts, we performed qRT-PCR with primers spanning exons 4–5, 10–12 and 16–17. In Ano5−/− mice, expression of Ano5 in skull and musculus biceps brachii dropped to almost zero (background level) when the primers span exons 10–12, suggesting complete knockout of Ano5. qRT-PCR with primers span exons 4–5 or exons 16–17 shows ~ 80% reduction of pre-deletion Ano5 transcripts in all tissues tested and > 99% reduction of post-deletion Ano5 transcript in calvariae and > 71% reduction of post-deletion Ano5 transcript in muscle (Fig. 1c). Behavior and overall appearance of Ano5−/− mice was comparable to normal littermates, and Ano5−/− male mice showed low fertility compared with heterozygotes and wild type mice (data not shown).
GDD-Like Skeletal Phenotype of Ano5−/− Mice
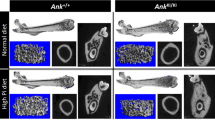
GDD is clinically diagnosed by histological analysis of bone lesions in the jaws combined with radiography [3, 8]. We performed radiography on mandibles, femurs and tibias of 12-week-old male littermates in more than 10 animals per group. Similar to GDD patients, Ano5−/− mice showed increased radiopacity of mandibles and cortical bone of femurs and tibiae. Mandibles of 12-week-old Ano5−/− mice were larger with increased bone density (Fig. 2a). Femoral length or width was not significantly different. To better illustrate the skeletal phenotype, we studied the bones by μCT. Ano5−/− mice had increased BMC and BMD in mandibles and in diaphyses of cortical bones (Table 1). Mandibles of Ano5−/− mice displayed hyperostosis (Fig. 2b), which is consistent with our X-ray results, whereas the bone volume fraction (BVF) in tibiae and femurs was reduced. Two notable characteristics of GDD patients are bowing of tibia, and bone fragility combined with cortical thickening of diaphyses. Ano5−/− mice exhibited abnormal shape of tibiae with increased diameter of diaphyseal cortices for both tibiae and femurs resulting in narrow medullary canals (Fig. 2c, d). The mandibles and diaphyses of femurs and tibiae were undertrabeculated in Ano5−/− mice with significantly reduced thickness and trabecular numbers compared to Ano5+/+ mice. The trabeculation of Ano5−/− mice was more significantly dispersed in mandibles and femoral diaphyses but normal in tibia (Table 2). In addition, heterozygous Ano5+/− mice were phenotypically similar to Ano5−/− mice and exhibited measurable GDD-like skeletal phenotypes as they aged.
GDD-like phenotype in 12-week-old Ano5−/− mice. (A) Representative radiographs of mandibles, tibias and femurs from 12-week-old Ano5+/+, Ano5+/−, and Ano5−/− male mice. (B) μCT images of vertical plane through mandibles and 3D reconstruction of mandibles from Ano5+/+ and Ano5−/− mice. White line points to sagittal plane through central fossa of first mandibular molar. White arrows points to the hyperostosis of mandibles. (C) Internal view of femurs in longitudinal section and 3D reconstructions of trabeculae in diaphysis from Ano5+/+ and Ano5−/− mice. (D) Internal view in longitudinal section and cross-section of cortical bone of tibia. 3D reconstructions of trabeculae in metaphysis of Ano5+/+ and Ano5−/− mice
Biochemical Analysis of Ano5−/− Mice
Serum alkaline phosphatase levels are variable but generally elevated in GDD patients [2, 8, 11]. Therefore, we tested the ALP activity of 12-week-old Ano5−/− mice and found that ALP in sera of Ano5−/− and Ano5+/− mice was increased by approximately 23% (Fig. 3a). To investigate the activity of osteoclasts, we measured the levels of TRACP 5b. Previously only one GDD patient was reported with elevated TRACP near the upper normal limits [27]. In the mouse model, we found comparable TRACP values in Ano5−/−, Ano5+/− and Ano5+/+ mice (Fig. 3b).
Morphological Analysis of Bone Tissues in Ano5−/− Mice
Histological sections stained with H&E confirmed that Ano5−/− mice had thicker cortical bones in cross sections of mandibles (Fig. 4a). Being consistent with the results from µCT, Ano5−/− mouse tibia exhibited cortical thickening and bowing. Tibial bowing is quite variable in Ano5−/− mice, as can be seen in Figs. 2a and 4b. Moreover, trabeculation is decreased drastically outside the metaphyseal region of femurs or tibiae of Ano5−/− mice (Fig. 4b). Ano5−/− mice have approximately threefold more blood vessels in the periodontal ligament of first molars than Ano5+/+ mice based on CD31 expression (Fig. 4c), and this may indicate beginning periodontal ligament hyperemia. Meanwhile, expression of TNF-α and IL-6 in blood vessels of the periodontal ligament of Ano5−/− mice was increased dramatically (P < 0.01 for TNF-α and IL-6), which suggest that Ano5−/− mice may suffer from periodontal inflammation (Fig. 4d).
Histology of Ano5+/+ and Ano5−/− male mice. (A) Mandible in vertical plane of from 12-week-old mice (H&E). (B) H&E staining of tibias and femurs of 12-week-old mice: tibias, scale bar = 1 mm; femurs, scale bar = 200 μm. (C) Representative image showing increased angiogenesis at first molars of Ano5−/− mice stained by CD31 and quantified area of blood vessels shown in bar graph below. D, dentin; B, alveolar bone; PDL, periodontal ligament. Scale bar = 100 μm. bP < 0.01. (D) Representative photomicrographs and quantitative immunopositive areas analysis of TNF-α and IL-6 staining in periodontal ligament of the first molars in Ano5+/+ and Ano5−/− mice. D, dentin; B, alveolar bone; PDL, periodontal ligament. TNF-α, scale bar = 100 μm; IL-6, scale bar = 50 μm. bP < 0.01
Bone Matrix and Mineral Formation
In consideration of the high expression of ANO5 protein in osteoblasts and evidence from our previous study suggesting a potential role of ANO5 in osteoblastogenesis [4], we determined the mineralization potential of cultured mCOBs by alizarin red staining. Osteoblast cultures from Ano5−/− mice developed more mineral nodule deposition over a 21-day culture period in osteogenic medium (Fig. 5a, b). Quantitative analysis also showed that the Ca2+ concentration was elevated in the Ano5−/− group after nodule dissolution (Fig. 5c). Ano5+/− mice showed intermediate levels of mineralization compared to Ano5+/+ and Ano5−/− mice. The highest ALP activity in osteoblasts occurred at culture day 5 and decreased by day 7 in both groups (Fig. 5d). However, Ano5−/− mice showed significantly higher ALP activity than Ano5+/+ mice at all time points (P < 0.01). Increased mineralization and ALP activity in Ano5−/− osteoblast cultures are consistent with our previous in vitro finding, where the overexpression of a GDD mutation resulted in increased mineralization [5].
Mineralization of mouse calvarial osteoblast cultures. (A) General observation and (B) microscopic observation of Ano5+/+ and Ano5−/− mCOBs stained for alizarin red S at culture days 7, 14 and 21. (C) Histogram representing corresponding calcium binding levels. (D) ALP detected in Ano5+/+ and Ano5−/− mCOBs at days 3, 5 and 7. aP < 0.05, bP < 0.01
Osteogenesis-Related Factors Increase in Ano5−/− Mice
To investigate whether deficit of Ano5 affects expression of osteoblast differentiation markers, we performed qRT-PCR of mRNA isolated from 0-, 3-, 7-, 14- and 21-day mCOB cultures of Ano5+/+, Ano5+/− and Ano5−/− mice in osteogenic medium. We found that expression of collagen I (Col1a1) and Bglap (osteocalcin, Ocn) in mCOBs gradually increased in Ano5+/+, Ano5+/− and Ano5−/− mice over time (Fig. 6a, b). Spp1 (osteopontin, Opn), Runx2, Sp7 (osterix) and Tnfrsf11b (osteoprotegerin, Opg) showed higher expression at day 7 with a peak at day 14 in mCOBs from Ano5−/− mice compared to Ano5+/+ mice (Fig. 6c–f). Interestingly, the ratio of Opg and Tnfsf11 (NF-kB ligand, Rankl) in Ano5−/− mouse osteoblasts significantly increased at days 7 and 14 compared to Ano5+/+ osteoblast cultures but was comparable at day 21 (Fig. 6h). This may be due to the high expression of Rankl at day 21 (Fig. 6g). It was recently reported that another member of the anoctamin family, ANO6, is involved in the control of bone mineralization [17, 18]. We found the expression of Ano6 elevated in Ano5+/− and Ano5−/− mCOBs at the time of osteoblast extraction and was lower starting at day 3 when compared Ano5+/+ cultures (Fig. 6i).
Osteogenesis-related gene expression in mCOBs from Ano5+/+ and Ano5−/− mice. qPCR analysis of Col1α1, Ocn, Runx2, SP7, Opn, Opg, Rankl, Opg/Rankl and Ano6 at days 0, 3, 7, 14 and 21 Ano5−/− calvarial osteoblast cultures normalized to expression levels of Ano5+/+ cells. Data from three independent experiments. aP < 0.05, bP < 0.01
Discussion
The ANO5 gene is abundantly expressed in skeletal and cardiac muscle as well as in chondrocytes and osteoblasts [16]. This suggests that ANO5 protein plays an essential role in muscle and bone development. Disruption of ANO5 is associated with autosomal dominant GDD and two autosomal recessive muscular dystrophies, limb girdle muscular dystrophy type2 (LGMD2L) and Miyoshi muscular dystrophy 3 (MMD3) [28,29,30,31,32]. Biochemical properties and physiological function of ANO5 are incompletely understood, and skeletal phenotypes in existing mouse models have not been investigated. We generated an Ano5 knockout mouse model to study the effect of reduced expression of ANO5 on bone development. We investigated Ano5+/+ and Ano5−/− mice as well as Ano5+/− mice at 12 weeks, which exhibit an intermediate phenotype. It will be interesting to follow the progression of their GDD-like phenotype to an older age. GDD in humans is an autosomal dominant disorder. However, it is not uncommon that the phenotypes of a dominant disorder are best replicated in homozygous mutant mouse models [33,34,35,36]. This discrepancy may be due to species-specific phenotypic thresholds and lifespan [33, 34]. Ano5−/− mice exhibit distinct features of GDD including elevated ALP levels in serum, mandibles with increased radiopacity, bowing and thicker cortical bone in long bones, which is also found in some clinical GDD reports [1, 2, 5, 8]. However, some mandibular phenotypes such as osteomyelitis, cemento-ossifying fibroma and cementum-like materials were not found in Ano5−/− mice at the age of 3 months. Some pathological features may require longer time to develop or may develop only after challenging the mouse. It is also possible that some additional factors that trigger manifestation of certain aspects of the disorder are different in mice and humans, such as the immune system, metabolism or other biological characteristics [37, 38]. Interestingly, we found increased blood vessel formation in the periodontal ligament (PDL) of Ano5−/− mice, which suggests a hyperemic state in the early stage of inflammation and therefore we considered the possibility that the mice are in the process of developing inflammatory signs of periodontitis. This speculation was confirmed by evidence of enhanced TNF-α and IL-6 expression in periodontal ligaments of Ano5−/− mice. Coincidentally, some adult patients with GDD suffer from painful swelling and purulent discharge from gingiva, loosening or displaced teeth and insufficient healing after tooth extraction [7, 20]. We also have to consider that knockout mice exhibit only those phenotypes that are due to loss of ANO5 function.
Besides increased cytokine expression in the jawbones, Ano5−/− mice display thickening of cortical diaphyses and trabecular abnormalities in tubular bones based on our μCT and histomorphometry findings. Decreased trabecular thickness and trabecular number suggest reduced bone remodeling activity that result in sparser trabecular bone with poor connectivity. These changes suggest that Ano5−/− mice may be more vulnerable to bone injury. Reduced cancellous bone plays a predominant role for susceptibility to osteoporotic fractures for GDD patients. Increased BMD and BMC in mandibles, femurs and tibiae of Ano5−/− mice are consistent with sclerosis and increased diameters of cortical bone found by histology. A recent study in a 13-year-old GDD patient showed that BMD in the radius increased by 20% within a year [8]. Overall bone strength is determined by structural and material components. Therefore, it is possible that cortical thickening in diaphyses is an attempt to compensate for poor bone quality induced by abnormal distribution of trabecular bone in Ano5−/− mice. However, over-ossification may inversely increase bone fragility.
Total serum ALP is widely used to assess bone metabolism. Three GDD patients have been reported with elevated levels of serum ALP [2, 8, 11]. While we found increased ALP in serum and in cultured calvarial osteoblasts from Ano5−/− mice, the serum TRACP5b level was comparable between Ano5+/+ and Ano5−/− mice. Therefore, we speculate that Ano5 deficiency may contribute to changes in osteoblastogenesis. Mineralization markers were increased in Ano5−/− mice during osteoblastic differentiation. Osteocalcin and Col1a1, which are associated with increased mineral deposition, are highly expressed at day 21 of Ano5−/− mCOB cultures [31]. This may be a result of increased expression of Runx2 and SP7, which are required for osteoblast differentiation by involving transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) signaling [39]. Interestingly, mutations in the TGF-β1 gene are associated with Camurati-Engelmann disease (CED), which is an autosomal dominant sclerosing bone dysplasia affecting the diaphysis of long bones. CED exhibits similar long bone manifestations as GDD [40]. Opg/Rankl ratio is a vital determinant of bone mass and skeletal integrity. In this study, we show that Ano5−/− mCOBs have an elevated Opg/Rankl ratio at an early differentiation stage. At later stages, the significant difference in Opg/Rankl ratio between wt and mutant mCOBs disappears. This finding could be due to an inhibitory effect on differentiation but not on osteogenic regulation at late stages. Similarly, we found highly expressed Opn, which acts as a mineralization inhibitor regulating hydroxyapatite crystal growth [41] in Ano5−/− mCOBs. In order to investigate compensatory roles of other anoctamins, we investigated the expression of anoctamin 6 (Ano6), the closest paralog of Ano5. Ano6 has been reported to up-regulate bone mineralization by activating a calcium transporter NCX1 [17, 28] or acting downstream of Runx2 and osterix. In this study, we found reduced Ano6 expression in differentiating osteoblasts for both wild type and Ano5−/− mCOBs indicating a possible interaction with the Ano5 gene product in calcium channel function and in matrix mineralization. Loss of Ano6 leads to skeletal deformities and mineralization defects and results in regions of uncalcified osteoid in newborn mice with delayed mineralization [17]. Interestingly, Ano6 expression decreased remarkably in Ano5−/− mCOB, accompanied by decreased osteoblast differentiation, and we suspect that this down-regulation of Ano6 transcripts may compensate for the hypermineralization caused by ablation of Ano5 in mice. Therefore, we hypothesize that Ano5 defects lead to GDD due to reduced osteoblast differentiation and deposition of abnormal bone together with increased bone remodeling activity.
Recently, four other laboratories have reported on Ano5 knockout mice/rabbit models. In two models with disruption of exon 1 or exon 2 of the Ano5 gene [24, 25] investigators concluded that the defect of Ano5, being a phospholipid scramblase, may alter lipid metabolism and inflammation signaling or affect sperm motility. The other two knockout animals were generated by deletion of exon 8 and 9 or ablating exon 12 and/or 13. These models display defective muscle membrane fusion and repair, which may be associated with two muscular disorders, LGMD2 and MMD3 [23, 26]. Our mice were generated with a deletion of exon 11 and exon 12, which causes a frameshift in a region where at least four GDD-related mutations are located. Therefore, we believe this region is important for the pathology of the disease. Although we find no transcripts corresponding to exons downstream of exon 12 in calvariae, we do find evidence of some expressed transcripts corresponding to the 5′ region of the mRNA by qPCR. However, there is some mRNA expression downstream of exon 12 detectable in muscle. We do not know whether any protein is translated from these transcripts, but this observation raised the possibility that partial transcripts may be produced in knockout animals, which may directly or indirectly have pathogenic consequences via instable or quickly degraded proteins. In addition, we found that our 16-week-old Ano5−/− mice began to show abnormal histology in skeletal muscle (Fig. S1a) and showed decreased expression of myopathy-related factors (Fig. S1b). Ano5−/− mice also had increased serum creatine kinase (CK) levels (Fig. S1c), similar to patients with limb girdle muscular dystrophy type 2L (LGMD2L) [20].
In summary, we successfully created a mouse model for the skeletal phenotype of GDD, which recapitulates principal features of the disorder, which is the beginning of further studies to elucidate the GDD pathogenesis on a cellular and molecular level. Our results suggest that the GDD phenotype closely relates to osteoblastogenesis and bone deposition. Further studies in a knockin mouse model carrying an Ano5 mutation for GDD will be used to study potential roles of Ano5 mutations in GDD and will supplement studies in this knockout model.
References
Akasaka Y, Nakajima T, Koyama K et al (1969) Familial cases of a new systemic bone disease, hereditary gnathodiaphyseal sclerosis. Nippon Seikeigeka Gakkai Zasshi 43:381–394
Riminucci M, Collins MA, Boyde A et al (2001) Gnathodiaphyseal dysplasia: a syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J Bone Miner Res 16:1710–1718
Ahluwalia J, Ly JQ, Norman E et al (2001) Gnathodiaphyseal dysplasia. Clin Imaging 31:67–69
Tsutsumi S, Kamata N, Maruoka Y et al (2003) Autosomal dominant gnathodiaphyseal dysplasia maps to chromosome 11p14.3–15.1. J Bone Miner Res 18:413–418
Jin L, Liu Y, Sun F et al (2017) Three novel ANO5 missense mutations in Caucasian and Chinese families and sporadic cases with gnathodiaphyseal dysplasia. Sci Rep 7:40935
Tsutsumi S, Kamata N, Vokes TJ et al (2004) The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am J Hum Genet 74:1255–1261
Marconi C, Brunamonti Binello P, Badiali G et al (2013) A novel missense mutation in ANO5/TMEM16E is causative for gnathodiaphyseal dysplasia in a large Italian pedigree. Eur J Hum Genet 21:613–619
Rolvien T, Koehne T, Kornak U et al (2017) A novel ANO5 mutation causing gnathodiaphyseal dysplasia with high bone turnover osteosclerosis. J Bone Miner Res 32:277–284
Vengoechea J, Carpenter L (2015) Gnathodiaphyseal dysplasia presenting as polyostotic fbrous dysplasia. Am J Med Genet A 167:1421–1422
Andreeva TV, Tyazhelova TV, Rykalina VN et al (2016) Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies. Sci Rep 6:26440
Duong HA, Le KT, Soulema AL et al (2016) Gnathodiaphyseal dysplasia: report of a family with a novel mutation of the ANO5 gene. Oral Surg Oral Med Oral Pathol Oral Radiol 121:123–128
Hartzell HC, Yu K, Xiao Q et al (2009) Anoctamin/TMEM16 family members are Ca2+-activated Cl– channels. J Physiol 587:2127–2139
Tran TT, Tobiume K, Hirono C et al (2014) TMEM16E (GDD1) exhibits protein instability and distinct characteristics in chloride channel/pore forming ability. J Cell Physiol 229:181–190
Duran C, Qu Z, Osunkoya AO et al (2012) ANOs 3–7 in the anoctamin/Tmem16 Cl– channel family are intracellular proteins. Am J Physiol Cell Physiol 302:C482–C493
Di Zanni E, Gradogna A, Scholz-Starke J et al (2018) Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell Mol Life Sci 75:1657–1670
Mizuta K, Tsutsumi S, Inoue H et al (2007) Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun 357:126–132
Ehlen HW, Chinenkova M, Moser M et al (2013) Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res 28:246–259
Ousingsawat J, Wanitchakool P, Schreiber R et al (2015) Anoctamin-6 controls bone mineralization by activating the calcium transporter NCX1. J Biol Chem 290:6270–6280
Zhao P, Torcaso A, Mariano A et al (2014) Anoctamin 6 regulates C2C12 myoblast proliferation. PLoS ONE 9:e92749
Hicks D, Sarkozy A, Muelas N et al (2011) A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain 134:171–182
Little AA, McKeever PE, Gruis KL (2013) Novel mutations in the Anoctamin 5 gene (ANO5) associated with limb-girdle muscular dystrophy 2L. Muscle Nerve 47:287–291
Magri F, Del Bo R, D’Angelo MG et al (2012) Frequency and characterisation of anoctamin 5 mutations in a cohort of Italian limb-girdle muscular dystrophy patients. Neuromuscul Disord 22:934–943
Griffin DA, Johnson RW, Whitlock JM et al (2016) Defective membrane fusion and repair in Anoctamin5-deficient muscular dystrophy. Hum Mol Genet 25:1900–1911
Xu J, El Refaey M, Xu L et al (2015) Genetic disruption of Ano5 in mice does not recapitulate human ANO5-deficient musculardystrophy. Skelet Muscle 5:43
Gyobu S, Miyata H, Ikawa M et al (2015) A role of TMEM16E carrying a scrambling domain in sperm motility. Mol Cell Biol 36:645–659
Sui T, Xu L, Lau YS et al (2018) Development of muscular dystrophy in a CRISPR-engineered mutant rabbit model with frame-disrupting ANO5 mutations. Cell Death Dis 9:609
Otaify GA, Whyte MP, Gottesman GS et al (2018) Gnathodiaphyseal dysplasia: Severe atypical presentation with novel heterozygous mutation of the anoctamin gene (ANO5). Bone 107:167–171
Schessl J, Kress W, Schoser B (2012) Novel ANO5 mutations causing hyper-CK-emia, limb girdle muscular weakness and Miyoshi type of muscular dystrophy. Muscle Nerve 45:740–742
Bolduc V, Marlow G, Boycott KM et al (2010) Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet 86:213–221
Witting N, Duno M, Petri H et al (2013) Anoctamin 5 muscular dystrophy in Denmark: prevalence, genotypes, phenotypes, cardiac findings, and muscle protein expression. J Neurol 260:2084–2093
Sarkozy A, Hicks D, Hudson J et al (2013) ANO5 gene analysis in a large cohort of patients with anoctaminopathy: confirmation of male prevalence and high occurrence of the common exon 5 gene mutation. Hum Mutat 34:1111–1118
Penisson-Besnier I, Saint-Andre JP, Hicks D et al (2012) Myopathy caused by anoctamin 5 mutations and necrotizing vasculitis. J Neurol 259:1988–1990
Liao BY, Zhang J (2008) Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci USA 105:6987–6992
Chipman SD, Sweet HO, McBride DJ Jr et al (1993) Defective pro alpha 2(I) collagen synthesis in a recessivemutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci USA 90:1701–1705
Ueki Y, Lin CY, Senoo M et al (2007) Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128:71–83
Chen IP, Wang CJ, Strecker S (2009) Introduction of a Phe377del mutation in ANK creates a mouse model for craniometaphysealdysplasia. J Bone Miner Res 24:1206–1215
Mestas J, Hughes CCW (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738
Terpstra AHM (2001) Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J Nutr 131:2067–2068
Wahbi K, Behin A, Becane HM (2013) Dilated cardiomyopathy in patients with mutations in anoctamin 5. Int J Cardiol 16876–16879
Campos-Xavier B, Saraiva JM, Savarirayan R et al (2001) Phenotypic variability at the TGF-beta1 locus in Camurati-Engelmann disease. Hum Genet 109:653–658
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant # 81570958); Beijing Natural Science Foundation (Grant #7162075); High-level Talents of Beijing Health System (Grant #2013-3-036); and Scientific Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant # 2015-1098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xiaoyu Wang, Xiu Liu, Rui Dong, Chao Liang, Ernst J. Reichenberger and Ying Hu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animal experimentation protocols were approved by the Institutional Animal Care and Use Committee of the Beijing Stomatological Hospital (the approval number: KQYY-201611-001) and were strictly undertaken in accordance with the ethical guidelines of the Caring for Laboratory Animals by the Ministry of Science and Technology of the People’s Republic of China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2019_528_MOESM1_ESM.docx
Supplementary material 1. Fig. S1 Skeletal muscle changes in Ano5+/+ and Ano5-/- mice. (A) H&E staining of gastrocnemius muscle of 16-week-old mice. Scale bar = 50 μm. (B) qPCR analysis of Dysferlin and Dystrophin in gastrocnemius (GAS), quadriceps (QD) and biceps muscles of 16-week-old mice. aP < 0.05, bP < 0.01. (C) Elevated serum creatine kinase (CK) level was detected in 16-week-old Ano5-/- mice. (n = 5). bP < 0.01 (DOCX 1084 KB)
Rights and permissions
About this article
Cite this article
Wang, X., Liu, X., Dong, R. et al. Genetic Disruption of Anoctamin 5 in Mice Replicates Human Gnathodiaphyseal Dysplasia (GDD). Calcif Tissue Int 104, 679–689 (2019). https://doi.org/10.1007/s00223-019-00528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00528-x