Abstract
Previous studies found adolescent idiopathic scoliosis (AIS) is associated with low bone mineral density (BMD) and abnormal bone quality, whilst the association between AIS and their bone strength is unknown. From high-resolution peripheral quantitative computed tomography-generated images, bone mechanical properties can be evaluated with finite element analysis (FEA), and trabecular rod-plate configuration related to trabecular bone strength can be quantified by structure model index (SMI). This study aimed to compare trabecular configuration and bone mechanical properties between AIS and the controls. 95 AIS girls aged 12–14 years and 97 age- and gender-matched normal controls were recruited. Bilateral femoral necks and non-dominant distal radius were scanned by dual-energy X-ray absorptiometry for areal BMD and HR-pQCT for SMI and FEA, respectively. Subjects were further classified into osteopenic and non-osteopenic group based on their areal BMD. Bone mechanical properties (stiffness, failure load and apparent modulus) were calculated using FEA. Linear regression model was used for controlling age, physical activity and calcium intake. AIS was associated with lower failure load and apparent modulus after adjusting for age, whereas AIS was associated with lower apparent modulus after adjusting for all confounders. Osteopenic AIS was associated with more rod-like trabeculae when compared with non-osteopenic AIS, whereas no difference was detected between osteopenic and non-osteopenic controls. This might be the result of abnormal regulation and modulation of bone metabolism and bone modelling and remodelling in AIS which will warrant future studies with a longitudinal design to determine the significance of micro-architectural abnormalities in AIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescent idiopathic scoliosis (AIS) is a complex 3-dimensional (3D) spinal deformity with unknown aetiology. It affects predominantly children aged 10–16 years old with a prevalence of 1–3 % [1]. AIS was associated with increased risk of osteopenia [2, 3]. In previous studies, osteopenia was found in up to 38 % in AIS girls with reference to a normative dataset of local ethnic girls [4–6] and it could persist in 86 % of subjects even after skeletal maturity [6]. Hung et al. reported that low bone mineral density (BMD) was one of the independent prognostic factors for curve progression in AIS [4].
Areal BMD (aBMD) measured by dual-energy X-ray absorptiometry (DXA) is currently the gold standard for the diagnosis of osteoporosis. The two-dimensional (2D) projectional images obtained from DXA provide limited insight into the compartmental 3D micro-structure of the bone, which is critical in the understanding of osteoporosis [7]. Based on conventional peripheral quantitative computed tomography (pQCT), high-resolution pQCT (HR-pQCT) has been developed as an advanced non-invasive modality capable of providing not only compartmental volumetric BMD (vBMD) but also micro-structural features of cortical and trabecular bone at peripheral skeleton with an isotropic resolution of 82 µm [8, 9]. Using HR-pQCT, we have previously reported in a case–control study that AIS patients have poorer bone quality at the distal radius after adjusted for age, physical activity level and dietary calcium intake, manifested as significantly lower cortical vBMD, lower trabecular number and greater trabecular separation [10]. Such observations are more pronounced in osteopenic AIS compared with non-osteopenic AIS [11].
Trabecular compartment consists of mixed plate and rod structures. Apart from trabecular number and separation, the rod-plate configuration contributes significantly to the ultimate strength of trabecular bone. Plate-like trabeculae can withstand higher load and deform in higher strain, whereas rod-like trabeculae deform at the beginning of compression [12]. With the high-resolution images provided by HR-pQCT, the rod-plate configuration of trabeculae can be quantified by structure model index (SMI). SMI lies between 0 and 3, with 0 representing an ideal plate-like structure and 3 representing an ideal rod-like structure. Moreover, the image dataset obtained from HR-pQCT can be subjected to micro-finite element (µFE) analyses to estimate the biomechanical properties of bone, including stiffness, failure load and apparent modulus. These bone biomechanical properties have shown to be associated with risk of fracture in postmenopausal women independent of aBMD [13].
Our previous report has shown the difference in primary HR-pQCT parameters between AIS and controls [10, 11]. To further evaluate the differences in bone biomechanical properties, this case–control study was carried out with the objectives of using HR-pQCT to characterize the rod-plate configuration of trabecular bone using SMI and the bone biomechanical properties of distal radius using µFE analysis in AIS girls and to compare them with normal healthy controls. Regression models were used to control confounding from age, physical activity level and dietary calcium intake. As reported in our previous paper, variation in profiles of primary HR-pQCT parameters from non-osteopenia to osteopenia is different between AIS and controls [11]. We, therefore, further investigated these features by subdividing the cohort into osteopenic and non-osteopenic group.
Materials and Methods
Subjects
In this case–control study, 95 AIS girls between 12 and 14 years of age were recruited. All AIS girls had maximum Cobb angle greater than 10 degrees without prior treatment with bracing. 97 age-matched healthy girls were recruited randomly from local secondary schools as the control group. All healthy controls were assessed by an experienced orthopaedic surgeon to rule out any spinal deformity. Subjects who had any disorders affecting bone metabolism, such as, congenital deformities, neuromuscular diseases, genetic diseases, chromosomal defects, autoimmune disorders, endocrine disturbances or medical conditions that affect bone metabolism, were excluded from this study. For both the AIS and control group, subjects with a previous history of fracture were excluded. All subjects and their parents provided written informed consent. The study was approved by The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee (CREC-2009.020).
Anthropometry, Pubertal Assessment and Curve Severity
Anthropometry assessment included body weight, standing height, sitting height and arm span. Body weight was measured by an electric balance and subjects were instructed to wear light clothes. Standing height without shoes was measured by a wall-mounted stadiometer. Sitting height was recorded with subjects on a Harpenden sitting height table. Arm span was measured with arms fully stretched horizontally [14]. Due to the spinal deformity, the standing height cannot reflect the true body height for AIS subjects [15]. Body mass index (BMI) was first calculated as body weight (kg)/standing height2 (m2) and then as body weight (kg)/arm span2 (m2). Age of menarche corrected to the nearest month and Tanner staging for the assessment of pubertal maturity were reported by each subject. For AIS subjects, standard standing anteroposterior radiograph of the whole spine was taken for the assessment of severity of the curvature using the Cobb method. In case of more than one curve, Cobb angle of the largest curve was recorded.
Physical Activity Level and Dietary Calcium Intake
The Chinese version of the Modified Baecke Questionnaire as adapted from Pols et al. [16] and validated by Lau with pregnant women in Hong Kong [17] was used to assess the physical activity level for the past 12 months. Total score was calculated from the indices of work, sport and leisure to represent the overall physical activity level of subjects. Modified food frequency questionnaire (FFQ) was used to determine the dietary calcium intake. The FFQ is based on data obtained from Hong Kong Adult Dietary Survey in 1995 and had been validated with the basal metabolic rate calculation and the 24-h sodium/creatinine and potassium/creatinine analysis [18, 19]. Subjects were asked to provide details on usual consumption and frequency in the past 12 months from the food list. Dietary nutrient intake was calculated by the Food Processor Nutrition Analysis and Fitness software version 7.9 (Esha Research, Salem, OR, USA), with addition of composition of some local foods based on food composition table from China (Institute of Nutrition and Food Safety, 2002) [20].
aBMD Measurement
aBMD of bilateral femoral neck (g/cm2) was measured by DXA (DXA, XR-46; Norland Medical Systems, Fort Atkinson, Wisconsin, USA). The detail of DXA measurement was presented in our previous study [5, 21]. Short-term precision error of aBMD of femoral neck expressed as coefficient of variation was 1.5 % [4]. Z-score was calculated with reference to a normative dataset of local ethnic Chinese girls. Subjects were classified as osteopenic if Z-score ≤ −1 and subjects with Z-score > −1 were classified as normal [11]. As reported previously, aBMD of the spine as measured by DXA should not be used in AIS because of the bias with axial vertebral rotation that is always present in AIS [22], hence spine was not measured with DXA in this study.
SMI and Micro-FE Analysis
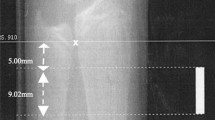
The distal radius of non-dominant forearm of subjects was scanned using HR-pQCT (XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland). The X-ray tube potential is 60 kVp and size of matrix is 1536 × 1536. The voxel size is 82 × 82 × 82 µm3. A dorsal-palmar projection image was obtained to define the region of interest. Scans started 5 mm proximal from the reference line, which was placed manually at the most proximal limit of the inner aspect of the epiphyseal growth plate of the radius, and spanned proximally for 9.02 mm, equivalent to 110 slices [10]. Measurements were repeated in case of severe motion artefact. A Gaussian filter was used to remove the noise signal and a threshold-based algorithm was used to separate the bone from background and cortical from trabecular bone. The threshold was set to one-third of apparent cortical bone density [23, 24].
SMI was used to quantify the trabecular bone into “rod-like” or “plate-like” by calculating the means of three-dimensional image under a differential analysis of the triangulated bone surface [25]. The equation of SMI is 12 (ε + ε 2)/[1 + 4 (ε + ε 2)], where ε is the rod-to-plate volume ratio. SMI lies between 0 and 3. Smaller SMI indicates more plate-like trabecular structure whereas greater SMI indicates more rod-like trabecular structure.
The subject-specific micro-FE model of the bone was converted from the images. The model contained eight-node brick elements with element size of 82 × 82 × 82 µm3. It is assumed that bone tissue is an isotropic and linear material with Young’s modulus as 10 GPa and a Poisson’s ratio as 0.3 [26]. In this linear finite element model, uniaxial compression test with 1 % strain along the axial direction was performed using software provided by manufacturer (µFE Element Analysis Solver v.1.15; Scanco Medical, Switzerland) [26]. Stiffness, failure load and apparent modulus were calculated in simulation. Failure load was determined when 1 mm3 elements in the model had an effective strain greater than 7000 microstrain [27].
Statistical Analysis
Statistical analyses were performed using the SPSS statistic software (version 20; SPSS Inc., Chicago, IL, USA). Data were first tested for normality. Normally distributed data were expressed as mean ± SD and compared between two groups (AIS and control groups) using Student’s t test. Skewed data were expressed as median and inter-quartile range and compared between two groups using Mann–Whitney U test. In order to adjust for potential confounders, multivariate linear regression analysis was used to test the difference in SMI and biomechanical properties between AIS subjects and controls. Age was adjusted in regression model 1, while age, calcium intake and total score of physical activity level were adjusted in regression model 2. Body weight was not included in the model as distal radius is a non-weight-bearing bone, its collinearity with age may inflate Type II error and that mechanical loading has been partially accounted for by inserting the physical activity level [10]. Subjects were further classified into osteopenic and non-osteopenic groups. One-way ANOVA with Bonferroni adjustment was performed to compare the subgroups (osteopenic AIS, non-osteopenic AIS, osteopenic controls and non-osteopenic controls). Two-way ANOVA was performed to investigate the interaction between groups (AIS vs. control) and the presence of osteopenia (osteopenic vs. non-osteopenic). An interactive p value < 0.05 by two-way ANOVA indicated that the difference between osteopenic AIS subject and osteopenic control differed significantly from that between non-osteopenic AIS subject and non-osteopenic control. All analyses were two-tailed and p value <0.05 was considered statistically significant.
Results
Characteristics of the Study Cohort
Demographic, anthropometric characteristics and aBMD of the study cohort are shown in Table 1. There was no significant difference between AIS subjects and controls in age, Tanner stage, standing height, sitting height and arm span. Compared with the controls, AIS subjects had significantly later onset of pubertal development, lower body weight and lower BMI. The mean ± SD of Cobb angle for AIS subjects was 21.2° ± 5.9°. Physical activities and nutrient levels were comparable between AIS subjects and the controls except that AIS subjects had significantly lower sport index. aBMD of bilateral femoral necks was on average −4.29 to −5.29 % significantly lower in AIS subjects than the controls. Z-scores were also lower in AIS subjects with significant difference in left femoral neck.
27 (28.4 %) AIS subjects and 18 (18.6 %) controls had osteopenia (Table 2). Cobb angle did not differ between osteopenic and non-osteopenic AIS subjects. In either AIS subjects or the controls, subjects with osteopenia had significantly later onset of menarche, lower Tanner stage, body weight, standing height, sitting height, arm span, BMI, aBMD and Z-scores compared with their counterparts (all p < 0.05 or p < 0.01).
SMI and Biomechanical Properties
Table 3 and Fig. 1 show the comparisons in SMI and biomechanical properties between AIS subjects and the controls. SMI was on average 3.98 % higher in AIS subjects than in controls but this difference did not reach significant level (p = 0.073). Stiffness, failure load and apparent modulus were on average 7.19 to 9.09 % significantly lower in AIS subjects (p = 0.035, 0.018 and 0.005, respectively).
Table 4 shows the results from the multivariate linear regression analyses. SMI and stiffness did not differ between AIS subjects and controls after adjusted for age (model 1) or after adjusted for age, level of physical activity and calcium intake (model 2). Between-group difference in failure load remained significant after adjusted for age (p = 0.042) but became insignificant after adjusted for age, level of physical activity and calcium intake (p = 0.072). Apparent modulus remained significantly lower in AIS subjects in both regression models (p = 0.013 and 0.024). The adjusted means and standard error of means on SMI and biomechanical properties for both AIS subjects and controls in model 1 and 2 are depicted in Table 5.
Subgroup Analysis by the Presence of Osteopenia
18 (18.6 %) of controls had osteopenia. According to the normal distribution, 16 % of subjects in the general population will have Z-score ≤ −1 [28]. This proportion was similar to that observed among the recruited controls who were, therefore, regarded as being representative of the healthy population. Table 6 shows the results of subgroup analysis by one-way ANOVA. In either AIS subjects or the controls, SMI was larger and stiffness, failure load and apparent modulus were significantly lower in subjects with osteopenia than in those with normal aBMD. The majority of these differences were significant except the difference in SMI between osteopenic and non-osteopenic controls (2.04 vs. 1.92, p = 0.636) indicating the variation in SMI, a secondary HR-pQCT parameter, from non-osteopenia to osteopenia was different between AIS and controls. There was no significant difference in SMI or biomechanical properties between osteopenic AIS subjects and osteopenic controls or between non-osteopenic AIS subjects and non-osteopenic controls (all p > 0.05). In two-way ANOVA, all interactive p values are >0.05, suggesting that the difference between osteopenic AIS subjects and osteopenic controls (SMI: 7.21 %, biomechanical properties: −4.98 to −0.57 %) did not differ from that between non-osteopenic AIS subjects and non-osteopenic controls (SMI: 1.71 %, biomechanical properties: −7.85 to −5.88 %).
Discussion
In this case–control study, we investigated the bone strength and trabecular rod-plate configuration in AIS. Bone strength was evaluated with FEA presented as bone mechanical properties including stiffness, failure load and apparent modulus. Higher bone mechanical properties mean the bone is stronger and more difficult to be deformed and broken. Trabecular rod-plate configuration was calculated by SMI [25]. Greater value means more rod-like trabeculae are present in trabecular compartment. Higher SMI was found in AIS which indicates that a relatively fewer plate-like trabeculae were found in AIS as compared with controls. Previous studies indicated that plate-like trabeculae can withstand larger force and only deform in the higher strain compared with rod-like trabeculae which is thinner or smaller [12, 29]. There is strong evidence that AIS was associated with lower mechanical properties. Carbonare and Giannini stated that cortical width is one of the main structural parameters for determining bone strength [30]. Putman et al. found that higher estimated bone strength is associated with favourable values for a variety of cortical and trabecular bone densitometric and micro-architectural indices even after adjusting for clinical covariates and aBMD [31]. The findings from this study are in compliance with our previous study that AIS was associated with lower cortical area, cortical vBMD and fewer trabeculae [10].
As noted in our previous report [10], puberty is associated with higher bone turnover [32]. Maturity is an important confounding factor for bone density, bone quality and bone strength [33, 34]. Results of this study were further analysed with adjustment of age using regression analysis (model 1). After adjusted for age, AIS was associated with lower failure load and lower apparent modulus. This indicates a risk of compromised attainment of peak bone mass in AIS subjects, which could be translated into an increased risk of bone fragility in these subjects in their adulthood and late life [6]. Improving the bone quality and bone structure for the better bone strength during pubertal growth is essential for AIS.
Calcium supplement and life style with sufficient physical activity are important determinants of bone quality [35, 36]. Subjects in this study were evaluated for their dietary calcium intake and physical activity level in the past 12 months using validated questionnaires [16, 18, 19]. The study by Lee et al. found that low bone mass in AIS was associated with inadequate weight-bearing physical activity level and calcium intake [21]. In our result, calcium intake and total score for physical activity level were numerically lower in AIS than in controls although the difference did not reach statistical significance. On the other hand, our AIS subjects did have statistically significant lower sport index in the physical activity level when compared with controls. Previous studies have mentioned physical activity and calcium intake had confounding effect on bone parameters including cortical area and total volumetric BMD [10]. Dietary calcium intake and physical activity level were included in the regression model for control of their confounding in addition to age (i.e. model 2). After adjusting for age, calcium intake and physical activity level, AIS remained associated with lower apparent modulus, suggesting that common risk factors may only partly explain low bone mass and poor bone quality in AIS. There may be an intertwined relationship between the disease course of AIS and the disturbed bone metabolism. Meanwhile, the difference in failure load between AIS and controls disappeared after including physical activity and calcium intake in the regression model. It reflects that those interventions can improve bone strength in AIS. Zhang et al. mentioned that bone mineral accretion in girl hip can be maximized by taking 1000 mg or more calcium daily [37]. But the efficacy of calcium supplement for AIS is unknown. Lam et al. also suggested that vibration therapy can improve the aBMD in osteopenic AIS, who were classified by their femoral neck aBMD [38]. Specker and Binkley conducted a randomized trial of physical activity and calcium supplementation in young children and found that calcium could modify the bone response to physical activity [39]. Further longitudinal or interventional study should be warranted to investigate the optimization of calcium intake and physical activity which can improve the bone strength and bone mass in AIS.
Low bone mass in AIS is multifactorial [21, 40–43]. Previous study found that osteopenia in AIS differed from osteopenia in normal controls with considering bone geometry and trabecular micro-architecture [11]. Our result also indicated that AIS subjects may have poorer trabecular micro-architecture, manifested as more rod-like trabeculae. This is supported by the findings that there was a trend towards greater SMI value in AIS subjects compared with healthy controls and the SMI value was the highest in AIS subjects with osteopenia. On the other hand, osteopenic AIS had the numerically lowest bone mechanical properties compared with controls and non-osteopenic AIS. The aetiopathogenesis of AIS is still unknown. A more severe form of the disease may imply a more severely perturbed bone metabolism, bone modelling or remodelling, leading to a more severe micro-architectural abnormality of the bone, as demonstrated in our study, a more rod-like trabecular structure conferring poorer mechanical properties. It is also possible that poor bone biomechanical properties might contribute to the curve progression.
Although AIS is a spinal deformity and we only compared their mechanical properties of distal radius instead of that of spine, Liu et al. suggested that HR-pQCT mechanical properties of the distal radius can reflect the mechanical competence of the central skeleton [44]. In our findings, poor bone mechanical properties of distal radius were found in both osteopenic subjects who were classified by their femoral neck BMD, which can further extend that low bone mass in AIS is a systemic disorder [41]. Therefore, we believe that distal radius findings may reflect similar changes in spine thus shedding lights on aetiopathogenesis of AIS from deranged bone quality and mechanical properties.
Our study had several limitations. Firstly, HR-pQCT was only performed at the distal radius but not at the distal tibia. Secondly, bone turnover markers which could potentially provide valuable information on bone metabolism were not assayed in this study. Thirdly, the physical activity level and dietary calcium intake analysed in this study were evaluated with questionnaires which might be subject to recall bias. In particular, the Modified Baecke Questionnaire could be more valid in men than in women and did not take into account the type of physical activity related to high ground reaction force sport which could have significant impacts on bone metabolism. Hence the data from lifestyle questionnaires on dietary intake and physical activity should be interpreted with caution. In addition, this is a case–control study with a cross-sectional design using threshold-based algorithm for differentiating cortical from trabecular bone and that the FEA analysis was based on algorithm adopted for the HR-pQCT machine used in this study. Currey et al. found that the bone mechanical properties are highly correlated with its material composition and structure [45]. Assigning different mineralizations with different mechanical properties in non-linear FEA may provide more insight on bone mechanical properties. Despite these limitations, results on the association between AIS and lower stiffness, lower failure load, lower apparent modulus and that osteopenia in AIS was characterized by higher SMI justify further prospective studies that longitudinally follow subjects preferably with physical activity evaluated with an accelerometer on a daily basis; with bone turnover markers assayed; and with multi-site HR-pQCT evaluation to be enhanced with advanced non-linear FEA and segmentation image analysis [12].
Conclusion
This study showed that as compared with the controls, AIS girls were associated with deranged trabecular micro-architecture and lower bone biomechanical properties. After adjusting for age, physical activity level and dietary calcium intake, apparent modulus remained significantly lower in AIS as compared with controls. These results suggest the presence of abnormal regulation and modulation of bone metabolism as well as bone modelling and remodelling in AIS. To address the potential correlation between curve severity and disease progression with severity of osteopenia or trabecular micro-architecture, further longitudinal studies are warranted to determine the significance of such micro-architectural abnormalities in AIS on curve progression during the course of the disease and the outcome for these patients with therapeutic interventions targeted for such.
References
Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. Lancet 371:1527–1537
Lonstein J (1995) Idiopathic scoliosis. Saunders, Philadelphia
Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, Burwell RG, Cheng JC (2011) Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop 31:S14–27
Hung VW, Qin L, Cheung CS, Lam TP, Ng BK, Tse YK, Guo X, Lee KM, Cheng JC (2005) Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 87:2709–2716
Cheng JC, Guo X (1997) Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine 22:1716–1721
Cheng JC, Hung VW, Lee WT, Yeung HY, Lam TP, Ng BK, Guo X, Qin L (2006) Persistent osteopenia in adolescent idiopathic scoliosis–longitudinal monitoring of bone mineral density until skeletal maturity. Stud Health Technol Inform 123:47–51
Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ 3rd, Riggs BL, Amin S, Muller R, Khosla S (2009) Bone structure at the distal radius during adolescent growth. J Bone Miner Res 24:1033–1042
Dambacher M, Neff M, Radspieler H, Rüegsegger P, Qin L (2007) In-vivo bone mineral density and structures in humans: from isotom over densiscan to xtreme-CT. In: Qin L, Genant H, Griffith J, Leung K (eds) Advanced bioimaging technologies in assessment of the quality of bone and scaffold materials. Springer, Berlin, Heidelberg, pp 65–78
Qin L, Choy W-Y, Hung VWY, Au S-K, Chan K-M, Leung K-S, Cheung W-H, Lam T-P, Cheng JCY (2014) Age-related vessel calcification at distal extremities is a risk factor of osteoporosis. J Orthop Transl 2:43–48
Yu WS, Chan KY, Yu FW, Ng BK, Lee KM, Qin L, Lam TP, Cheng JC (2014) Bone structural and mechanical indices in Adolescent Idiopathic Scoliosis evaluated by high-resolution peripheral quantitative computed tomography (HR-pQCT). Bone 61:109–115
Yu WS, Chan KY, Yu FW, Yeung HY, Ng BK, Lee KM, Lam TP, Cheng JC (2013) Abnormal bone quality versus low bone mineral density in adolescent idiopathic scoliosis: a case-control study with in vivo high-resolution peripheral quantitative computed tomography. Spine J 13:1493–1499
Liu XS, Bevill G, Keaveny TM, Sajda P, Guo XE (2009) Micromechanical analyses of vertebral trabecular bone based on individual trabeculae segmentation of plates and rods. J Biomech 42:249–256
Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD (2008) Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399
Yim AP, Yeung HY, Hung VW, Lee KM, Lam TP, Ng BK, Qiu Y, Cheng JC (2012) Abnormal skeletal growth patterns in adolescent idiopathic scoliosis—a longitudinal study until skeletal maturity. Spine 37:E1148–1154
Cheng JC, Leung SS, Lau J (1996) Anthropometric measurements and body proportions among Chinese children. Clin Orthop Relat Res 323:22–30
Pols MA, Peeters PH, Bueno-De-Mesquita HB, Ocke MC, Wentink CA, Kemper HC, Collette HJ (1995) Validity and repeatability of a modified Baecke questionnaire on physical activity. Int J Epidemiol 24:381–388
Lau J (2005) The Knowledge and practice of physical activities amongst the pregnant women in Hong Kong. The Chinese University of Hong Kong, Hong Kong
Leung S, Ho S, Woo J, Lam T, Janus E (1997) The Hong Kong Adult Dietary Survey 1995–1996. Chinese University of Hong Kong, Hong Kong
Woo J, Leung SSF, Ho SC, Lam TH, Janus ED (1997) A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr Res 17:1633–1641
Yang Y (2002) China food composition table. Beijing Medical University Press, Beijing
Lee WT, Cheung CS, Tse YK, Guo X, Qin L, Ho SC, Lau J, Cheng JC (2005) Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int 16:1024–1035
Cheng JC, Sher HL, Guo X, Hung VW, Cheung AY (2001) The effect of vertebral rotation of the lumbar spine on dual energy X-ray absorptiometry measurements: observational study. Hong Kong Med J 7:241–245
Kim S, Macdonald HM, Nettlefold L, McKay HA (2013) A comparison of bone quality at the distal radius between Asian and white adolescents and young adults: an HR-pQCT study. J Bone Miner Res 28:2035–2042
Laib A, Hauselmann HJ, Ruegsegger P (1998) In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6:329–337
Hildebrand T, Ruegsegger P (1997) Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Eng 1:15–23
Hansen S, Brixen K, Gravholt CH (2012) Compromised trabecular microarchitecture and lower finite element estimates of radius and tibia bone strength in adults with turner syndrome: a cross-sectional study using high-resolution-pQCT. J Bone Miner Res 27:1794–1803
Mueller TL, Christen D, Sandercott S, Boyd SK, van Rietbergen B, Eckstein F, Lochmuller EM, Muller R, van Lenthe GH (2011) Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone 48:1232–1238
Daniel WW (2005) Probability Distributions. Biostatistics—a foundation for analysis in the health sciences. John Wiley & Sons Inc, Hoboken, pp 87–128
Brandi ML (2009) Microarchitecture, the key to bone quality. Rheumatology 48:3–8
Dalle Carbonare L, Giannini S (2004) Bone microarchitecture as an important determinant of bone strength. J Endocrinol Invest 27:99–105
Putman MS, Yu EW, Lee H, Neer RM, Schindler E, Taylor AP, Cheston E, Bouxsein ML, Finkelstein JS (2013) Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res 28:2177–2185
van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA (2002) Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol 57:107–116
Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E (2010) Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res 25:1521–1526
Magarey AM, Boulton TJ, Chatterton BE, Schultz C, Nordin BE, Cockington RA (1999) Bone growth from 11 to 17 years: relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr 88:139–146
Nguyen TV, Center JR, Eisman JA (2000) Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res 15:322–331
Ondrak KS, Morgan DW (2007) Physical activity, calcium intake and bone health in children and adolescents. Sports Med 37:587–600
Zhang ZQ, Ma XM, Huang ZW, Yang XG, Chen YM, Su YX (2014) Effects of milk salt supplementation on bone mineral gain in pubertal Chinese adolescents: a 2-year randomized, double-blind, controlled, dose-response trial. Bone 65:69–76
Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC (2013) Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int 24:1623–1636
Specker B, Binkley T (2003) Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res 18:885–892
Cheung CS, Lee WT, Tse YK, Lee KM, Guo X, Qin L, Cheng JC (2006) Generalized osteopenia in adolescent idiopathic scoliosis–association with abnormal pubertal growth, bone turnover, and calcium intake? Spine 31:330–338
Li XF, Li H, Liu ZD, Dai LY (2008) Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J 17:1431–1440
Nguyen TV, Howard GM, Kelly PJ, Eisman JA (1998) Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol 147:3–16
Sadat-Ali M, Al-Othman A, Bubshait D, Al-Dakheel D (2008) Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J 17:944–947
Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, Kokolus SL, McMahon DJ, Lappe JM, Recker RR, Lang T, Guo XE (2010) Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25:2229–2238
Currey JD (2002) Bones: structure and mechanics. Princeton University Press, Princeton
Acknowledgments
This study was supported by Research Grants Council of the Hong Kong S.A.R., China (Project no: 468809 and 468411).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed and written consent was obtained from parents or guardians of all participating children included in the study.
Rights and permissions
About this article
Cite this article
Cheuk, K.Y., Zhu, T.Y., Yu, F.W.P. et al. Abnormal Bone Mechanical and Structural Properties in Adolescent Idiopathic Scoliosis: A Study with Finite Element Analysis and Structural Model Index. Calcif Tissue Int 97, 343–352 (2015). https://doi.org/10.1007/s00223-015-0025-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0025-2