Abstract
Due to the increasing survival of thalassemic patients, osteopathy is a mounting clinical problem. Low bone mass alone cannot account for the high fracture risk described; impaired bone quality has been speculated but so far it cannot be demonstrated noninvasively. We studied bone quality in thalassemia major using trabecular bone score (TBS), a novel texture measurement extracted from spine dual-energy X-ray absorptiometry (DXA), proposed in postmenopausal and secondary osteoporosis as an indirect index of microarchitecture. TBS was evaluated in 124 adult thalassemics (age range 19–56 years), followed-up with optimal transfusional and therapeutical regimens, and in 65 non-thalassemic patients (22–52 years) undergoing DXA for different bone diseases. TBS was lower in thalassemic patients (1.04 ± 0.12 [range 0.80–1.30]) versus controls (1.34 ± 0.11 [1.06–1.52]) (p < 0.001), and correlated with BMD. TBS and BMD values correlated with age, indicating that thalassemia negatively affects both bone quality and quantity, especially as the patient gets older. TBS was 1.02 ± 0.11 [0.80–1.28] in the osteoporotic thalassemic patients, 1.08 ± 0.12 [0.82–1.30] in the osteopenic ones and 1.15 ± 0.10 [0.96–1.26] in those with normal BMD. No gender differences were found (males: 1.02 ± 0.13 [0.80–1.30], females 1.05 ± 0.11 [0.80–1.30]), nor between patients with and without endocrine–metabolic disorders affecting bone metabolism. Our findings from a large population with thalassemia major show that TBS is a valuable tool to assess noninvasively bone quality, and it may be related to fragility fracture risk in thalassemic osteopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conventional transfusional protocols and optimal chelation, with new oral chelating drugs, have produced a significant change in the clinical features of thalassemia syndromes. The survival expectation in thalassemia major patients has significantly increased, but the longer life itself allowed the appearance of complications closely linked to the chronic course of the disease. Among them, osteopathy or osteoporosis–osteopenia syndrome (OSS), characterized by bone mass reduction and alteration of bone microarchitecture, is very common and has a prominent impact on life quality linked to the high risk of fragility fractures [1, 2]. Bone mineral density estimate by dual-energy X-ray absorptiometry (DXA) is currently considered the gold standard to define bone involvement, and osteopathy severity in thalassemic patients is usually stated by interpreting DXA results similar to post-menopausal population.
However, several studies showed limitations of DXA in thalassemia [3], and it provides no information about bone architecture, a well-recognized contributing factor to bone strength, independent of bone mass. It is well known that fractures are associated with microarchitecture damage besides quantitative alteration in several secondary osteoporosis conditions [4]; therefore, the evaluation of microarchitecture seems to be necessary to get adequate information about bone health.
Trabecular bone score (TBS) is a gray-level texture measurement based on the use of experimental variograms of 2D projection images acquired during a DXA lumbar spine scan. Although not a direct measurement, it has been demonstrated to be strongly correlated with bone microarchitecture, regardless of BMD [5], and several studies documented the added value of TBS in bone mineral densitometry for fracture risk assessment in postmenopausal osteoporosis [6]. So, this textural index has been suggested for routine clinical evaluation of bone micro-architecture in addition to BMD, to subcategorize the patients according to their level of fracture risk, as those with low BMD and low TBS seem to present more likely fractures than patients with low BMD but high TBS [7–9].
TBS values in thalassemia have never been reported; we hypothesized that TBS can be a useful tool to evaluate bone quality also in this disease. Therefore, we assessed TBS in a large group of adult patients with thalassemia major, and compared them with the values obtained in a control group of non-thalassemic patients with osteoporosis. We investigated the correlations between bone condition, expressed by densitometric quantitative indices and by TBS as a qualitative index, and disease-related physiological and pathological factors, including iron overload, pre-transfusional hemoglobin levels, bone metabolism markers, and endocrine complications.
Materials and Methods
Patients
In a cross-sectional study, we evaluated the densitometric measures as well as the clinical, hematological, and endocrinological data in 124 adult patients with thalassemia major. These were extracted at the corresponding time from the clinical charts of the patients, followed-up at our single tertiary outpatient clinic in Milan. Approval of the ethical committee of our institution (number 2013-841) was obtained.
The periodic routine checks for these patients include an annual endocrinological evaluation (clinical examination and tests for thyroid, parathyroid, adrenal, gonadic, pancreatic function, bone metabolism indices), while bone densitometry of the femur and lumbar spine is performed every 2 years with DXA. TBS was calculated from DXA routinely performed.
In addition to the above data, the collected information included age, gender, body mass index (BMI), history of fractures, auxologic and sexual development, splenectomy, liver function and disease, drugs, starting age of transfusion regimen, mean values of pre-transfusional hemoglobin, and ferritin in the previous year. The compliance to chelation was estimated on the basis of mean ferritin levels evaluated every 3 months and liver iron content (LIC) by MRI when available.
All the enrolled hypogonadal patients were on hormonal replacement therapy with appropriate sex steroids and the hypothyroid patients were on replacement with levothyroxine. The hypoparathyroid patients were on treatment with calcitriol and calcium supplements at the doses maintaining serum calcium levels within the normal range or at lower normal limit.
Controls
In the control group, 65 non-thalassemic females were enrolled among the patients referred to the Bone Metabolic Unit of our Hospital to perform DXA for the following clinical reasons: postmenopausal decrease in bone mineral density (osteoporosis 29 pts, 44.6 %, osteopenia 2 pts, 3.1 %), early menopause (14 pts, 21.5 %), family history of osteoporosis (11 pts, 16.9 %) or fractures (2 pts, 3.1 %), and secondary osteoporosis due to antiestrogenic treatment (7 pts, 10.8 %).
Methods
Blood cell count was performed by the Coulter Counter. Automated routine procedures were used for liver function tests and assays of calcium and phosphorus in serum (normal values, n.v.: 2.1–2.5 and 0.8–1.4 mmol/L, respectively) and 24-h urine (n.v.: 2.5–7.5 and 12.9–42 mmol/day, respectively), intact parathyroid hormone (n.v.: 1.5–6.6 pmol/L), osteocalcin (n.v.: females 5.3–23.6, mcg/L; males, 4.4–26.1), 25-hydroxy vitamin D (n.v.: >72 nmol/L), ferritin (n.v.: 30–400 mcg/L), glucose (n.v. 70–110 mg %), insulin (n.v. 2.6–25 mU/L), and TSH (ECLIA, n.v. 0.3–4.2 mU/L). Serum albumin concentrations were normal in all the patients, therefore not corrected serum calcium levels were considered.
Alkaline phosphatase was measured by the International Federation of Clinical Chemistry liquid (n.v.: 40–129 U/L); the bone isoenzyme was assessed by semiquantitative electrophoretic method using a scanning densitometer (normal values, 20–75 %). Serum CTX was assayed by the Serum CrossLaps One Step ELISA (IDS, Boldon, Tyne and Wear, UK; n.v.: women, postmenopausal, 0.142–1.351; premenopausal, 0.112–0.73; males 0.115–0.748 ng/mL).
Iron overload was estimated by LIC derived from T2* according to Wood et al. [10], MRI was performed at CMR Unit Department of Cardiology “A. De Gasperis” at Niguarda Ca’ Granda Hospital in Milan), using a 1.5 T MR scanner (Avanto Siemens, Erlangen). All T2* images were analyzed using post-processing software (CMR Tools, Imperial College, London).
Iron overload was estimated by LIC as measured by MRI, performed at CMR Unit Department of Cardiology “A. De Gasperis” at Niguarda Ca’ Granda Hospital in Milan, using a 1.5 Tesla MR scanner (Avanto Siemens, Erlangen). All T2* images were analyzed using post-processing software (CMR Tools, Imperial College, London).
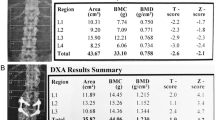
Bone mineral density was measured by dual X-ray photon absorptiometry (Hologic Bone Densitometer, QDR Discovery A, Version 12.7.3.1), and BMD values were expressed as T score and Z score; the former was calculated as SDs from the normal reference population database, the latter as SDs from age and sex matched population. According to the WHO report (WHO Technical Report, ISCD Official Position Paper 2007), the data were classified as follows: normal: T score > −1; low bone density (osteopenia): −1 > T score > −2.5; osteoporosis: T score < −2.5.
Lumbar spine TBS was derived for each spine DXA examination blinded to clinical parameters and outcomes using the TBS iNsight® software (medimaps SASU, Merignac, France).
Statistical Analysis
The data were statistically analyzed using “SPSS®” programs (version 20.0). Due to the small number of subjects with normal bone mass, the three groups of patients with normal bone mineral density, osteopenia, and osteoporosis were compared for continuous variables using the Kruskal–Wallis and Mann–Whitney non-parametric tests, for dichotomic variables using the χ 2 test. Pearson correlation index was calculated between TBS scores and the other studied variables. A multivariate analysis (MANOVA) was performed to evaluate the effects of age, gender, hematologic, and endocrine parameters on TBS values. A p value <0.05 was considered statistically significant.
Results
Demographic Data
124 adult Caucasian patients homozygous or double heterozygous for severe beta-thalassemia mutations were enrolled (51 males, 73 females, mean age 36.1 ± 7 years, range 19–56). All the patients (pts) received regular blood transfusions every 20–25 days, to maintain a pre-transfusional hemoglobin around 9–9.5 g/dL according to the international guidelines [9].
Seventy-nine pts (63.7 %) were taking cholecalciferol supplements for vitamin D deficiency, producing 25OH-vitamin D serum concentrations between 20 and 30 ng/mL; 21 pts (16.9 %) were also on treatment with a bisphosphonate (alendronate). There were 87 (70.2 %) hypogonadic pts, all replaced with adequate sex steroid treatment.
The hematologic variables in the whole series of thalassemic patients were as follows: pre-transfusional Hb (9.50 ± 0.6 4, range 8.00–11.4 g/dL), ferritin (1,168.58 ± 1,137.54, range 104–5,886 mcg/L), fibroscan (8.03 ± 3.72, range 3.90–19.80 kPa), T2* heart (32.47 ± 11.91, range 5.18–57.68 ms) and liver (9.08 ± 6.80, range 0.96–34.45 ms), and LIC (5.42 ± 4.81, range 0.94–26.66 mg Fe/g of liver dry weight).
Bone mineralization of the pts at DXA was as follows: 26 pts (21 %) osteoporosis, 90 pts (72.6 %) osteopenia, and 8 pts (6.4 %) normal; 18 pts (14.5 %) had previous bone fractures, but only 5 could be labeled as fragility fractures.
No significant differences were found between osteoporotic, osteopenic pts, and pts with normal bone mineral density for the hematologic parameters, age (36.31 ± 6.86 vs 34.81 ± 7.98 years, respectively), BMI (22.70 ± 3.45 vs 23.66 ± 3.16), nor prevalence of endocrine–metabolic disorders related to bone mineralization: diabetes (13.3 vs 19.2 %), hypothyroidism (24.4 vs 34.6 %), hypogonadism (71.1 vs 61.5 %), and hypercalciuria (54.3 vs 38.1 %). Conversely, the two groups differed for prevalence of hypoparathyroidism (8.9 vs 34.6 %, p = 0.003).
The control group included 65 non-thalassemic, untreated females; BMI was 23.88 ± 5.33, mean age 45.71 ± 6.20 years (range 22–52 years), so significantly higher than in the thalassemic group (p < 0.001). On the basis of DXA, bone mineralization state was as follows: osteoporosis in 27 pts (41.5 %), osteopenia in 20 pts (30.8 %), and normal bone mass in 18 pts (27.7 %).
Bone Quantity Evaluation (DXA)
Significant differences emerged in bone mass measurements between the thalassemic population and controls. The results of DXA assessment in the two groups are shown in Table 1.
No gender differences were found. When considering the endocrine and metabolic disorders affecting bone condition, the presence of hypoparathyroidism appeared to influence significantly all DXA measures, especially at the vertebral site (Table 2). Hypercalciuria was correlated with a reduction of bone mineral density at all sites, though statistical significance was reached only at the vertebral site (T score −2.63 ± 0.97 in hypercalciuric pts vs −2.12 ± 1.00 in normocalciuric pts, p = 0.011; Z score −2.55 ± 0.98 vs −2.04 ± 0.99, p = 0.009).
Bone Quality Evaluation (TBS)
The mean value of TBS in the thalassemic population was 1.04 ± 0.12 (range 0.80–1.30); no differences were found between genders (1.02 ± 0.13 [range 0.80–1.30] in males vs 1.05 ± 0.11 [range 0.80–1.30] in females). TBS was significantly higher in the control group: 1.34 ± 0.11 (range 1.06–1.52), p < 0.001; see Fig. 1.
When the thalassemic pts were divided on the basis of bone mineral density at DXA, the TBS values were 1.02 ± 0.11 [range 0.80–1.28] in osteoporotic pts, 1.08 ± 0.12 [range 0.82–1.30] in osteopenic pts, and 1.15 ± 0.10 [range 0.96–1.26] in patients with normal bone mineral density. TBS was significantly different in osteoporotic pts versus the osteopenic (p = 0.026) as well as the normal mineral density patients (p = 0.002), while the difference was not significant between the osteopenic patients and those with normal bone mineral density.
Table 3 shows the TBS values in thalassemic patients with different bone mass, divided in three percentile groups.
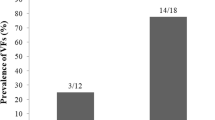
No significant differences were found in TBS values between the groups of patients with and without fragility fractures (1.067 ± 0.12 vs 1.034 ± 0.11, respectively, p = 0.2); moreover, no correlation was found between TBS and previous fracture events.
When the TBS scores were compared in the groups of patients with and without endocrine–metabolic disorders affecting bone metabolism—hypoparathyroidism, hypercalciuria, and hypogonadism—none of them influenced significantly TBS values (p = ns).
The correlations of TBS with the DXA indices, the demographic parameters, and the biochemical variables expressing bone mineral metabolism were studied: among all the analyzed variables (age, gender, hematologic and endocrine variables) only age (p = 0.007) and bone mineralization status (normal, osteopenia, osteoporosis; p < 0.001) reached the statistical significance. More precisely, no correlation was found between TBS value and PTH, calcium, and 25OH vitamin D serum levels. Figure 2 shows the correlation between TBS and BMD at the vertebral site.
Discussion
Our study provides the first noninvasive evaluation of bone quality in a large group of adult patients with thalassemia major. Thalassemia major is a rare condition, but it somehow represents a “model” disease due to the impressive array of complications and to the fast change of clinical picture associated to recent therapeutical advancements. Osteoporosis has emerged as a major health concern in thalassemic patients, especially with aging [2]; it currently represents a clinical challenge that likely will aggravate along with patient longer survival.
TBS is a gray-level texture measurement extracted from DXA images and well correlated with the three-dimensional bone microarchitecture; TBS evaluation could represent a useful adjunct to areal BMD measured by DXA, which is currently considered the gold standard for thalassemic osteopathy [3, 11] in spite of well-recognized limitations. First, densitometric values are considerably overlapping in thalassemic patients with and without fragility fractures, and despite the low BMD values commonly found, fracture prevalence actually seems to be lower than expected from densitometric assessment. This finding might be explained as DXA measures areal BMD without taking into account changes in the third dimension, so that BMD could be underestimated in smaller bones; this could represent a confounding factor in thalassemic patients, whose spine is frequently shorter than in non-thalassemic individuals [12]. Furthermore, BMD measurement loses precision in older patients with associated degenerative spinal bone changes. To overcome the drawbacks of DXA, high-resolution quantitative computed tomography (QTC) was proposed as a more valid tool to determine bone strength, providing a volumetric measure and allowing the separate determination of cortical and trabecular bone mineral density. Interestingly, BMD values are higher when measured by QCT compared with DXA; this discrepancy could hypothetically be ascribed to the different involvement of cortical and trabecular bone [3, 11]. However, this methodic has reduced reliability in inadequately chelated thalassemic patients, as local iron deposition may increase the X-ray attenuation values in trabecular bone; moreover, it appears impractical for routine clinical management, due to the much higher radiation exposition and the limited availability [11].
From all the above observations and clinical evidence, some authors [3] hypothesized that BMD is only one of the determinants of skeletal strength, and other factors, not adequately considered by densitometry and included in the concept of “bone quality,” may play a crucial role. Scientific evidence shows that microarchitecture deterioration together with macrogeometry of cortical bone and bone turnover represent prominent contributing features, independent of BMD. These features are not assessed by DXA [13, 14].
Bone quality could not be widely estimated so far, as bone biopsy was the only mean for providing detailed and reliable information on bone microarchitecture, and obviously the role of invasive methods is strictly limited in clinical practice [1, 15, 16]. The interest of TBS resides in allowing a noninvasive evaluation of bone texture (related to microarchitecture); so, it promises to be an innovative tool for the assessment of fracture risk in diseases impairing bone quality. In addition, TBS value can be calculated retrospectively from previously performed DXA, without further imaging [9].
The present study shows that both BMD and TBS are significantly lower in the thalassemic patients, directly demonstrating the assumption that both bone quantity and bone quality are negatively affected by this disease [2, 13, 14, 17–19].
In the thalassemic studied patients, TBS was correlated with BMD, and both were correlated with age; therefore, bone quality appears to worsen as the patient gets older, with a trend similar to bone quantity. This behavior is in agreement with that reported in postmenopausal women by Leslie et al. [20]. Dividing the TBS values in percentiles, it emerged that even the highest values, found in thalassemic patients with normal BMD, were below the cut-off of normality for postmenopausal women (i.e., 1.350) [16, 21]. This finding seems to indicate that trabecular microstructure is abnormal even in the thalassemic patients who, on the basis of DXA estimate, would not be considered osteopenic.
Bone quality expressed by TBS appeared not to be influenced by hypoparathyroidism. Conversely, BMD was more severely reduced in patients with normal parathyroid function than in hypoparathyroid ones, confirming the previously described protective role of hypoparathyroidism against bone demineralization [13, 14]. Indeed, vitamin D is used as replacement therapy for parathyroid defect, and it can normalize serum calcium concentrations and favor calcium integration into bone matrix, but it does not replace the physiological action of PTH on the osteoclasts. It follows that bone resorption processes are reduced in hypoparathyroid thalassemic patients, but probably bone quality does not change.
Most of our patients were hypogonadic, but no correlation was found between BMD nor TBS values and gonadic function. In view of the commonly recognized role of sex-steroid hormones in maintaining both bone quantity and bone quality, we believe that this finding is consistent with an early and adequate sex-steroid replacement.
Besides the original data, obtained using an innovative approach to bone disease, a noticeable strength of the study is the large size of the cohort, for a rare disease as thalassemia major. In addition, it is a homogeneous group followed-up at a single tertiary outpatient clinic with optimal transfusional and therapeutical regimens, including correction of vitamin D deficiency and endocrine defects.
Limitations of the Study
One limitation of the present study was the lack of a healthy control group matched for age and gender. The choice of controls was highly problematic, as it appeared unethical to perform a radiological test in young patients without a clinical indication. Therefore, we chose non-thalassemic patients undergoing DXA for bone disease different from thalassemia; their mean age was a little higher than that of thalassemic patients.
Another limitation of the study is the cross-sectional design, as well as the fact that in the medical history of our 124 thalassemic patients there were only five fragility fractures throughout life. We could not identify distinctive features of the patients with fractures on the basis of DXA nor TBS, and it seems likely that a higher number of events or a longitudinal study including fracture events appearing over an adequate observation time would be more appropriated to demonstrate any statistical correlation.
Conclusions
The evaluation of TBS in a large group of adult thalassemic patients confirmed with a noninvasive approach the impairment of bone microstructure demonstrated so far only by bone biopsy. It has been proven that trabecular texture assessment by TBS is a useful adjunctive tool in defining fracture risk in patients with secondary osteoporosis characterized by impairment of bone quality [21, 22]. An interesting use of TBS in adult thalassemic patients could be risk fracture stratification, as well as identification of those who most likely would benefit from treatment, with significant savings of resources.
Further studies are needed to confirm our experience and hopefully to develop clinical recommendations for the use of TBS in this disease.
References
Chatterjee R, Shah FT, Davis BA, Byers M, Sooranna D, Bajoria R, Pringle J, Porter JB (2012) Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to pamidronate in beta-thalassaemia presenting with osteopenia–osteoporosis syndrome. Br J Haematol 159:462–471
Haidar R, Musallam KM, Taher AT (2011) Bone disease and skeletal complications in patients with beta thalassemia major. Bone 48:425–432
Mylona M, Leotsinides M, Alexandrides T, Zoumbos N, Dimopoulos PA (2005) Comparison of DXA, QCT and trabecular structure in beta-thalassaemia. Eur J Haematol 74:430–437
Ulivieri FM, Silva BC, Sardanelli F, Hans D, Bilezikian JP, Caudarella R (2014) Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine (in press)
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
Rabier B, Heraud A, Grand-Lenoir C, Winzenrieth R, Hans D (2010) A multicentre, retrospective case–control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): analysing the odds of vertebral fracture. Bone 46:176–181
Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D (2009) Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case–control study. J Clin Densitom 12:170–176
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42:775–787
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a non-invasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Wood JC, Enriquez C, Ghugre N, Tyzka JM et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle-cells disease patients. Blood 106:1460–1465
Angastiniotis M, Eleftheriou A (2008) Thalassaemic bone disease. An overview. Pediatr Endocrinol Rev 6(Suppl 1):73–80
Toumba M, Skordis N (2010) Osteoporosis syndrome in thalassaemia major: an overview. J Osteoporos 2010:537673
Baldini M, Forti S, Orsatti A, Ulivieri FM, Airaghi L, Zanaboni L, Cappellini MD (2011) Bone disease in adult patients with beta-thalassaemia major: a case–control study. Intern Emerg Med 9:59–63
Baldini M, Forti S, Marcon A, Ulivieri FM, Orsatti A, Tampieri B, Airaghi L, Zanaboni L, Cappellini MD (2010) Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann Hematol 89:1207–1213
Chatterjee R, Katz M, Bajoria R (2011) Use of hormone replacement therapy for correction of high turnover bone disease in hypogonadal beta-thalassemia major patients presenting with osteoporosis: comparison with idiopathic premature ovarian failure. Hemoglobin 35:653–658
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769
Origa R, Fiumana E, Gamberini MR, Armari S, Mottes M, Sangalli A, Paglietti E, Galanello R, Borgna-Pignatti C (2005) Osteoporosis in beta-thalassemia: clinical and genetic aspects. Ann N Y Acad Sci 1054:451–456
Gaudio A, Morabito N, Xourafa A, Macri I, Meo A, Morgante S, Trifiletti A, Lasco A, Frisina N (2008) Bisphosphonates in the treatment of thalassemia-associated osteoporosis. J Endocrinol Invest 31:181–184
Skordis N, Toumba M (2011) Bone disease in thalassaemia major: recent advances in pathogenesis and clinical aspects. Pediatr Endocrinol Rev 8(Suppl 2):300–306
Leslie WD, Krieg MA, Hans D, Manitoba Bone Density Program (2013) Clinical factors associated with trabecular bone score. J Clin Densitom 16:374–379
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Breban S, Briot K, Kolta S, Paternotte S, Ghazi M, Fechtenbaum J, Roux C (2012) Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J Clin Densitom 15:260–266
Conflict of Interest
Didier Hans is co-owner of the TBS patent and has corresponding ownership shares in Medimaps group. Marina Baldini, Fabio Massimo Ulivieri, Stella Forti, Serena Serafino, Sonia Seghezzi, Alessia Marcon, Federico Giarda, Carmelo Messina, Elena Cassinerio, Bérengère Aubry-Rozier, and Maria Domenica Cappellini declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study (retrospective) formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldini, M., Ulivieri, F.M., Forti, S. et al. Spine Bone Texture Assessed by Trabecular Bone Score (TBS) to Evaluate Bone Health in Thalassemia Major. Calcif Tissue Int 95, 540–546 (2014). https://doi.org/10.1007/s00223-014-9919-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9919-7