Abstract
Low-energy laser irradiation (LELI) accelerates wound healing and is thought to accelerate bone formation. However, the mechanism of laser healing is not clear. To clarify the biological mechanism of LELI healing, we investigated the effects of LELI on rat osteoblasts in vitro. Osteoblastic cells from 3-day-old Wistar rat calvaria were irradiated using a low-energy gallium-aluminum-arsenide (Ga-Al-As) diode laser. Bone formation, osteoblast differentiation, and cell proliferation were evaluated by von Kossa staining, reverse-transcription polymerase chain reaction, alkaline phosphatase (ALP) staining, 5-bromo-2′-deoxyuridine (BrdU) uptake, and fluorescence-activated cell sorter (FACS) analysis. At 21 days after LELI, the greatest bone formation was observed with irradiation energy of 3.75 J/cm2 and the first week after seeding. LELI (3.75 J/cm2) induced an increased number of cells at day 3. LELI-stimulated differentiation in osteoblastic cells was demonstrated by the increases of Runx2 expression and ALP-positive colonies. By contrast, at 1 day after laser irradiation, the number of cells in the irradiation group was significantly lower than that in the control group. BrdU uptake indicated lower proliferation 12 and 24 hours after irradiation compared with the control. Furthermore, FACS data demonstrated a higher proportion of cells in the G2/M phase of the cell cycle 12 hours after irradiation compared with the control. G2/M arrest was confirmed by the appearance of G2/M arrest marker 14-3-3-σ or phospho-p53. These results demonstrate that LELI induces not only acceleration of bone formation but also initial G2/M arrest, which may cause wound healing like tissue repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When laser irradiation was invented in 1960, its application to medical treatment was for light coagulation treatment of retinodialysis by ruby laser [1]. Subsequently, CO2, argon, or Nd:YAG lasers were used for surgical treatment. Previous investigators have reported various biostimulatory effects of laser treatment, such as pain relief [2], effect on chondral proliferation [3], regeneration of severed nerves [4–6], anti-inflammation [7], wound healing [8–11], and collagen synthesis [12–16]. In general, high-energy lasers are used for surgical treatment and low-energy lasers are used for biostimulation.
Some in vivo studies of low-energy laser irradiation (LELI) use in bone regeneration have demonstrated accelerated regeneration in tibia fractures in mice and rats [17, 18], in mid-palatal suture expansion in rats [19], and during tooth movement in rats [20]. The stimulatory effect of LELI on bone regeneration in vitro has also been reported. Gallium-aluminum-arsenide (Ga-Al-As) laser irradiation caused a significant increase in the total area of bone nodules in a dose-dependent manner [21–23], and Ozawa et al. [23] demonstrated increased alkaline phosphatase (ALP) activity and osteocalcin expression following LELI in the early stages of cell culture.
Though these findings indicated that LELI effects are seen mainly at early stages of cell proliferation or differentiation, only a few studies have focused on the effects of LELI on cell proliferation, especially the cell cycle. LELI has been reported to promote cell cycle entry of skeletal muscle cells [24] and induce the mitogenic potential of lymphocytes [25]. Regarding the effects of LELI on osteoblastic cells, only Yamamoto et al. [26] demonstrated stimulating effects of LELI on expression of the minichromosome maintenance (MCM) 3 gene, which has been identified as a license factor involved in the initiation of replication. On the contrary, Coombe et al. [27] reported that cellular proliferation or activation of osteoblastic cells was not significantly affected by LELI. Therefore, precise investigations are needed to clarify the effects of LELI on the cell cycle of osteoblastic cells.
In the present study, we examined the effects of LELI on the proliferation and cell cycle of primary cultured rat osteoblastic cells to find the optimal condition of LELI for the bone formation of primary rat osteoblastic cells. The aim of this study was to investigate the effects of LELI on cellular proliferation and the cell cycle of primary osteoblastic cells under optimal conditions of LELI.
Materials and Methods
Cell Isolation and Primary Culture of Osteogenic Cells
Rat calvarial cells were isolated essentially as described by Bellows et al. [28]. Calvaria were dissected aseptically from 3-day-old Wistar rats, and loosely adherent soft connective tissues were removed. Calvaria were minced and digested in a collagenase-containing enzyme mixture at 37°C for 10, 20, 30, 40, or 50 minutes, yielding populations I through V as previously described [28]. Cells retrieved from each step of the digestion sequence were plated in 60 mm culture dishes in α-minimal essential medium (α-MEM) containing 10% heat-inactivated fetal bovine serum (FBS; Wako Pure Chemical Industries, Osaka, Japan) and antibiotics (100 μg/mL penicillin G [Wako], 50 μg/mL gentamicin [GIBCO, Grand Island, NY], and 0.3 μg/mL fungizone [GIBCO]). After 1 week, the cultures were washed with phosphate-buffered saline (PBS) to remove nonviable cells and other debris, then incubated with 0.25% trypsin in PBS and counted using a hemocytometer. Cells from populations II through V were pooled, resuspended in α-MEM containing 10% FBS and antibiotics, and plated in 24-well plates at 1 × 104/well. The culture medium was replaced after 24 hours with the same medium supplemented with 50 mg/mL ascorbic acid (Wako), 10 nM dexamethasone (Wako), and 10 mM β-glycerophosphate (Nacalai Tesque, Kyoto, Japan), which is optimal for the formation of mineralized osteoid nodules. At 21 days, the cultures were processed for bone nodule staining.
Laser Irradiation
A low-power Ga-Al-As diode laser apparatus (Trinple-D; Yoshida, Tokyo, Japan) with a wavelength of 905 nm and energy density of 1.25 J/cm2 (150 seconds) was used in this study. The tip of the hand piece was placed 10 mm above the basal surface of 24-well plates [21], and LELI was performed in a tissue culture hood. The 24-well plates were placed on a thermal insulation plate (Cherry Shoji, Tokyo, Japan), and the temperature of the culture fluid was maintained at 37°C.
To establish the optimal time to irradiate, the culture period was divided into three phases: the proliferation phase in the first week after cell plating, matrix maturation phase in the second week, and mineralization phase in the third week, as described by Stein et al. [29]. Laser irradiation was performed at 3.75 J/cm2 (450 seconds) every day, once per day, during each period.
Furthermore, to establish the optimal irradiation dose, we irradiated cells at 1.25, 3.75, and 6.25 J/cm2 (150, 450, and 750 seconds, respectively) at the optimal irradiation period, as determined above.
To investigate cell proliferation, 5-bromo-2′-deoxyuridine (BrdU) uptake, and cell cycle, rat calvarial cells were cultured for 1 day after cell seeding. Then, cells were irradiated once and cultured additionally for up to 6 days.
Quantification of Bone Nodules
After 21-day cell culture, specimens were stained using the von Kossa technique to detect the mineral in bone nodules. In brief, specimens were washed in PBS three times, fixed with 3.7% formaldehyde in PBS for 10 minutes, washed in distilled water three times, and then incubated in 0.5% silver nitrate (Wako) for 1 hour. They were then washed in distilled water three times, incubated in 0.3% sodium thiosulfate pentahydrate (Nacalai Tesque) for 3 minutes, washed in distilled water, and dried. The stained mineralized bone nodules were placed under a charge-coupled device camera and the images transferred to a computer-assisted image analysis system (MCID; Interfocus Ltd., Linton, England).
Reverse Transcription-Polymerase Chain Reaction Analysis
To examine the effects of LELI on the differentiation of rat osteoblastic cells, levels of Runx2 expression were determined by reverse transcription-polymerase chain reaction (RT-PCR). After the single LELI, at 0, 12, and 24 hours of culture, total RNAs were extracted using the Total RNA Extraction Miniprep System (Viogene, Sunnyvale, CA), according to the manufacturer’s protocol. To reduce DNA contamination, the RNA samples were treated with RNase-free DNaseI (Takara Bio, Shiga, Japan) for 3 hours at 37°C; cDNA was synthesized from total RNA in 30 μL of a reaction buffer composed of 500 μM deoxynucleotide triphosphates, 20 U ribonuclease inhibitor (Promega, Madison, WI), and 200 U Superscript II reverse transcriptase (Invitrogen Life Technology, Carlsbad, CA). The reaction was carried out at 70°C for 7 minutes, then at 45°C for 60 minutes, followed by 10 minutes at 70°C, with final cooling to 4°C. The primers used to amplify Runx2 were 5′-GCTCCGGAATGCCTCTGCTGTTAT-3′ and 5′-CACCT GCCTGGCTCTTCTTACTGA-3′ [30]. The primers for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal control were 5′-TGAAGGTCGGTGTCAACGGATTTG GC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′. Each cycle consisted of denaturation (Runx2, 94°C for 1 minute; GAPDH, 95°C for 1 minute), annealing (Runx2, 52°C for 1 minute; GAPDH, 65°C for 1 minute), and 72°C for 40 cycles for Runx2 and 38 cycles for GAPDH, with a final extension step (72°C for 9 minutes). To confirm its reproducibility, we replicated the RT-PCR experiment three or more times. Each PCR mixture consisted of cDNA (reverse-transcribed RNA), Taq polymerase buffer, and 1 μL each of the sense and antisense primers, for a total volume of 20 μL. The PCR products were electrophoresed in 2% agarose gels and visualized with ethidium bromide. The data were analyzed using NIH Image (National Institutes of Health, Bethesda, MD).
The oligonucleotide RT-PCR primer sequences were designed and their specificity was confirmed using a BLAST-assisted Internet search of nonredundant nucleotide sequence databases (National Library of Medicine, Bethesda, MD).
ALP Staining
To examine the effects of LELI on the differentiation of rat osteoblastic cells, ALP staining was performed in 1-, 3-, and 6-day cultures of calvarial cells on 24-well plates. In brief, the specimens were washed in PBS three times, fixed with 3.7% formaldehyde in PBS for 10 minutes, and washed in distilled water three times. Then, Tris-HCl buffer (0.1 M, pH 8.2, 7.8 mL) containing naphthol AS-BI phosphate (Wako, 2 mg), dimethyl formamide (Sigma, St. Louis, MO; 200 μL), 0.1 M levamisole (Sigma, 1 mL), and fast red violet (Sigma, 10 mg) were added; and the cells were incubated for 15 minutes in shaded conditions. The area of stained ALP-positive nodules was measured by computer-assisted image analysis (MCID).
Cell Proliferation
At 1, 3, and 6 days after the single LELI, cultures were washed with PBS three times and incubated with 0.25% trypsin (GIBCO) in PBS at 37°C for 5 minutes, and the number of cells was counted using a hemocytometer.
BrdU Uptake
BrdU uptake was determined by applying the methods of Boulton and Marshall [31] and Shiraiwa et al. [32]. Circular cover glasses (12 mm) were cleaned ultrasonically, air-dried, sterilized by exposure to ultraviolet light for 24 hours, and placed on the bottom of each well. After the single LELI, at 6, 12, 24, 36, 48, and 60 hours of culture, proliferating cells were detected using cell proliferation kits (Amersham Pharmacia Biotech, Little Chalfont, UK). Cultured cells were systematically exposed to 1 μM BrdU in the culture medium. Following a 2-hour incubation at 37°C, the specimens were washed twice with 0.1 M PBS, fixed in 90% ethanol for 10 minutes, and washed in PBS. The fixed cells were incubated with anti-BrdU monoclonal antibody (1:100 dilution) for 1 hour at room temperature, washed in PBS, and incubated with fluorescein-conjugated goat anti-mouse immunoglobulin G (IgG, 1:40 dilution) for 1 hour. To confirm the presence of cells, the specimens were washed three times with cytoskeleton-stabilizing (CS) buffer and incubated for 30 minutes at 4°C with tetramethyl rhodamine isothiocyanate (TRITC) phalloidin (Molecular Probes, Eugene, OR; 1:40 dilution). After three additional washes in 0.1 M PBS, the cells were enclosed with glycerol-PBS solution containing 1,4-diazabicyclo [2.2.2] octane (Sigma, 100 mg/mL) to prevent fluorescence decay. The samples were examined using a fluorescence microscope (Olympus Optical, Tokyo, Japan), and the proliferation rate (BrdU-positive cells per phalloidin-positive cells) was calculated in 10 randomly selected fields of each of the two samples.
Cell Cycle Analysis
Cell cycle analysis was carried out using the modified method of Sato et al. [33]. The cells were suspended in a hypotonic solution (0.1% Triton X-100 [Research Organics, Cleveland, OH], 1 mM Tris-HCl [pH 8.0], 3.4 mM sodium citrate, 0.1 mM ethylenediaminetetraacetic acid [EDTA]), stained with 5 μg propidium iodide/mL (Sigma), and analyzed with a fluorescence-activated cell sorter (FACS; Becton Dickinson Immunocytometry Systems, San Jose, CA). The population of cells in each cell cycle phase (G1, G2/M, or S phase) was determined using a computer-assisted image analysis system (ModFit LT, Becton Dickinson Immunocytometry Systems). Some cells were treated for 1 hour with 10 ng/mL doxorubicin hydrochloride (Adriamycin, Wako) to pharmacologically induce G2/M arrest. After 1-hour incubation, the cells were washed with PBS and re-fed with fresh medium.
Furthermore, to confirm that cells were in G2/M arrest, the appearance of two G2/M arrest markers, 14-3-3-σ and phospho-p53, was examined immunocytochemically. Briefly, the specimens were washed twice with 0.1 M PBS, fixed in 90% ethanol for 10 minutes, and washed in PBS. The fixed cells were incubated with anti-14-3-3-σ goat polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-phospho-p53 goat polyclonal antibody (1:100 dilution, Santa Cruz Biotechnology) for 1 hour at room temperature, washed in PBS, and incubated with fluorescein-conjugated sheep anti-goat IgG (1:40 dilution) for 1 hour. The samples were examined using a fluorescence microscope, as mentioned above.
Statistical Analysis
The data were analyzed using StatView (Abacus Concepts, Berkeley, CA). One-way analysis of variance was used to evaluate the influence of laser irradiation on bone formation, osteoblast differentiation, and cell number. Significant differences were examined by post-hoc Scheffé tests, and unpaired t-tests were used to compare proliferation rates.
Results
Optimal Conditions for Laser Irradiation
Osteoblastic cells isolated from rat calvaria in medium containing 10% FCS, dexamethasone, ascorbic acid, and β-glycerophosphate formed many mineralized bone nodules on 24-well plates by day 21 (Fig. 1a, b). These were recognized as dark brown spots using von Kossa staining under normal light.
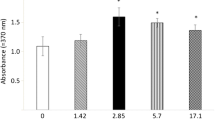
The effects of laser irradiation on bone nodule formation by rat calvarial osteoblasts. (a, b) Photographs of bone nodules produced by rat osteoblasts cultured for 21 days without laser irradiation (a) and with 3.75 J/cm2 laser irradiation in the first week of cell culture (b). (c, d) The effects of laser irradiation on bone nodule formation by primary osteoblastic cells cultured for 21 days. The bone nodule areas formed by osteoblasts irradiated with 3.75 J/cm2 every day during the first, second, or third week (c) and with 1.25, 3.75, or 6.25 J/cm2 every day only in the first week after subculture (d). The average control bone nodule area is equivalent to 100. Data are means ± standard error of the mean of five specimens. *Statistically significant differences from control value (P < 0.01).
To establish the optimal time for LELI, cultured osteoblastic cells were irradiated every day in the first, second, or third week and maintained for 21 days. Following laser irradiation (3.75 J/cm2) in the first week, the total bone nodule area was significantly greater than the control value. In contrast, no significant increase in bone nodule area was observed in specimens that were irradiated in the second or third week compared with controls (Fig. 1c).
When osteoblastic cells were irradiated for 1.25, 3.75, or 6.25 J/cm2 every day in the first week after subculture and maintained for 21 days, it was observed that the total area of bone nodule was significantly stimulated by 3.75 J/cm2 laser irradiation compared with control (Fig. 1d). However, no significant increase in the bone nodule area was observed with 1.25 or 6.25 J/cm2 laser irradiation.
Effects of LELI on Osteoblastic Cell Differentiation
To examine the effects of LELI on the differentiation of osteoblastic cells, cells were irradiated once for 3.75 J/cm2 at 1 day after subculture and the expression of Runx2 was detected at 0, 12, and 24 hours after LELI. RT-PCR for Runx2 generated two products. Sequencing confirmed that the smaller product was generated from amplification of an alternatively spliced Runx2 transcript [30]. The relative density of Runx2 expression was greater with LELI than without LELI at both 12 and 24 hours after laser irradiation (Fig. 2a). We then examined the effects of laser irradiation on the initial differentiation of osteoblastic cells by measuring ALP activity. Laser irradiation (3.75 J/cm2) was performed at 1 day after subculture (day 0). To evaluate ALP activity, we measured the number of ALP-positive colonies. No significant difference in the number of ALP-positive colonies was observed at day 1 or 3 (data not shown) compared with control. In the 6-day culture, there was a significant increase in ALP-positive colonies following 1.25 or 3.75 J/cm2 laser irradiation compared with the control culture, whereas no significant increase in the number of ALP-positive colonies was observed following 6.25 J/cm2 laser irradiation (Fig. 2b).
The effects of laser irradiation on cell differentiation. (a) RT-PCR analysis of Runx2 mRNA with or without LELI. Total RNAs were harvested at 0, 12, and 24 hr after 3.75 J/cm2 LELI. MW, molecular weight markers. RT-PCR for Runx2 generated two products. The smaller product (420 bp) was generated from amplification of an alternatively spliced Runx2 transcript. Runx2 590 /GAPDH and Runx2 420 /GAPDH indicate the relative density of Runx2 (590, 420 bp) expression. Similar results were obtained in three independent experiments. (b) Effects of laser irradiation on the number of ALP-positive colonies at 6 days after laser irradiation. The cells were irradiated at 1 day after subculture (day 0), with fixation and ALP staining at 1, 3, and 6 days of culture. In 1- and 3-day culture, there were no ALP-positive cells (data not shown). *Statistically significant differences (P < 0.01).
Precursor Cell Proliferation
To examine the effects of LELI on the proliferation of osteoblastic cells, cells were irradiated once for 1.25-6.25 J/cm2 at 1 day after subculture (day 0) and counted on days 1, 3, and 6 (Fig. 3). Following 3.75 J/cm2 of LELI, the number of cells was initially lower at day 1 and then significantly greater at day 3 compared to control. Following 1.25 or 6.25 J/cm2 LELI, the number of cells did not differ significantly from control values during the research period.
We then evaluated BrdU uptake to determine cell proliferation (Fig. 4). Without laser irradiation, cells gradually increased BrdU uptake up to 12 hours (36 hours after plating), then decreased uptake by 36 hours. There was no initial increase in proliferation in cells that were laser-irradiated (3.75 J/cm2), but a proliferation rate of 25–45% was maintained. Therefore, although the initial proliferation rate was greater for control cells than for laser-irradiated cells, a higher proliferation rate was seen in irradiated cells at 36 hours and after (Fig. 4d).
The effects of laser irradiation on the proliferation of rat calvarial cells. (a–f) Fluorescence micrographs stained to identify cells using TRITC phalloidin (a, d) and proliferating cells using an anti-BrdU antibody (b, e) and a merged image (c, f) at 12 hours after laser irradiation (a–c) and control (d–f). Scale bar = 30 μm. (g) The proliferation rate of cells (BrdU-positive cells per phalloidin-positive cells) in the control and 3.75 J/cm2 irradiated groups at 6, 12, 24, 36, 48, and 60 hours after laser irradiation.
Cell Cycle Analysis
FACS analysis with propidium iodide staining demonstrated the phase of the cells in the cell cycle [33]. At 24 and 36 hours (data not shown) after laser LELI (3.75 J/cm2), FACS analysis showed no difference in the number of G2/M phase cells (fluorescence intensity >140) between laser-irradiated and control cells. However, at 12 hours, more laser-irradiated than control cells were in G2/M phase (Fig. 5b). For the positive control, doxorubicin also induced cells in G2/M phase (Fig. 5c). The percentage of cells in G2/M phase was significantly greater by treatments with LELI or doxorubicin than control values (Fig. 5d).
FACS analysis of the cell cycle on osteoblastic cells. (a) Control. (b) At 12 hours after LELI (3.75 J/cm2). (c) At 12 hours after 1-hour doxorubicin (Adraimycin) treatment (10 ng/mL). (d) The population of cells in G2/M phase (fluorescence intensity >140) from 10 samples in each group was determined using an image-analyzing system. *Statistically significant differences from control value (P < 0.01).
Immuocytochemical appearances of 14-3-3-σ and phospho-p53 were observed at 12 hours after LELI (Fig. 6a–h). The percentages of 14-3-3-σ-positive cells were 5.3% and 8.9% by LELI and doxorubicin treatment, respectively (Fig. 6i). The percentage of phospho-p53-positive cells was 5.0% and 7.1% by LELI and doxorubicin treatment, respectively (Fig. 6j). These results confirmed that LELI and doxorubicin treatment induced G2/M phase.
The effects of laser irradiation and doxorubicin treatment on the appearance of G2/M arrest markers 14-3-3-σ; and phospho-p53. The presence of cells was indicated by actin staining with TRITC-conjugated phalloidin (a, c, e, g). Localization of 14-3-3-σ; (b, d) and phospho-p53 (f, h) at the same area of (a, c) and (e, g), respectively. Cells were at 12 hours after LELI (a, b, e, f) and without LELI (c, d, g, h). Scale bar = 20 μm. The rate of 14-3-3-σ; (i) and phospho-p53 (j) was calculated in 10 randomly selected fields for each condition.
Discussion
Although various studies of the healing effect of LELI have been reported, its precise mechanism remains controversial. In this study, we demonstrated that the effects of LELI (3.75 J/cm2) are not only to accelerate cell proliferation but also to induce temporal G2/M arrest.
It is important for the clinical application of LELI that the stage of osteoblastic bone formation that is most appropriate for treatment and the most effective dose for bone formation are known. In this study, LELI (3.75 J/cm2) was effective only in the initial phase of osteoblastic bone formation. By the second and third weeks, which correspond to the matrix maturation phase and mineralization phase, respectively, of osteoblastic bone formation [29], LELI failed to stimulate bone nodule formation. These results are consistent with results reported by Ozawa et al. [23], who demonstrated that laser irradiation at an early stage of culture stimulates a greater number of bone nodules and a larger area of bone nodule formation by rat calvarial osteoblasts. The study suggested that 3.82 J/cm2 laser irradiation is optimal for the stimulation of bone formation by cultured osteoblastic cells. In our study, as in that previous study [23], 3.75 J/cm2 laser irradiation caused the greatest stimulation of bone nodule formation. We also found that neither lower (1.25 J/cm2) nor higher (6.25 J/cm2) laser irradiation stimulated bone formation. These results imply that laser irradiation is effective on bone formation only if it is performed at the initial phase and with an optimal dose (3.7–3.8 J/cm2) to bone-forming cells.
To confirm the effects of LELI on the differentiation of osteoblastic cells, we performed RT-PCR and ALP staining. Though a previous study demonstrated that LELI increased osteocalcin expression [23], examining the expression of Runx2, an osteoblastic transcription factor on osteoblastic genes, is more suitable for the evaluation of the early differentiation of osteoblastic cells. In the present study, we found the relative density of Runx2 expression was stimulated by LELI. The LELI-stimulated differentiation on osteoblastic cells was confirmed by the increase of the number of ALP-positive colonies. Thus, optimal LELI is found to stimulate bone-related gene expression.
Increases in cell number following LELI suggest that osteoblastic cell proliferation is stimulated. One possible factor affecting cell proliferation is heat. However, in this study, there was no temperature change in the medium after laser irradiation (1.25–6.25 J/cm2) (data not shown). By investigating proliferation after laser irradiation, we found that it was initially inhibited and then stimulated. This proliferation pattern was confirmed by BrdU uptake; without laser irradiation, proliferation increased until 12 hours (36 hours after plating), then decreased to a lower level than that seen at time 0. In contrast, proliferation in laser-irradiated cells remained constant from time 0 to 48 hours, then slightly increased. The proliferation pattern shown by BrdU uptake was consistent with the changing cell numbers after laser irradiation. These proliferation patterns are quite similar to those seen after mechanical loading [34]. Expression of the cox-2 gene is initially inhibited and then stimulated after mechanical loading. The molecular mechanism of constant proliferation by LELI is still unclear. One possible explanation is the effects of contact inhibition; i.e., initial inhibition of cell proliferation by LELI may delay contact inhibition.
To further investigate the effects of LELI, we performed FACS analysis. We found an increased ratio of G2/M phase cells from 19.0% (control) to 31.5% (with LELI) in the cell cycle at 12 hours after laser irradiation but no significant difference at 24 and 36 hours. This result means that the effect of LELI is limited at less than 24 hours. Though we have not examined the change in duration of the cell cycle, LELI may accelerate the cell cycle after temporal inhibition. The increase of G2/M phase cells has also been reported in the process of wound healing in corneal epithelial cells [35]. These findings suggest that more cells could be in M (mitotic) phase by the action of G2/M checkpoint after 3.75 J/cm2 laser irradiation, which results in the increase of cell number after 24 hours. Furthermore, Kim et al. [36] demonstrated that cohesion, which is a conserved protein complex in cell-cycle specific DNA double-strand break repair, is induced after laser-induced DNA damage. Possibly these results imply that LELI causes light damage to DNA, then the cell cycle is suspended at the G2/M checkpoint and DNA repair occurs. These processes may cause an initial decrease of cell proliferation. On the other hand, some previous studies demonstrated activation of cell proliferation after LELI. Yamamoto et al. [26] demonstrated that LELI stimulates expression of the MCM-3 gene, which has been identified as a license factor involved in the initiation of replication. Shefer et al. [24] demonstrated that LELI promotes skeletal muscle cell proliferation by increasing expression of the antiapoptotic protein Bcl-2 and decreasing expression of the proapoptotic protein BAX. They also demonstrated the reduction of p53 and the cyclin-dependent kinase inhibitor p21. Laser-induced lymphocyte proliferation was observed throughout the 4 consecutive days post-laser irradiation [25]. In this study, though similar G2/M arrest also occurred by pharmacological treatment with doxorubicin, the increase of cell proliferation was not induced (data not shown). The difference of cell proliferation between LELI and doxorubicin treatment may be due to the differences between physical and pharmacological treatments. Taken together, these findings suggest that optimal laser irradiation may cause initial cell cycle depression and then result in increased cell proliferation, similar to mechanical loading or wound healing.
In conclusion, LELI initially depressed the cell cycle of osteoblastic cells and then maintained constant cell proliferation, whereas transcription of bone-related genes is stimulated by LELI. Alternatively, optimal LELI temporally inhibits the cell cycle but stimulates osteoblast differentiation. After temporal arrest of the cell cycle, greater cell proliferation with higher bone-related gene expression by LELI resulted in increased bone nodule formation. We found that G2/M arrest induced by LELI is one of the key phenomena for the accelerated bone formation induced by LELI.
References
Maiman TH (1960) Stimulated optical radiation in ruby. Nature 187:493–494
Walker J (1983) Relief from chronic pain by low power laser irradiation. Neurosci Lett 43:339–344
Schultz RJ, Krishnamurthy S, Thelmo W, Rodriguez J, Harvey G (1985) Effects of varying intensities of laser energy on articular cartilage. Lasers Surg Med 5:577–588
Rochkind S, Nissan M, Razon N, Schwarz M, Bartal A (1986) Electrophysical effect in He-Ne laser on normal and injured sciatic nerve in the rat. Acta Neurochir 83:125–130
Assia E, Rosner M, Belkin M, Soloman A, Schwartz M (1989) Temporal parameters of low energy laser irradiation for optical delay of posttraumatic degeneration of rat optic nerve. Brain Res 476:205–212
Anders JJ, Borke RC, Woolery SK, Von de Merwe WP (1993) Low power laser irradiation alter the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82
Honmura A, Yanase M, Obata J, Haruki E (1992) Therapeutic effect of Ga-Al-As diode laser irradiation on experimentally induced inflammation in rats. Lasers Surg Med 12:441–449
Mester E, Spiry T, Szende B, Tota JG (1971) Effects of laser rays on wound healing. Am J Surg 122:532–535
Kana JS, Hutschenreiter G, Haina D, Waidelich W (1981) Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg 116:293–296
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Conlan MJ, Rapley JW, Cobb CM (1996) Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol 23:492–496
Mester E, Jaszagi-Nagy E (1973) The effects of laser irradiation on wound healing and collagen synthesis. Stud Biophys 35:227–234
Abergel RP, Meeker CA, Lam TS, Dwyer RM, Lesavoy MA, Uitto J (1984) Control of connective tissue metabolism by laser: recent developments and future prospects. J Am Acad Dermatol 11:1142–1150
Balboni GC, Brandi ML, Zonefrati R, Repice F (1986) Effects of He-Ne/I.R. laser irradiation on two lines of normal human fibroblasts in vitro. Arch Ital Anat Embriol 91:179–188
Bosatra M, Jucci A, Olliaro P, Quacci D, Sacchi S (1984) In vivo fibroblast and dermis fibroblast activation by laser irradiation at low energy. An electron microscopic study. Dermatologica 168:157–162
Lam TS, Abergel RP, Meeker CA, Castel JC, Dwyer RM, Uitto J (1986) Laser stimulation of collagen synthesis in human skin fibroblast cultures. Lasers Life Sci 1:61–77
Trelles MA, Mayoyo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop 111:525–532
Kawasaki K, Shimizu N (2000) Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Laser Surg Med 26:282–291
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21:271–277
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stage of cell culture in rat calvarial cells. Bone 22:347–354
Shefer G, Partridge TA, Heslop L, Gross JG, Orin U, Halevy O (2002) Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci 115:1461–1469
El Batanouny M, Korraa S, Fekry O (2002) Mitogenic potential inducible by He:Ne laser in human lymphocytes in vitro. J Photochem Photobiol B 68:1–7
Yamamoto M, Tamura K, Hiratsuka K, Abiko Y (2001) Stimulation of MCM3 gene expression in osteoblast by low level laser irradiation. Lasers Med Sci 16:213–217
Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4:3–14
Bellows CG, Aubin JE, Heersche JNM, Antosz ME (1986) Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int 38:143–154
Stein GS, Lian JB, Stein JL, van Wijnen AJ, Frenkel B, Montecino M (1996) Mechanisms regulating osteoblast proliferation and differentiation. In: Bilezikian JP, Raisz LG, Rodan GA (eds), Principles of Bone Biology. Academic Press, San Diego, pp 69–86
Bertrand-Philippe M, Ruddell RG, Arthur MJ, Thomas J, Mungalsingh N, Mann DA (2004) Regulation of tissue inhibitor of metalloproteinase 1 gene transcription by RUNX1 and RUNX2. J Biol Chem 279:24530–24539
Boulton M, Marshall J (1986) He-Ne stimulation of human fibroblast proliferation and attachment in vitro. Lasers Life Sci 1:125–134
Shiraiwa M, Goto T, Yoshinari M, Koyano K, Tanaka T (2002) A study of the initial attachment and subsequent behavior of rat oral epithelial cells cultured on titanium. J Periodontol 73:852–860
Sato T, Koseki T, Yamato K, Saiki K, Konishi K, Yoshikawa M, Ishikawa I, Nishihara T (2002) p53-independent expression of p21CIP1/WAF1 in plasmacytic cells during G2 cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect Immun 70:528–534
Kawata A, Mikuni-Takagaki Y (1998) Mechanotransduction in stretched osteocytes – temporal expression of immediate early and other genes. Biochem Biophys Res Commun 246:404–408
Thompson HW, Malter JS, Steinemann TL, Beuerman RW (1991) Flow cytometry measurements of the DNA content of corneal epithelial cells during wound healing. Invest Ophthalmol Vis Sci 32:433–436
Kim JS, Krasieva TB, LaMorte V, Taylor AM, Yokomori K (2002) Specific recruitment of human cohesin to laser-induced DNA damage. J Biol Chem 277:45149–45153
Acknowledgment
We thank Dr. Tatsuji Nishihara (Department of Oral Microbiology, Kyushu Dental College) for technical advice on the FACS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuhara, E., Goto, T., Matayoshi, T. et al. Optimal Low-Energy Laser Irradiation Causes Temporal G2/M Arrest on Rat Calvarial Osteoblasts. Calcif Tissue Int 79, 443–450 (2006). https://doi.org/10.1007/s00223-006-0072-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-006-0072-9