Abstract
We studied bone mass and metabolism in 30 adult women (age 28.5 ± 1.3) with thalassemia major (TM) and evaluated whether prolonged hormone replacement therapy (HRT) was able to optimize bone accrual. TM patients had reduced bone mass, increased bone turnover and lower serum gonadotropin and estradiol levels compared with 10 normal women of similar age. A significant correlation was found between bone mass and sex hormone levels. Six TM patients with normal ovarian function had normal bone turnover markers and modestly low bone mass (lumbar spine −1.29 ± 0.31; femoral neck −0.60±0.21; Z-score). The other 24 TM women were hypogonadic and had significantly lower bone mass for age (lumbar spine −2.35 ± 0.2, femoral neck −1.83 ± 0.2) and increased bone turnover relative to eugonadal women. Of the hypogonadal patients, 13 had taken HRT since age 15 ± 1 years, but their bone mass and turnover markers were not different than untreated hypogonadal patients. In conclusion, while hypogonadism negatively affects bone mass acquisition in adult TM women, HRT at the standard replacement doses is not sufficient to secure optimal bone accrual.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reduced bone mass is common among patients with thalassemia major (TM) reaching adult age [1, 2, 3]. In young adult TM patients, the prevalence of osteoporosis and osteopenia is above 50%, and vertebral fractures have been reported in up to 20% of patients [2]. Since hypogonadism is also prevalent in adult TM patients [4], sex hormone deficiency has been proposed as a mechanism for reduced bone mass in this condition [5, 6]. However, low bone density also occurs in prepubertal TM patients [7] and it has been generally difficult to correlate low bone mass with hormonal deficiency [8]. Thus, other potential pathogenetic factors have been proposed, such as low IGF-I levels [7, 8] and polymorphism of genes related to collagen production [8]. In this study, we evaluated the effect of estrogen deficiency and prolonged hormonal replacement therapy (HRT) on bone mass and bone metabolism in adult TM women.
Materials and Methods
Experimental Subjects
Thirty women (mean age 28.5±1.3 years, range 21–41) with established TM were studied. All received blood transfusions within the first 2 years of life. Mean BMI was 23 ± 0.5. Fourteen women had spontaneous menarche at age 15.1 ± 0.3 (range 12–18 years). In 10 TM patients, menarche was induced by hormonal therapy and 6 TM patients had primary amenorrhea at the time of the study. In 24 TM patients, hypogonadism had been diagnosed during adolescence (between 15 and 18 years of age), and among these 13 were on treatment with HRT since age 15.5 ± 1 years. In 10 patients, treatment consisted of daily oral estro-progestins (20 pg ethynylestradiol, norethindrone acetate 1.0 mg) and 3 patients received 0.625 mg conjugated equine estrogens and 5 mg/day medroxyprogesterone acetate. The other 11 hypogonadic TM patients underwent HRT for only short periods of time (less than one year) during adolescence, and at the time of the study they had been off HRT for 11.6 ± 0.5 years. Six TM patients reported normal menses since menarche and 5 were on replacement treatment with thyroxin with normal TSH levels.
Controls were 10 normal female subjects of similar age and weight (age 29.5 ± 1 years, BMI 23 ± 1). In all patients and controls, informed consent was obtained before the study. The protocol was approved by the Ethics Committee of University of Palermo.
Study Design
Blood samples were obtained in the morning after overnight fasting. In HRT-treated TM patients, therapy was withdrawn at least 1 month before blood sampling for hormone assays, while the other biochemical parameters were assessed during therapy. In menstruating TM patients and controls, blood samples were drawn during the mid-follicular phase of the cycle (days 5–8). In menstruating TM patients, a blood sample was also obtained during the mid-luteal phase of the cycle (days 21–22) for measurement of progesterone.
Bone Densitometry
Bone mineral density of the lumbar spine (L1–L4) and femoral neck (FN) was determined by dual X-ray absorptiometry (DEXA, Lunar DPX-Plus). In our center, the precision of this technique is 1.83 (CV).
Biochemistry
Estradiol, LH and FSH were determined by RIA using previously described methods [9, 10]. Estradiol was measured after extraction with diethyl ether and separation by celite column partition chromatography, whereas a direct radioimmunoassay was used for LH, FSH and progesterone measurements. Intact PTH was measured by enzyme-linked immunosorbent assay (ELISA) using materials provided by Biosource, Belgium. Osteocalcin and C-Telopeptides (CTX) were measured by ELISA (Biotech A/S, Herlev, Denmark). Bone alkaline phosphatase (BAP) was measured by ELISA (Beckmann-Coulter, CA, USA). Ferritin was determined by ELISA (RAMCO, TX, USA). In all assays, the intra-assay coefficient of variation was 6% or less, and the interassay was 15% or less.
Statistical Analysis
Analysis of variance and the Mann-Whitney U test were used to compare patients and controls. Correlations were calculated by Pearson correlation index. P less than 0.05 were considered statistically significant. Normal ranges were calculated as mean ± 2 SD of the values obtained in normal controls. Results are expressed as mean ± SEM.
Results
Compared with controls, TM patients had significantly lower serum levels of LH (2.2 ± 0.46 vs. 8.9 ± 0.6 mUI/ml, P < 0.01), FSH (5 ± 1.2 vs. 8.5 ± 0.5 mUI/ml, P < 0.01) and estradiol (29 ± 7 vs. 52 ± 6 pg/ml, P < 0.05), but higher serum levels of ferritin (888 ± 119 vs. 140 ± 34 µg/L, P < 0.01). As shown in Table 1, TM patients also had significantly lower bone mass and higher BAP and CTX than control subjects, but normal PTH and osteocalcin levels. Six adult TM women (age 29 ± 2, BMI 24.2 ± 0.6) had normal ovulatory cycles, normal serum progesterone in the follicular phase (>7 ng/ml), and normal serum estradiol (48 ± 5 pg/ml), LH (8.1 ± 1 mUI/ml) and FSH (8 ± 1 mUI/ml). Two of these TM patients previously had spontaneous successful pregnancies. Thus, this subset of TM patients was defined as eugonadal. Table 2 shows the differences between eugonadal and lypogonadal TM patients.
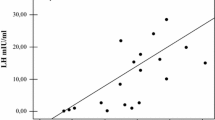
Hypogonadal adult TM patients treated with HRT had levels of estradiol, LH, FSH and ferritin similar to untreated hypogonadal adult TM patients. As shown in Table 3, there were no statistical differences in serum levels of PTH and bone markers, and bone density was only marginally lower in untreated relative to treated patients. A significant correlation was found between bone density and sex hormone levels (L1–L4 and serum estradiol: r = 0.51, P < 0.01; FN and serum estradiol r = 0.39, P < 0.05), whereas no correlation was found between bone density and serum PTH or bone markers, or between bone density and age, BMI or ferritin.
Discussion
We found that adult TM women with estrogen deficiency have a lower bone mineral density than adult eugonadal TM patients, and this reduced bone mass is associated with increased markers of bone turnover. This result corroborates previous observations in similar adult populations of patients with TM [1, 2, 3]. Though it has been suggested that hypogonadism plays a major role in determining the low bone mass of TM patients [5], a cause–effect relationship has not been unambiguously demonstrated. Many other factors may be involved including the severity of the hemoglobinopathy, and concomitant treatments that may affect bone metabolism. Gender differences in bone mass acquisition and maintenance may also contribute to the inconsistency among different studies. In this work, we selected a population of adult TM women in a small age range. In these subjects, we confirmed a very high prevalence (80%) of hypogonadotropic hypogonadism, whose pathogenesis may be related to iron deposits in the pituitary [13]. However, our TM patients with normal ovarian function had the same serum ferritin levels as the estrogen-deficient TM women. Moreover, we found a significant correlation between low bone mass and reduction of serum estradiol, adding evidence to the hypothesis that reduced bone density in TM women is mostly dependent on sex hormone deficiency.
Hypogonadism might be the main determinant of reduced bone mass of these patients, but other factors certainly play a role [8]. In fact, compared to controls of the same age and weight, lumbar spine bone mineral density was lower in our eugonadal TM patients, in agreement with findings of reduced bone mass in prepubertal TM childrens [7]. Therefore, factors related to the genetic disease, probably acting during childhood, may negatively affect achievement of peak bone mass. On the other hand, a subtle deficieny in ovarian function cannot be ruled out even in eugonadal women, as suggested by a delay in age of menarche which we observed in our patients.
We had anticipated a higher bone mass in estrogen-deficient TM patients treated with HRT. Somewhat surprising, these subjects had a reduced bone mass and increased bone turnover, relative to normal controls, a picture quite similar to that seen in untreated adult TM women. There is conflicting evidence on the clinical effectiveness of HRT in maximizing bone mass in TM [3, 6]. Younger patients (around 20 years of age), who presumably have not achieved peak bone mass seem to benefit more from HRT [6] than older subjects (30–35 years of age) [3]. A simpler explanation for the apparent failure of HRT to maximize bone density in TM may be related to the type and doses of hormonal preparations used. The preparation we used was probably not sufficient to fully compensate for the effect of estrogen deficiency on bone, as suggested by the lack of suppression of bone resorption markers we observed in our treated patients. In general, young women treated with GnRH agonists require higher doses of estrogen to prevent bone loss than do older women [11, 12].
In conclusion, we have shown that adult TM women have substantially reduced bone mass for their age, which is partly related to sex hormone deficiency. In hypogonadic TM patients, prolonged HRT at the doses used for postmenopausal women is not sufficient to optimize peak bone mass.
References
R Orvieto I Leichter A Rachmilewitz JY Margulies (1992) ArticleTitleBone density, mineral content, and cortical index in patients with thalassemia major and the correlation to their bone fractures, blood transfusions, and treatment with desferrioxamine. Calcif Tissue Int 50 397–399 Occurrence Handle1:STN:280:By2B1c3ksFM%3D Occurrence Handle1596775
CE Jensen SM Tuck JE Agnew S Koneru RW Morris A Yardumian E Prescott AV Hoffbrand B Wonke (1998) ArticleTitleHigh prevalence of low bone mass in thalassemia major. Br J Haematol 103 901–915 Occurrence Handle10.1046/j.1365-2141.1998.01108.x
E Voskaridou MC Kyrtsonis E Terpos M Skordili I Theodoropoulos A Bergele E Diamanti A Kalovidouris A Loutradi D Loukopoulos (2001) ArticleTitleBone resorption is increased in young adults with thalassemia major. Br J Haematol 112 36–41 Occurrence Handle10.1046/j.1365-2141.2001.02549.x Occurrence Handle1:STN:280:DC%2BD3M7ltFaksw%3D%3D Occurrence Handle11167780
C Wang SC Tso D Todd (1989) ArticleTitleHypogonadotropic hypogonadism in severe thalassemia: effect of chelation and pulsatile gonadotropin-releasing hormone therapy. J Clin Endocrinol Metab 68 511 Occurrence Handle1:STN:280:BiaC3sbns1I%3D Occurrence Handle2493034
ML Anapliotou IT Kastanias P Psara EA Evangelou M Lipakari P Dimitriou (1995) ArticleTitleThe contribution of hypogonadism to the development of osteoporosis in thalassemia major: new therapeutic approaches. Clin Endocrinol 42 279–287 Occurrence Handle1:STN:280:ByqB2sjpvVU%3D
A Lasco N Morabito A Gaudio M Bruno M Wasniewska N Frisina (2001) ArticleTitleEffects of hormonal replacement therapy on bone metabolism in young adults with beta-thalassemia major. Osteoporos Int 12 570–575 Occurrence Handle10.1007/s001980170079 Occurrence Handle1:CAS:528:DC%2BD3MXns1yqtLk%3D Occurrence Handle11527055
AT Soliman N El Banna M Abdel Fattah MM El Zalabani BM Ansari (1998) ArticleTitleBone mineral density in prepubertal children with beta-thalassemia: correlation with growth and hormonal data. Metabolism 47 541–548 Occurrence Handle1:CAS:528:DyaK1cXjtFertLw%3D Occurrence Handle9591744
PJ Giardina R Schneider M Lesser B Simmons A Rodriguez J Gertner M New MW Hillgartner (1995) Abnormal bone metabolism in thalassemia. S Ando (Eds) Endocrine disorders in thalassemia. Springer, Berlin, 39
FZ Stanczyk L Chang E Carmina Z Putz RA Lobo (1991) ArticleTitleIs 11 hydroxyandrostenedione a better marker of adrenal androgen excess than dehydroepiandrosterone sulfate? Am J Obstet Gynecol 166 1837–1842
E Carmina M Ferin RA Lobo (1999) ArticleTitleEvidence that insulin and androgens may participate in the regulation of serum leptin levels. Fertil Steril 72 426–431 Occurrence Handle10.1016/S0015-0282(99)00387-8
AK Sugimoto AB Hodsman JA Nisker (1993) ArticleTitleLong-term gonadotropin releasing hormone agonist with standard postmenopausal estrogen replacement failed to prevent vertebral bone loss in premenopausal women. Fertil Steril 60 672–674 Occurrence Handle1:STN:280:ByuD3cbnslU%3D Occurrence Handle8405523
E Carmina RA Lobo (1996) Steroid supplementation of GnRH analog in ovarian hyperandrogenism. M Filicori C Flamigni (Eds) Treatment with GnRH analogs: controversies and perspectives. Parthenon Pub. London, 115–121
C Wang SC Tso D Todd (1989) ArticleTitleHypogonadotropic hypogonadism in severe beta thalassemia: effect of chelation and pulsatile gonadotropin-realising hormone therapy. J Clin Endocrinol Metab 68 511 Occurrence Handle1:STN:280:BiaC3sbns1I%3D Occurrence Handle2493034
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carmina, E., Di Fede, G., Napoli, N. et al. Hypogonadism and Hormone Replacement Therapy on Bone Mass of Adult Women with Thalassemia Major . Calcif Tissue Int 74, 68–71 (2004). https://doi.org/10.1007/s00223-002-1044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-002-1044-3