Abstract
GABAB and 5-HT2C agonists are effective in attenuating the behavioral effects of psychostimulants. However, they induce adverse side effects when used in high doses. The previous evidence has suggested that the 5HT2C receptor activation effect could be produced by an increased release of GABA in the ventral tegmental area (VTA) and the consequent activation of GABAergic receptors. Therefore, the objective of this study was to evaluate the effects of joint administration of an intermediate dose of the GABAB agonist baclofen (3.0 mg/kg) with different doses of the 5HT2C agonist Ro60-0175 (0.3, 1.0, and 3.0 mg/kg) on the locomotor sensitization expression induced by the repeated administration of amphetamine (1.0 mg/kg). Our results showed an attenuation of the expression of sensitization in a dose-dependent manner with both agonists. In both cases, we observed a complete blockade at the highest dose. In addition, the intermediate dose of baclofen increased the effects of the three doses of Ro60-0175. These results support the role of the joint action of GABAB and 5-HT2C receptors in the effects of psychostimulants. However, it remains to be explored whether the observed effect can be attributed to receptors located in the VTA or the nucleus accumbens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the neurobiological level, addictive drugs exert their effects by activating the brain reward system, whose primordial element is the mesolimbic dopaminergic pathway that projects from the ventral tegmental area (VTA) to the nucleus accumbens (nAcc). Psychostimulants such as amphetamine (AMPH) increase the release of dopamine (DA) in the nAcc through the action on DA transporters. There have been attempts to use DA antagonists as pharmacological treatments for addictions; however, because of the side effects associated with the direct manipulation of DA concentrations, alternatives with different mechanisms have been evaluated and involve the manipulation of other neurotransmitters systems with the possibility of indirectly regulating the dopaminergic pathway. Recent suggestions have included the activation of neurotransmitter systems, such as the GABA or serotonin systems.
The GABAB agonist baclofen (BCF) (2.5–5.6 mg/kg i.p.) has been shown to attenuate self-administration (i.v.) of psychostimulants (Roberts et al. 1996; Roberts and Andrews 1997; Shoaib et al. 1998; Campbell et al. 1999; Brebner et al. 2000, 2005) and other drugs such as nicotine (Fattore et al. 2002). BCF (3.2–5.6 mg/kg i.p.) also reduces the breakpoints generated with progressive ratio schedules reinforced with cocaine (Brebner et al. 2000), and blocks the development and expression of locomotor sensitization induced by morphine (Bartoletti et al. 2007). In addition, we reported that BCF (3.0–5.6 mg/kg i.p.) partially reduces the discriminative signal of AMPH (Miranda et al. 2009), and blocks the development and expression of AMPH-induced locomotor sensitization (3.0–4.0 mg/kg i.p.) (Cedillo and Miranda 2013). The effects of BCF on addiction-related behavior could be mediated by the activation of GABAB receptors on the DAergic neurons of the VTA. This mediation has been supported by findings that GABAB receptors are located on the body of the DAergic neurons in the VTA (Nagai et al. 1983; Bowery et al. 1987; Johnson and North 1992; Kalivas 1993). However, because of the high distribution of GABAB receptors in the CNS, the use of high doses of BCF (e.g., 4 mg/kg) induces sedation, hypothermia, and alterations in sexual behavior, which, together with its short half-life, limit its application as a therapeutic agent for addiction treatment in humans.
On the other hand, evidence has shown that 5HT2C receptors modulate the behavioral and neurochemical effects of cocaine, since the systemic administration of 5HT2C agonists (1.0 mg/kg i.p.) inhibits and antagonists enhance behavioral responses produced by cocaine, such as the locomotor-stimulating effects and the discriminative and reinforcing properties (Filip et al. 2012). This effect may involve the activation of the 5HT2C receptors located on cell bodies of the GABAergic interneurons in the VTA; activation of these receptors could increase GABA in the VTA and, consequently, decrease DA release in the nAcc (Devroye et al. 2013; Nocjar et al. 2015). Although 5HT2C receptors are currently considered to have the potential to improve treatment for the abuse of and dependence on addictive drugs such as cocaine (Cathala et al. 2015), the activation of these receptors with Ro60-0175 has been shown to have a sedative profile and potential anxiogenic effects at doses exceeding 0.5 mg/kg (Kennett et al. 2000).
In summary, in separate studies, it has been proven that activation of GABAB and 5HT2C receptors in the VTA can modulate the DAergic neurons in the nAcc, and this modulation may be of therapeutic value in the field of addictions. However, the use of high and effective doses of BCF and Ro60-0175 has been shown to produce side effects. These side effects may be reduced using lower doses of both agonists. In line with this, the aim of the present work is to evaluate the effect of the joint administration of an intermediate dose of the GABAB agonist BCF and low doses of the 5HT2C agonist Ro60-0175 on the development of locomotor sensitization induced by the repeated administration of AMPH.
Materials and methods
Animals
A total of 210 male Wistar rats that were 120 days old and weighed 200–250 g at the beginning of the experiment were obtained from the breeding colony of the FES-Iztacala-UNAM, México. They were individually housed in stainless-steel cages with freely available food (Teklad LM485 Rat Diet by Harlan, Mexico City, Mexico) and water, and were maintained under a 12 h light/dark cycle, with the lights turned on at 08:00 h. The room was maintained at a temperature of 21 ± 1 °C. All experiments were conducted during the light phase (11:00–13:00 h). Animal care and handling procedures were performed in accordance with the Official Mexican Norm (NOM-062-ZOO-1999), and the local bioethics committee approved all procedures.
Drugs
The drugs used in these experiments were d-amphetamine sulfate, the GABAB receptor agonist (±)-baclofen (Sigma-Aldrich, St. Louis, USA), and the 5-HT2C receptor agonist Ro60-0175 (Sigma-Aldrich, St. Louis, USA). The drugs were dissolved in 0.9% saline solution (Pisa, Jalisco, México), freshly prepared daily, and administered to rats via intraperitoneal injection (i.p.) (1 mL/kg).
Apparatus
Locomotor activity was measured with an open-field activity monitoring system (ENV-515 model from Med Associates, St. Albans, USA). Each box (40 × 40 × 30 cm) was equipped with 2 sets of 8 photobeams that were located at 2.5 cm above the surface of the floor on opposite walls to record x–y ambulatory movements. Photobeam interruptions were recorded and translated by software in real time to yield the horizontal distance traveled (cm), which was the dependent measure used for analysis.
Experimental procedure
Acute effects of BCF and Ro60-0175 on locomotor activity
To investigate the acute effects of BCF and Ro60-0175 on locomotor activity, we conducted an initial experiment. Separate groups of rats (n = 10 rats per group) were assessed once in response to the different doses of BCF (0.0, 2.0, 3.0, and 4.0 mg/kg), Ro60-0175 (0.0, 0.3, 1.0, and 3.0 mg/kg), and BCF (3.0 mg/kg) + Ro60-0175 (0.0, 0.3, 1.0, and 3.0 mg/kg).
Effect of the GABAB agonist BCF, the 5-HT2C agonist Ro60-0175, and the coadministration of the GABAB agonist BCF, and the 5-HT2C agonist Ro60-0175 on locomotor sensitization expression
The experimental design (Table 1) consisted of 11 sessions. For all sessions, rats were removed from their home cages and introduced into the activity boxes. Each session began with a habituation period of 10 min in the locomotor activity boxes, after which time, the rats were removed from the activity boxes, were administered drugs or saline, and then returned to the activity boxes, where locomotor activity was recorded for 60 min. For habituation (day − 1) and baseline (day 0) sessions, all rats received three saline injections (i.p.) before introduction into the activity boxes to record their activity for 60 min. During the sensitization development period (days 1–5), all rats, except ten randomly selected rats (SAL group), received AMPH (1.0 mg/kg), and then, their locomotor activity was recorded. The SAL group was administered saline again. Once sensitization developed, on day 6, rats that had been administered AMPH during the 5 previous days were randomly assigned to one of the ten experimental groups. On days 6 and 7, all rats remained in their home cages without manipulation. Finally, two different pharmacological challenges were carried out. For the saline challenge (S challenge) (day 8), rats received three saline injections (i.p.). For the AMPH challenge (day 9), rats received three injections (i.p.) of the drug or drugs to be evaluated depending on the group to which they had been assigned.
Data analysis
Locomotor activity (distance traveled in cm) is expressed as the mean ± SEM. Data obtained during the development of locomotor sensitization were analyzed via two-way ANOVA with repeated measures, with group as factor 1 and day as factor 2. The data obtained during the baseline, testing days, and the acute effects of BCF and Ro60-0175 were analyzed with one-way ANOVA. When the ANOVA results were significant, Tukey’s test (p < 0.05) was used to perform a posteriori comparisons.
Results
Acute effects of the GABAB agonist BCF and the 5-HT2C agonist Ro60-0175 on locomotor activity
The results of this experiment are shown in Fig. 1. The data revealed that administration of different doses of BCF, Ro60-0175, and BCF + Ro60-0175 did not alter rat locomotor activity (F[9,99] = 0.690, p = 0.716).
Results for the acute effects of BCF and Ro60-0175 on locomotor activity. Bars are mean ± SEM of the distance traveled (n = 10). All groups received an injection of one of the different doses of baclofen (B groups), one of the different doses of Ro60-0175 (R groups), or the medium dose of BCF plus different doses of Ro60-0175
Sensitization development
The development of sensitization is shown in Fig. 2. First, there were no differences between the baseline values from the AMPH and SAL groups (t[108] = 2.25, p = 0.299). Repeated AMPH administration resulted in the development of locomotor sensitization, while saline administration did not produce any changes. Two-way repeated-measures ANOVA indicated significant differences between groups (F[1108] = 46.53, p = 0.000), days (F[4432] = 3.26, p = 0.012), and the interaction group × day (F[4.432] = 4.58, p = 0.001).
Effect of sensitization development on the saline challenge
During the saline (S) challenge on day 8 (Fig. 3), no significant differences were found between the groups (F[10,109] = 1.216, p = 0.290).
Effects of the GABAB agonist BCF, agonist Ro60-0175, and coadministration of the GABAB agonist BCF, and the 5-HT2C agonist Ro60-0175 on locomotor sensitization expression
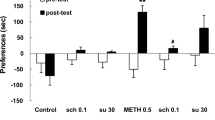
Data obtained with different doses of BCF, different doses of Ro60-0175, and coadministration of BCF and Ro60-0175 are shown in Fig. 4. On day 9, we found that the GABAB agonist BCF and the 5-HT2C agonist Ro60-0175 dose-dependently decreased the expression of locomotor sensitization produced by repeated AMPH administration, and BCF increased the effect of Ro60-0175 (F[10,109] = 9.973, p = 0.0001). Multiple comparisons also indicated that the AMPH (p = 0.035) and R0.3 (p = 0.024) groups were significantly higher than the SAL group. In contrast, we found significant differences between the AMPH and SAL (p = 0.035), B4.0 (p = 0.000), B3.0 + R0.3 (p = 0.002), B3.0 + R1.0 (p = 0.000), and B3.0 + 3.0 (p = 0.000) groups. A posteriori comparison also showed that the B3.0 group was different from the B3.0 + R3.0 group (p = 0.045). The B2.0 group was not different from the AMPH group (p = 0.413), but was also not different from the SAL group (p = 0.991).
Results for AMPH challenge on day 9. Bars are mean ± SEM of the distance traveled (n = 10). All groups received AMPH injection, AMPH injection plus one of the different doses of baclofen (B groups), AMPH injection plus one of the different doses of Ro60-0175 (R groups) or AMPH plus the medium dose of BCF and different doses of Ro60-0175. *Indicate significant differences with SAL group, +significant differences with AMPH group, and δsignificant differences with B3.0 group after Tukey’s test
Discussion
The main objective of the present study was to evaluate the coadministration of low doses of the GABAB agonist BCF and the 5HT2C agonist Ro60-0175 on the expression of AMPH-induced locomotor sensitization. During the experiments, we observed that the repeated administration of AMPH (1.0 mg/kg) increased the locomotor activity of rats in a progressive way. In addition, both the GABAB agonist BCF and the 5-HT2C agonist Ro60-0175 prevented the expression of locomotor sensitization in a dose-dependent manner. In the case of BCF, the 4.0 mg/kg dose produced significant differences with respect to the AMPH group. The 2.0 and 3.0 mg/kg doses did not produce significant differences with respect to the AMPH group, but did not produce significant differences with respect to the SAL group. Although the medium and lowest dose of BCF reduced the expression of locomotor sensitization produced by AMPH, it was not able to completely block its expression. In the case of Ro60-0175, we only found differences with the highest dose (3.0 mg/kg). In addition, the effect of all doses of Ro60-0175 was increased by administering the intermediate dose of BCF, and the effect of the combination of the highest doses of both compounds was significantly higher than the effect of 3.0 mg/kg BCF alone.
Our findings are consistent with the previous results in which it has been reported that BCF can prevent both the development (Bartoletti et al. 2005; Cedillo and Miranda 2013) and the expression (Bartoletti et al. 2004; Cedillo and Miranda 2013) of locomotor sensitization produced by AMPH, cocaine (Frankowska et al. 2009), morphine (Bartoletti et al. 2007; Fu et al. 2012), and ethanol (Broadbent and Harless 1999).
Similarly, it has been reported that 5-HT2C receptor pharmacological activation with lorcaserin suppresses the development and expression of heroin-induced behavioral sensitization (Wu et al. 2015).
These results could be explained by the modulation of DA release. In the nAcc, DA is principally released by neurons projecting from the VTA. This DAergic pathway, known as the mesolimbic system, plays an important role in the production of rewarding and motor effects of psychostimulants (Di Chara 1995; Kalivas and Nakumara 1999; Koob 1992). The mesolimbic system has also been implicated in the development and expression of locomotor sensitization (Pierce and Kalivas 1997). In animals that express locomotor sensitization, pre- and postsynaptic neuroadaptations have been found in the nAcc DAergic system (Robinson and Berridge 2000). However, some reports have shown that neural adaptations in DA terminal fields in the nAcc are sufficient for the expression of AMPH sensitization, although an action on DA cell bodies may be required for the induction of AMPH sensitization (Paulson and Robinson 1991). Regardless, DAergic projections from the VTA to the nAcc are thought to be the major component in the development and expression of locomotor sensitization, and there is a clear role of DA release for AMPH-induced sensitization (Vanderschuren and Kalivas 2000).
There is considerable evidence to support the hypothesis that GABAB receptors are located on the cell bodies of the DAergic neurons in the VTA (Nagai et al. 1983; Bowery et al. 1987; Johnson and North 1992; Kalivas 1993) where they are activated by GABA released from GABAergic interneurons or by nAcc projections. Evidence has shown that BCF infusions in the VTA decreased DA release in the nAcc (Westerink et al. 1996; Yoshida et al. 1994). In addition, it has been shown that BCF infusions in the VTA reduced heroin (Xi and Stein 1999) and cocaine (Shoaib et al. 1998) self-administration under fixed-ratio and progressive ratio schedules (Brebner et al. 2000), as well as the motor activity produced by AMPH (Kalivas et al. 1990). Although the evidence seems to support this mechanism as an explanation of the observed results, there is also evidence of the presence of GABAB receptors in the nAcc, and this potential influence would have to be analyzed (Pitman et al. 2014).
In the case of the 5-HT2C agonist Ro60-0175, the effect could be due to activation of 5-HT2C receptors located on the cell bodies of GABAergic interneurons in the VTA. 5-HT2C receptor activation could produce excitation of the interneurons and, therefore, an increase in VTA GABA release. This GABA increase would result in a decrease in the DA release in the nAcc (Devroye et al. 2013; Nocjar et al. 2015). This hypothesis is supported by studies in which it has been found that Ro60-0175 infusions (5 μg/0.2 μL) in the VTA can significantly reduce the DA release produced by systemic administration of cocaine (Navailles et al. 2008). In addition, in behavioral studies, it has also been found that Ro60-0175 injections in the VTA can attenuate the locomotor activity increase produced by cocaine, as well as its self-administration (Fletcher et al. 2004). Similar to the reports of GABAB receptors, there are also reports that indicate the presence of 5-HT2C receptors in the nAcc shell (Navailles et al. 2008).
Finally, with respect to the lack of conditioned locomotion observed in the present experiment, there are conflicting results reported in different papers. Some authors (Vezina and Leyton 2009; Cedillo and Miranda 2013) have reported conditioned locomotor activity; however, there are also authors who have reported that there is no conditioned locomotion (Acosta-García et al. 2017), as in the present results. In this line, some authors have suggested that psychostimulant behavioral sensitization is a composite of conditioned locomotion and increased unconditioned drug responses. While these authors have reported that the conditioned response can be statistically robust, the response was only a very small fraction of the sensitized response (Pinheiro et al. 2011). The expression or absence of conditioned locomotion can be influenced by different factors, such as the number of days of testing or the intensity of the stimulus. This has been the subject of previous investigations (Pinheiro et al. 2011; Vezina and Leyton 2009).
Taken together, evidence shows that the increase in behavioral effects observed after administering the GABAB agonist BCF and the 5-HT2C agonist Ro60-0175 could be due to the simultaneous stimulation of both types of receptors. These results support the role of GABAB and 5-HT2C receptors in the behavioral effects of psychostimulants and reaffirm the idea of these agonists as possible therapeutic agents in the treatment of psychostimulant addiction. However, it is still necessary to analyze the actual contribution of the GABAB and 5-HT2C receptors in the VTA and nAcc.
References
Acosta-García J, Jimenez JC, Miranda F (2017) Additive effects of coadministration of A2A receptor agonist CGS-21680 and mGluR5 antagonist MPEP on the development and expression of methamphetamine-induced locomotor sensitization in rats. J Drug Alcohol Res 6:1–11. https://doi.org/10.4303/jdar/236038
Bartoletti M, Gubellini C, Ricci F, Gaiardi M (2004) The GABAB agonist baclofen blocks the expression of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol 15:397–401
Bartoletti M, Gubellini C, Ricci F, Gaiardi M (2005) Baclofen blocks the development of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol 16:553–558. https://doi.org/10.1097/01.fbp.0000179279.98029.e9
Bartoletti M, Ricci F, Gaiardi MA (2007) GABA(B) agonist reverses the behavioral sensitization to morphine in rats. Psychopharmacology 192:79–85. https://doi.org/10.1007/s00213-006-0693-8
Bowery NG, Hudson AL, Price GW (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383. https://doi.org/10.1016/0306-4522(87)90098-4
Brebner K, Phelan R, Roberts DCS (2000) Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology 148:314–321
Brebner K, Ahn S, Phillips AG (2005) Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology 177(4):409–417. https://doi.org/10.1007/s00213-004-1968-6
Broadbent J, Harless WE (1999) Differential effects of GABAA y GABAB agonist on sensitization to the locomotor stimulant effects of ethanol in DBA/2J mice. Psychopharmacology 141(2):197–205. https://doi.org/10.1007/s002130050825
Campbell UC, Lac ST, Carroll ME (1999) Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology 143:209–214. https://doi.org/10.1007/s002130050937
Cathala A, Devroye C, Maitre M, Piazza PV, Abrous DN, Revest JM, Spampinato U (2015) Serotonin2C receptors modulate dopamine transmission in the nucleus accumbens independently of dopamine release: behavioral, neurochemical and molecular studies with cocaine. Addict Biol 20(3):445–457. https://doi.org/10.1111/adb.12137
Cedillo LN, Miranda F (2013) Effects of co-administration of the GABAB receptor agonist baclofen and a positive allosteric modulator of the GABAB receptor, CGP7930, on the development and expression of amphetamine-induced locomotor sensitization in rats. Pharmacol Rep 65(5):1132–1143
Devroye C, Filip M, Przegalinski E, McCreary AC, Spampinato U (2013) Serotonin2C receptors and drug addiction: focus on cocaine. Exp Brain Res 230:537–545. https://doi.org/10.1007/s00221-013-3593-2
Di Chara G (1995) The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depen 38:95–137
Fattore L, Cossu G, Martellotta MC, Fratta W (2002) Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol 37(5):495–498. https://doi.org/10.1093/alcalc/37.5.495
Filip M, Spampinato U, McCreary AC, Przegaliński E (2012) Pharmacological and genetic interventions in serotonin (5-HT) (2C) receptors to alter drug abuse and dependence processes. Brain Res 1476:132–153
Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA (2004) Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology 29:308–318
Frankowska M, Nowak E, Filip M (2009) Effects of GABAB receptor agonists on cocaine hyperlocomotor and sensitizing effects in rats. Pharmacol Rep 61:1042–1049
Fu Z, Yang H, Xiao Y, Zhao G, Huang H (2012) The gamma-aminobutyric acid type B (GABAB) receptor agonist baclofen inhibits morphine sensitization by decreasing the dopamine level in rat nucleus accumbens. Behav Brain Funct 8:20. https://doi.org/10.1186/1744-9081-8-20
Johnson SW, North RA (1992) Two types of neuron in the rat ventral tegmental area and their synaptic inputs. J Physiol (Lond) 450:455–468
Kalivas PW (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev 18:75–113. https://doi.org/10.1016/0165-0173(93)90008-N
Kalivas PW, Nakumara M (1999) Neural systems for behavioral activation and reward. Curr Opin Neurobiol 9:223–227. https://doi.org/10.1016/S0959-4388(99)80031-2
Kalivas PW, Duffy P, Eberhardt H (1990) Modulation of A10 neurons by gamma-aminobutyric acid agonist. J Pharmacol Exp Ther 253:858–866
Kennett G, Lightowler S, Trail B, Bright F, Bromidge S (2000) Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur J Pharmacol 387(2):197–204. https://doi.org/10.1016/S0014-2999(99)00706-2
Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184. https://doi.org/10.1016/0165-6147(92)90060-J
Miranda F, Jiménez JC, Cedillo LN, Sandoval-Sánchez A, Millán-Mejía P, Sánchez-Castillo H, Velázquez-Martínez DN (2009) The GABA-B antagonist 2-hydroxysaclofen reverses the effects of baclofen on the discriminative stimulus effects of d-amphetamine in the conditioned taste aversion procedure. Pharmacol Biochem Behav 93:25–30. https://doi.org/10.1016/j.pbb.2009.04.002
Nagai T, McGeer PL, McGeer EG (1983) Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol 218:220–238. https://doi.org/10.1002/cne.902180209
Navailles S, Moison D, Cunningham KA, Spampinato U (2008) Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology 33:237–246. https://doi.org/10.1038/sj.npp.1301414
Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA (2015) Serotonin-2C and -2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience 297:22–37. https://doi.org/10.1111/j.1471-4159.2010.06694.x
Paulson PE, Robinson T (1991) Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequent intra-accumbens amphetamine challenge in rats. Psychopharmacology 104:140–141
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res 25:192–216. https://doi.org/10.1016/S0165-0173(97)00021-0
Pinheiro CM, Carey RJ, Cruz DFR, Wermelinger ML (2011) Reversal of apomorphine locomotor sensitization by a single post-conditioning trial treatment with a low autoreceptor dose of apomorphine: a memory re-consolidation approach. Pharmacol Biochem Behav 99:29–34. https://doi.org/10.1016/j.pbb.2011.03.018
Pitman KA, Puil E, Borgland SL (2014) GABAB modulation of dopamine release in the nucleus accumbens core. Eur J Neurosci 40:3472–3480. https://doi.org/10.1111/ejn.12733
Roberts DCS, Andrews MM (1997) Baclofen suppression of cocaine self-administration: demonstration using a discrete trial procedure. Psychopharmacology 131:271–277. https://doi.org/10.1007/s002130050293
Roberts DC, Andrews MM, Vickers GJ (1996) Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15:417–423. https://doi.org/10.1016/0893-133X(96)00002-4
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95:91–117
Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW (1998) The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol 9:195–206
Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120
Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56:160–168. https://doi.org/10.1016/j.neuropharm.2008.06.070
Westerink BH, Kwint HF, De Vries JB (1996) The pharmacology of mesolimbic dopamine neurons: a dual probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci 16:2605–2611
Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW, Zhang G (2015) Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett 607:23–28. https://doi.org/10.1016/j.neulet.2015.09.013
Xi ZX, Stein EA (1999) Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther 290:1369–1374
Yoshida M, Yokoo H, Tanaka T, Emoto H, Tanaka M (1994) Opposite changes in the mesolimbic dopamine metabolism in the nerve terminal and cell body sites induced by locally infused baclofen in the rat brain. Brain Res 636:111–114. https://doi.org/10.1016/0006-8993(94)90183-X
Acknowledgements
This study was supported by grant IN301717 from PAPIIT-UNAM (Mexico).
Author information
Authors and Affiliations
Contributions
LNC designed the experiments, wrote the manuscript, and performed experiments RIR performed experiments, JCJ performed experiments, and FM designed and directed the experiments and wrote the manuscript. All of the authors analyzed the data, discussed the results and commented on the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cedillo, L.N., Ruíz-García, R.I., Jiménez, J.C. et al. Effect of coadministration of the GABAB agonist baclofen and the 5-HT2C agonist Ro60-0175 on the expression of amphetamine-induced locomotor sensitization. Exp Brain Res 237, 1691–1697 (2019). https://doi.org/10.1007/s00221-019-05540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05540-z