Abstract
The pathophysiological mechanisms leading to dyskinesias in Parkinson’s disease (PD) after long-term treatment with levodopa remain unclear. This study investigates the neuronal firing characteristics of the entopeduncular nucleus (EPN), the rat equivalent of the human globus pallidus internus and output nucleus of the basal ganglia, and its coherence with the motor cortex (MCx) field potentials in the unilateral 6-OHDA rat model of PD with and without levodopa-induced dyskinesias (LID). 6-hydroxydopamine-lesioned hemiparkinsonian (HP) rats, 6-OHDA-lesioned HP rats with LID (HP-LID) rats, and naïve controls were used for recording of single-unit activity under urethane (1.4 g/kg, i.p) anesthesia in the EPN “on” and “off” levodopa. Over the MCx, the electrocorticogram output was recorded. Analysis of single-unit activity in the EPN showed enhanced firing rates, burst activity, and irregularity compared to naïve controls, which did not differ between drug-naïve HP and HP-LID rats. Analysis of EPN spike coherence and phase-locked ratio with MCx field potentials showed a shift of low (12–19 Hz) and high (19–30 Hz) beta oscillatory activity between HP and HP-LID groups. EPN theta phase-locked ratio was only enhanced in HP-LID compared to HP rats. Overall, levodopa injection had no stronger effect in HP-LID rats than in HP rats. Altered coherence and changes in the phase lock ratio of spike and local field potentials in the beta range may play a role for the development of LID.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), which leads to the depletion of dopamine in the striatum, the entrance region of the basal ganglia (BG) motor loop, is one of the pathophysiological hallmarks of Parkinson’s disease (PD). Chronic replacement therapy with levodopa relieves symptoms, however, eventually may lead to abnormal involuntary movements, termed dyskinesias, which become treatment-limiting. It has been thought that levodopa-induced dyskinesias (LIDs) develop as a consequence of pulsatile stimulation of dopamine receptors, with consequent dysregulation in downstream neurons resulting in changes in neuronal firing patterns (Obeso et al. 2000).
In patients with PD, abnormal neuronal activity has been found in the globus pallidus internus (GPi) and the subthalamic nucleus (STN), and especially, an increase in synchronized oscillatory beta band activity (13–30 Hz) has been noted, along with enhanced neuronal firing rates and burst activity (Brown 2003; Obeso et al. 2006; Wichmann and Dostrovsky 2011; Weinberger et al. 2012). Recordings from patients undergoing pallidotomy or deep brain stimulation have shown that dyskinesias after chronic levodopa treatment are accompanied by reduced oscillatory beta band activity and enhanced theta band activity (4–10 Hz), together with an extensive decrease in firing rates and abnormal firing patterns (Lozano et al. 2000; Obeso et al. 2000; Alonso-Frech et al. 2006). These studies, however, did not address differences of neuronal single units and oscillatory activity in patients with drug-naïve advanced PD with or without peak-dose dyskinesias, since almost all patients undergoing neurosurgical treatment have received chronic treatment with levodopa and therefore developed levodopa-induced dyskinesias (LID) at least to some extent at the time of surgery.
Injection of 6-hydroxydopamine (6-OHDA) into the rat nigrostriatal system leads to degeneration of dopaminergic neurons in the SNc together with concomitant abnormal neuronal activity in the BG, which closely parallels the findings in PD patients. When chronically treated with levodopa, 6-OHDA-lesioned rats exhibit a broad range of behavioral, physiological, and biochemical features that are similar to LIDs in human patients (Lundblad et al. 2002; Picconi et al. 2005; Marin et al. 2008, 2009; Alam et al. 2014). The oscillatory theta band activity recorded in different basal ganglia regions was significantly more pronounced in 6-OHDA-lesioned animals with LIDs than in drug-naïve 6-OHDA-lesioned rats (Alam et al. 2014; Meissner et al. 2006).
In order to better understand the neuronal mechanisms involved in the development of LIDs, we investigated the neuronal firing activity of the entopeduncular nucleus (EPN), the rat equivalent to the human GPi, and its coherence with the motor cortex (MCx) field potentials in 6-OHDA-lesioned hemiparkinsonian (HP) rats with LIDs on/off levodopa, i.e., a model for advanced PD with peak-dose dyskinesias. Measures were compared with the neuronal activity of HP rats without dyskinesias and naïve rats, which served as controls.
Materials and methods
Animals
Thirty-eight adult male Sprague–Dawley rats (Charles River Laboratories, Germany) were used in this study. They were housed in groups of three-to-four animals per cage (Macrolon Type IV) and kept under controlled environmental conditions (temperature 22 °C, relative humidity 45–55 %, 14-/10-h light/dark cycle) and fed with laboratory rat chow and water ad libitum. All animal procedures were in accordance with the European Council Directive of November 24, 1986 (86/609/EEC) and were approved by the local animal ethic committee. All efforts were made to minimize the number of animals used and their suffering.
Thirty-two rats were rendered hemiparkinsonian by unilateral injection of 6-OHDA in the medial forebrain bundle (MFB). Subsequently, these HP rats were divided into two groups. One group (n = 24) were rendered dyskinetic by long-term injections of levodopa, in the following termed HP-LID rats, while the other HP group (n = 8) received no levodopa injection. Another group of rats (n = 6) without surgery served as naïve controls.
6-OHDA lesion
For surgery, rats were anaesthetized with 3.6 % chloral hydrate (1 ml/100 g body weight, i.p., Sigma, Germany) and placed in a stereotaxic frame (Stoelting, Wood Dale, Illinois, USA). Two holes were drilled over the targets above the right medial forebrain bundle, and the dura was exposed. 6-OHDA was dissolved in 0.02 % ascorbate saline at a concentration of 3.6 µg/µl and was injected (1 µl/min) in two deposits (2.5 and 3 µl, respectively) at the following coordinates in mm relative to bregma and to the surface of the dura mater: anterior–posterior (AP) = 4.0; lateral (L) = ±0.8; ventral (V) = −8.0; tooth bar at +3.4 and AP = 4.4; L = ±1.2; V = −7.8; tooth bar at −2.4, respectively. Sham-lesioned rats received only the vehicle (0.02 % ascorbic acid in physiological saline) at the same coordinates. After infusion, the incision was closed by stitches, and the animals were returned to their home cages for recovery.
The efficacy of the 6-OHDA-induced lesion was validated 3 weeks after surgery by injection of apomorphine (0.05 mg/kg, s.c.; Sigma) as previously described (Alam et al. 2014). The lesion was considered successful in those animals that made more than 80 net contraversive rotations in 20 min. To induce dyskinesias, the rats were treated for four weeks with 6 mg/kg L-DOPA methylester (Sigma-Aldrich, Germany) plus 12 mg/kg benserazide-HCl. Both drugs were dissolved in physiological saline and injected subcutaneously (s.c.) with a volume of 1 ml/kg body.
Dyskinesias were scored by the Abnormal Involuntary Movements (AIM) scale as described earlier (Alam et al. 2014). The different subtypes of AIMs: orolingual, forelimb, and axial dyskinesias, were scored separately for 2 h after levodopa injection on an ordinal scale from 0 to 4, respectively, for 1 min every 10 min (i.e., 12 monitoring periods from 10 to 120 min post-injection). The mean value of these measures was used for further analysis. Only rats with total AIMs scores higher than 4 were included in HP-LID group.
Electrophysiology
Neuronal activity was recorded in the EPN in naïve controls, HP, and HP-LID rat groups before and after levodopa injections. Recordings were done under urethane anesthesia (1.4 g/kg i.p. with additional 25 % doses as needed) as described previously (Alam et al. 2012). The temperature of the anesthetized animals was constantly controlled with a rectal probe and maintained at 37.2° to 37.6° C with a heating pad (Harvard Apparatus). Electrocardiographic (ECG) activity was monitored constantly to ensure the animals’ well-being. A drop of silicon oil was applied to all areas of the exposed cortex to prevent dehydration. Depth of anesthesia was monitored by examination of the reflex answer to a toe pinch.
The recordings of extracellular single-unit (SU) activity were performed in the EPN (coordinates relative to bregma AP): −2.3 to −2.8 mm posterior to bregma: L: −2.6 to −3.0 mm from the midline; V: 7.5–8.0 mm from the dura, tooth bar at −3.3 mm. Spike train recordings from the EPN were paired with simultaneous recordings of the MCx-ECoG.
Extracellular SU recordings were taken by quartz-coated pulled and ground platinum–tungsten alloy core (95–5 %) microelectrode with a diameter of 80 μm, and an impedance of 1–2 MΩ at 1 kHz. The electrode was advanced using a microdrive (Thomas Recording GmbH, Giessen, Germany) in the ipsilateral EPN. The SU signals were digitized at a sampling rate of 25 kHz with 0.5- to 5-kHz band-pass filter and amplification of signals from × 9500 to × 19,000. Additionally, the MCx-ECoG was recorded via a 1-mm-diameter jeweler’s screw, which was positioned on the dura mater above the frontal cortex ipsilateral to the lesioned or sham-lesioned hemisphere (AP, +2.7 mm; ML, 2.0 mm; which corresponds to the primary motor cortex region). Two additional screws, serving as MCx-ECoG reference and ground, were placed over the parietal lobe and cerebellum and band-pass-filtered (0.5–100 Hz) with a sampling rate of 1 kHz (Alam et al. 2012). All signals were digitized with a CED 1401 (Cambridge Electronic Design (CED), Cambridge, UK). The firing of each neuron was recorded for 8–10 min after signal stabilization. After termination of the experiment, electrical lesions were made at the recording sites (10 μA for 10 s; both negative and positive polarity), and the rat was perfused with 4 % paraformaldehyde. Each brain was then cut into 20-μm sections and stained with a standard hematoxylin and eosin (HE) stain protocol to verify the position of each electrode. For verification of loss of dopaminergic neurons tyrosine hydroxylase (TH), immunohistochemistry was used in 40-µm coronal striatal and SNpc free-floating tissue sections with a standard avidin–biotin immunohistochemical protocol previously described (Lindemann et al. 2013; von Wrangel et al. 2015).
Analysis of electrophysiological data
Rats anaesthetized with urethane demonstrate spontaneous and cyclical alternations of brain state that resembles sleep state alternations. These fluctuations between different brain states are known as active state (rapid eye movement: REM) and quiet slow-wave (<1 Hz rhythm or non-REM) stages. In our previous study, we have shown higher beta oscillations in 6-OHDA in the motor cortex and the subthalamic nucleus compared to naïve control rats only under the active state of urethane anesthesia (Alam et al. 2012; Rumpel et al. 2013). Therefore, only spike and LFP activity recorded during the active state of the brain were used for analysis, which was determined by visual inspections of MCx field potentials. One epoch of 300-s recordings was analyzed and sorted on the base of a 3:1 signal-to-noise ratio. Neuronal firing activity arising from a single neuron was discriminated by threshold spike detection and template-matching, controlled by cluster analysis with principal component analysis and final visual inspection by using the template-matching function of the spike-sorting software (Spike2; Cambridge Electronic Design, Cambridge, UK).
The EPN/GPi spikes exhibited the electrophysiological characteristics of GABAergic neurons in the output structures of basal ganglia as reported previously (Benhamou and Cohen 2014). The waveform duration was measured from the first valley to the following peak in tonic and bursts firing neurons with a mean duration of (214.4 ± 14.36 μs). All recorded spikes showed a biphasic (positive/negative) neuronal activity with 0.35- to 0.5-mV amplitudes.
The firing rate was calculated with the firing rate histograms generated in NeuroExplorer version 4 (NEX Technologies, NC). The coefficient of variation (CV) of the spike inter-spike interval sequence was computed for each recording as a measure of the regularity of the spike firing. CV is a measure of spike train irregularity defined as the standard deviation divided by the mean inter-spike interval. Exponential distributions have a CV of 1, i.e., describe more irregular discharge patterns, whereas distributions derived from more regular ISIs have CV values below 1.
An asymmetry index was computed as the mode inter-spike interval divided by the mean inter-spike interval. It provides information on the shape of the ISI histogram and the regularity of the discharge pattern. An asymmetry index close to 1 reveals a relatively regular firing pattern, whereas the more the index differs from unity, the more irregular the spike trains. A ratio of less than 1 reflects an asymmetrical shape, indicating a larger fraction of short inter-spike intervals (positively skewed), as is expected when there is bursting activity.
Firing patterns of spikes events
The analysis classified discharge patterns into 1 of 3 basic categories, i.e., regular, irregular, and bursty firing. Its discharge density histogram was estimated on the base of three reference probability density functions (PDFs) as proposed by Labarre et al. (2008). This method is a comparison of the density histogram d (λ) to a reference density function p x(λ). For the reference functions, (1) a Gaussian PDF with mean 1 and variance 0.5, (2) a Poisson PDF with mean 1, and (3) a Poisson PDF with mean 0.8 were used to represent regular, irregular, and bursting activity, respectively (Lourens et al. 2013). The smallest distance of the estimated discharge density histogram of the neuron to the three reference PDFs determined the type of neuron.
Field potentials of MCx-ECoG and its coherence and phase lock with EPN spikes
Representative epochs of 300 s without major artifacts were used for the frequency-domain signal processing for the ECoGs. A finite impulse response (FIR) 50-Hz notch filter and 100-Hz low- pass filter were used. Autospectra of ECoG were derived by discrete Fourier transformation with blocks of 1024 samples using a Welch periodogram in a custom MATLAB (MathWorks, Inc.) script, which resulted in a frequency resolution of 0.9766 Hz. Hanning’s window function was applied to overcome spectral leakage phenomenon. For comparison of powers at different frequency bands, the area under the computed power density spectrum in specified frequency ranges were calculated and averaged.
Since several studies in human PD patients have suggested that low and high beta activities may have a different functional signaling (Priori et al. 2004; Marceglia et al. 2006, 2007; Lopez-Azcarate et al. 2010), we additionally analyzed low (12–19 Hz) and high (19–30 Hz) beta band coherence in order to determine any possible changes within the beta frequency range.
The duration of 300- s simultaneously recorded EPN neuronal spikes and MCx-ECoG signals was used to determine coherence between a point process and a field potential using the neurospec toolkit (version 2.0) in MATLAB, as described in Halliday et al. (1995). Mean coherences were calculated for the theta (4–8 Hz), low beta (12–19 Hz) and high beta (19–30 Hz), and total beta (12–30 Hz) frequency ranges.
Additionally, phase relationships between spikes and MCx-ECoG field potentials were assessed using spike-triggered waveform averages (STWA). The ECoG channels were band-pass-filtered at different bands with an ideal (noncausal) filter to prevent phase distortions. STWAs were calculated for 150 ms before and after the spike trigger over a 300-s epoch. Spike trains of each neuron were shuffled 20 times to create a null hypothesis for a non-phase-locked spike train with the same first-order statistics as the original spike train. The phase-locked ratio was obtained by dividing the peak-to-trough amplitude of the unshuffled spike trains STWA by the mean of the shuffled distribution. A comparison of the mean ratios was analyzed for the EPN single-unit firing neuron referenced to the MCx-ECoG filtered in theta (4–8 Hz) and beta (12–30 Hz) frequency ranges, and beta activity was further divided into low (12–19 Hz) and high beta (19–30 Hz).
Statistical analysis
Two-way analysis of variance (two-way ANOVA) was used to test for significant differences among the groups followed by post hoc Tukey’s Test for multiple comparisons between groups for detection of significance (P value less than 0.05). Pearson’s Chi-square test was used to determine differences in the distribution of firing patterns. All data are expressed as the mean ± SEM.
Results
Two of the 24 animals in the HP-LID group were euthanized because of severe and continuous loss of body weight after surgery for unilateral 6-OHDA lesions. All remaining 6-OHDA-lesioned rats showed more than 80 contraversive rotations during the apomorphine challenge and were thus considered suitable for the experiments. After 4 weeks of chronic levodopa injection, 12 of the 22 animals in the HP-LID group showed dyskinesias as determined by the AIM (mean score of 7.53 ± 0.71, range 4.33–11.5) and were thus used for the electrophysiological recordings. All rats operated for the HP group had appropriate 6-OHDA lesions, i.e., showed more than 80 contraversive rotations during the apomorphine challenge, and were used for electrophysiological recordings. Additionally, a nearly complete loss of dopamine was found in both striatum and SNpc tissue sections (see Fig. 2).
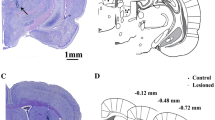
The neuronal activity of 307 single units (SUs) before and of 217 SU after levodopa injection was recorded. In control group the number of SU recorded neurons were (n = 140), HP group (n = 196) and HP-LID group (n = 188). The average units number (mean and SEM) recorded per individual rat was 21.83 ± 1.90. Examples of single-unit activity (SUA) raw data of spontaneous neuronal discharges in the EPN and simultaneously recorded motor cortex ECoG field potential sweeps over a 3-s in control, HP, and HP-LID is shown in (Fig. 1). An illustration of histological-based reconstruction from the rat brain atlas (Paxinos and Watson 1998) and of the localization of recordings electrode in HE-stained coronal section of the EPN is shown (see Fig. 2 for examples).
Examples of spontaneous single-unit activity (SUA) in the EPN and spikes wave form parameters, together with simultaneously recorded motor cortex ECoG field potential sweeps over a 3-s and the mean EPN spike-triggered waveform average at beta (12–30 Hz) range for duration of 300 s in the motor cortex ECoG for naïve control (black), HP group (blue) and HP-LID group (red) in off (without levodopa, a, b and c) and on (with levodopa, a′, b′ and c′), respectively
Histological pictures showing examples of recording trajectories in the EPN magnified from 10× and 50× (a and b). White arrows indicate the 200-μm-distanced electrolytic coagulations (10 µA bipolar current for 10 s) along the recording trajectories. Corresponding schematic reconstructions from Paxinos and Watson (1998; c showing the recording sites in the EPN in different Groups (naïve control: O; HP: black circle; HP-LID: black triangle) and examples of TH-immunostained coronal tissues sections in 6-OHDA-lesioned rat striatum (d) and subtantia nigra pars compacta (SNpc; e). Densities of TH immunoreactivity in striatum and SNpc are clearly reduced after the 6-OHDA injection (left side) as compared to the densities of striatal TH immunoreactivity fibers the unlesioned control hemisphere (right side)
Firing rate
In the EPN the firing rate was higher in 6-OHDA-lesioned than in naïve rats, but long-term treatment with levodopa; however, had no additional effect. Acute levodopa injection reduced firing rates in HP and HP-LID rats without difference, but increased this measure in naïve rats (Fig. 3a). The 25th and 75th percentiles of neuronal firing rate were calculated in the naive group (13.7 and 26.7 before levodopa, 12.0 and 35.0 after levodopa), the HP group (17.9 and 33.2 before levodopa, 9.3 and 24.2 after levodopa), and the HP-LID group (19.9 and 34.9 before levodopa, 4.7 and 25.1 after levodopa), respectively. Statistical analysis with two-way ANOVA showed an effect for the factor drug (F 2, 523 = 16.12, P < 0.001), and an interaction between the factors drug and group (F 2, 523 = 17.72, P < 0.001), but no effect for the factor group (F 2, 523 = 1.68, P = 0.19). Post hoc testing showed that the mean firing rates were enhanced in HP and HP-LID rats as compared to naïve controls without difference (all P values <0.05). Injection of levodopa increased the firing rates in naïve controls, but reduced this measure in HP and HP-LID rats without difference (all P values <0.05).
CV and asymmetry index
The CV of HP and HP-LID groups was higher as compared to naïve controls. Levodopa injection further enhanced this measure in both groups. Statistical analysis with ANOVA revealed a significant effect for the factor group (F 5, 523 = 34.48, P < 0.001), the factor drug (F 2, 523 = 27.23, P < 0.001) and the interaction between factors (F 2, 523 = 5.21, P < 0.006). Post hoc analysis revealed an enhanced CV in HP and HP-LID rats (P < 0.05) without difference between these groups. Levodopa significantly increased CV in HP and HP-LID groups. This enhancement was less in HP-LID rats, leading to a significant lower CV in HP-LID rats after levodopa injection compared to HP rats (P < 0.05; Fig. 3b).
The asymmetry index of HP and HP-LID groups was lower as compared to naïve controls. Levodopa injection further decreased this measure in both groups. Two-way ANOVA revealed significant effects on asymmetry index for the factor group (F 2, 523 = 40.409, P < 0.001), the factor drug (F 2, 511 = 29.968, P < 0.001), and interaction between factors (F 2, 511 = 7.609, P < 0.001). Both HP and HP-LID rats showed a lower asymmetry index compared to naïve controls (P < 0.001), but without difference between groups. Injection of levodopa significantly reduced the asymmetry index in both groups, but to a lesser extent in HP-LID rats, leading to a significant lower asymmetry index in HP compared to HP-LID rats (P < 0.05; Fig. 3c).
Firing patterns
The percentage of bursty pattern neurons was higher, and the percentage of regular pattern neurons was lower in HP and HP-LID rats as compared to naïve control rats (P < 0.01). Administration of levodopa increased the number of bursty neurons only in HP rats (Fig. 3d).
MCx-ECoG oscillatory activity
Since on spectral analysis we did not observe any differences in the gamma range in our recordings, we restrained analysis of oscillatory activity on theta and beta band activity. The recorded MCx-ECoG oscillatory activity revealed enhanced theta band (4–8 Hz) activity in HP rats after levodopa injection, while theta band activity of HP-LID rats did not differ from naïve controls, and levodopa had no effect. ANOVA revealed a significant effect for the factor group (F 2, 499 = 9.93; P < 0.001) and the interaction between factors (F 2, 499 = 4.31; P < 0.05) but not for the factor drug (F 1, 499 = 1.86; P = 0.17). Post hoc testing showed that theta band activity was higher in the HP group after levodopa injection compared to both naïve controls (P < 0.05) and HP-LID (P < 0.001).
The beta frequency band (12–30 Hz) activity was higher in HP and HP-LID rats and levodopa injection reduced beta band activity in both groups. ANOVA showed significant differences for the factor group (F 2, 499 = 7.26; P < 0.001) and the factor drug (F 1, 499 = 10.53; P < 0.001), but not the interaction between factors (F 2, 499 = 2.29; P = 0.10). The beta band activity was higher in HP and HP-LID groups compared to naïve controls (P < 0.001 and 0.05, respectively). Injection of levodopa decreased the beta activity in the HP and HP-LID group (P < 0.05 and 0.001, respectively). Low beta band (12–19 Hz) activity showed a significant decrease in HP-LID group after levodopa injection but not altered in other groups. ANOVA showed a significant effect for the factor group (F 2, 499 = 7.52; P < 0.001) and the interaction between two factors (F 2, 499 = 3.59; P < 0.05), but not for the factor drug (F 1, 499 = 1.94; P = 0.17). Post hoc test showed that injection of levodopa significantly decreased the low beta band activity in HP-LID group (P = 0.004; Table 1).
Inspection of the power spectrum showed that the altered beta frequency activity was focused in the high beta band (19–30 Hz). Two-way ANOVA showed significant effect for the factor group (F 2, 499 = 8.09; P < 0.001), the factor drug (F 1, 499 = 16.04; P < 0.001), and the interaction between groups (F 2, 499 = 3.51; P < 0.05). Post hoc test showed that high beta band activity was higher in HP and HP-LID groups compared to naïve controls (Table 1).
Coherence of EPN spikes and MCx-ECoG
Analysis of the coherence of theta band activity between EPN spikes and MCx-ECoG showed enhanced coherences in HP and HP-LID rats compared to naïve controls, but no difference between groups. Levodopa injection reduced this measure only in HP-LID rats, without affecting HP rats (Fig. 4a). The statistical analysis with ANOVA of EPN spikes and MCx-ECoG coherence of theta band showed a significant effect for the factor group (F 2, 523 = 7.35, P < 0.001) and for the interaction for the factors group and drug (F 2, 523 = 6.14, P < 0.01), but no statistical difference for the factor drug (F 1, 523 = 1.16, P = 0.28). Post hoc tests confirmed that compared to controls the theta frequency band coherence was higher in the HP rats (P < 0.05) and in the HP-LID rats (P < 0.001), without differences between groups. Injection of levodopa decreased theta band coherence only in HP-LID rats (P < 0.001; Fig. 4b).
Neuronal firing rates, (a) coefficient variation of inter-spike intervals (CV; b), asymmetry index (AI; c) and the percentage of three different discharge patterns (burst, irregular, and regular; d) of the EPN neuronal activity. Significant differences in comparison with naïve control group is indicated by asterisks (*), differences within group after treatment of L-DOPA with ($) and differences between HP and HP-LID comparisons by (#; P < 0.05; two-way ANOVA and post hoc Tukey’s test for the neuronal firing rate; Chi-square test with Bonferroni adjustment for the distributions of discharge patterns)
Analysis of the coherence of beta band activity between EPN spikes and MCx-ECoG showed that beta band activity was more enhanced in HP than in HP-LID rats. Injection of levodopa decreased beta band coherence in both groups. Statistical analysis with ANOVA showed a significant effect for the factor group (F 2, 523 = 6.57, P < 0.002), for the factor drug (F 1, 523 = 17.70, P < 0.001), and for the interaction between factors (F 2, 523 = 4.82, P < 0.008). The beta modulating spike coherence with the MCx was significantly higher in HP rats compared to both naïve and HP-LID rats (P < 0.001). Levodopa injection decreased beta coherence in HP rats (P < 0.001), while this effect did not reach the level of significance in HP-LID rats (P = 0.25; Fig. 4c).
The coherence spectrum analysis that two frequency peaks dominated the spectrum of the beta range, one in the low beta (12–19 Hz) and another in the high beta range (19–30 Hz), i.e., the EPN spikes and motor cortex ECoG coherence showed a shift in the beta spectrum between HP and HP-LID rats (Fig. 4a). Statistical analysis of the low and high beta bands with ANOVA showed a significant effect for the factor group (low: F 2, 523 = 7.05, P < 0.001; high: F 2, 523 = 7.80, P < 0.001), the factor drug (low: F 1, 523 = 18.98, P < 0.001; high: F 1, 523 = 10.28, P < 0.001), and the interaction between factors (low: F 2, 523 = 6.14, P < 0.01; high: F 2, 523 = 5.67, P < 0.01). Post hoc analysis revealed that the low beta range frequency-modulating spike coherence was higher in both HP and HP-LID groups as compared to naïve control rats (P < 0.05; P < 0.001), but also higher in HP-LID rats compared to HP rats (P < 0.05). Levodopa injection decreased the low beta coherence in both HP (P < 0.05) and HP-LID rats (P < 0.001; Fig. 4d). The high beta frequency (19–30 Hz) spikes coherence was higher in HP rats as compared to HP-LID rats (P < 0.001), which did not differ from naïve control rats. Treatment with levodopa significantly decreased the high beta coherence only in HP rats (P < 0.001; Fig. 4e).
EPN spikes and MCx-ECoG phase relation
The EPN spikes and MCx phase-locked ratio of theta band activity was only enhanced in HP-LID rats. Injection with levodopa had a different effect on HP and HP-LID rats. While it reduced the phase-locked ratio in HP-LID rats, this measure was enhanced in HP rats. Statistical analysis with ANOVA showed a significant effect for the factor group (F 2, 523 = 3.74, P < 0.05) and for the interaction between factors (F 2, 523 = 8.59, P < 0.001), while the factor drug had no effect (F 2, 523 = 0.27, P = 0.61). Post hoc testing showed that the theta phase-locked ratio was significantly enhanced only in the HP-LID group (P < 0.001; Fig. 5a). Treatment with levodopa increased the theta phase-locked ratio in the HP group, but decreased this measure in HP-LID rats (P < 0.001).
Coherence of EPN spikes and MCx-ECoG spectral power as shown within the frequency range of 1–40 Hz (a). The bar plot shows the mean ± SEM ratio-transformed coherence of the theta (a) and beta oscillatory coherence (b), as well as for the low- and high beta oscillatory coherence across experimental groups (c and d), respectively. Significant differences in comparison with naïve control group is indicated by asterisks (*), differences within group after treatment of L-DOPA with ($), and differences between HP and HP-LID comparisons by (#; P < 0.05; two-way ANOVA and post hoc Tukey’s test)
The EPN spikes and MCx phase-locked ratio of beta oscillatory activity were enhanced in both HP and HP-LID rats without difference, and injection of levodopa reduced this measure in both groups. Statistical analysis with ANOVA showed a significant effect for the factor group (F 2, 523 = 8.58, P < 0.001), the factor drug (F 1, 523 = 17.75, P < 0.001), and interaction between factors (F 2, 523 = 4.58, P < 0.05). Post hoc analysis showed that the beta phase-locked ratios were higher in both HP and LID groups without difference, and treatment with levodopa decreased beta phase-locked ratio in both groups (P < 0.001; Fig. 5b).
Similar to the coherence of EPN spike and MCx-ECoG, two-way ANOVA of the low and high beta band showed a significant effect for the factor group (low: F 2, 523 = 11.24, P < 0.001; high: F 2, 523 = 8.47, P < 0.001), the factor drug (low: F 1, 523 = 27.24, P < 0.001; high: F 1, 523 = 7.453, P < 0.01), and the interaction between factors (low: F 2, 523 = 6.53, P < 0.01; high: F 2, 523 = 4.73, P < 0.01). Post hoc analysis showed a higher spike-ECoG phase-locked ratio at low beta band in both HP and HP-LID groups compared to naive controls (P < 0.001), but also higher activity in HP-LID rats compared to the HP group (P < 0.01). Levodopa injection significantly decreased the phase-locked ratios in both HP and HP-LID groups (P < 0.001). EPN spike–ECoG phase-locked ratio at high beta frequency band was significantly higher in the HP group compared to both naïve (P < 0.001) and HP-LID rats (P < 0.01); however, the HP-LID rats did not differ from naive controls. Levodopa injection significantly reduced the phase-locked ratio only in the HP group (P < 0.001) (Fig. 5c and d).
Bar graphs show a comparison of the mean ratios between peak-to-trough amplitudes of the original STWA and the mean of 20 shuffled STWAs for MCx-ECoG frequency ranges for the theta (4–8 Hz) (a), beta (12–30 Hz) (b), low beta (12–19 Hz) (c) and high beta (19-30 Hz) (d) for EPN neuronal firing activity
Discussion
Analysis of single-unit activity in the EPN of 6-OHDA-lesioned rats showed enhanced neuronal firing rates, which were reduced by levodopa. This is in line with the classical rate-coding model of PD, which predicts that the loss of nigrostriatal dopamine leads to disinhibition of the STN, which subsequently results in overactivity of the GPi. Dopamine replacement therapy normalizes GPi activity by its action through the direct and indirect striatal output pathway. Consistent with this concept, enhanced neuronal firing rates have been found in the STN and GPi of patients with PD (Rodriguez-Oroz et al. 2001, 2011 ; Brown 2003; Obeso et al. 2006; Wichmann and Dostrovsky 2011; Weinberger et al. 2012) and in experimental studies using the 6-OHDA rat model and the MPTP monkey model of PD (Hollerman and Grace 1992; Wichmann et al. 1994; Hassani et al. 1996; Ni et al. 2001). Further, neurophysiological studies in parkinsonian monkeys (Filion and Tremblay 1991; Boraud et al. 1998) and patients during pallidotomy (Hutchison et al. 1997) have shown a reduction in the firing rate in the GPi after application of dopamine agonists. In PD patients with LID, neuronal activity of the STN and GPi also shifts from increased neuronal firing in the parkinsonian state to marked hypoactivity during expression of dyskinesias (Merello et al. 1999; Lozano et al. 2000). This corroborates findings of Papa et al. (1999), showing that the firing rates in monkeys expressing dyskinesias after administration of levodopa were substantially more reduced than in monkeys without expression of dyskinesias (Papa et al. 1999). These studies used a low dose of levodopa to examine the “on” state without dyskinesias versus the “dyskinesia” state after high dose of levodopa, but did not differentiate between drug-naïve PD and PD with LID after long-term treatment with levodopa. In contrast, in the present study, we measured neuronal activity in drug-naïve HP rats compared to HP-LID rats “off” and “on” levodopa, i.e., did not merely differentiate between the effects of high- and low-dose levodopa in HP-LID rats. With this study design, in the “off” state the EPN firing rate was enhanced in both drug-naïve HP and HP-LID rats without difference. Further, the firing rate was reduced in the “on” levodopa state in both groups without difference, i.e., levodopa did not have a stronger effect on the firing rate in HP-LID rats than on drug-naïve HP rats.
The induction of LIDs by dopamine agonists has not only been associated with a mean reduction in the firing rate, but also with an increase in burst activity in the STN and GPi when patients express dyskinesias (Lozano et al. 2000; Obeso et al. 2000; Levy et al. 2001). In the present study, we found enhanced bursty pattern neurons in HP and HP-LID rats, which did not differ between groups. Treatment with levodopa, however, increased the bursty pattern neurons only in HP rats. Likewise, in both HP and HP-LID rats the irregularity of firing activity, as measured by the CV, was increased with no difference between HP and HP-LID groups, and the effect of levodopa injection was more severe in HP than in HP-LID rats.
Overall, using the 6-OHDA rat model with LID, we did not find differences between HP and HP-LID rats, and acute levodopa injection did not have a stronger effect on neuronal activity in HP-LID rats. The effects seen in the human and monkey studies may therefore be more related to different dosages of dopamine agonists, than to the underlying neurophysiological changes induced by long-term application of levodopa.
Besides the classical rate model, the oscillatory activity in different spectral bands and their synchronization provide information about brain network activity. With that regard, beta oscillations are enhanced in akinetic PD and possibly contribute to bradykinesia and rigidity (Brown 2003; Kuhn et al. 2006; Chen et al. 2007; Kuhn et al. 2008; Ray et al. 2008). Injection of dopamine agonists have been reported to reduce beta band activity in patients with PD (Kuhn et al. 2006; Weinberger et al. 2006; Ray et al. 2008) and in patients expressing dyskinesias (Alonso-Frech et al. 2006). Experimental studies under active state of urethane anesthesia also have also reported a higher beta oscillations in 6-OHDA in the motor cortex and subthalamic nucleus compared to naïve control rats (Alam et al. 2012; Rumpel et al. 2013).
Oscillatory activity of total beta frequency band in the dyskinetic state, however, is not well understood. Over the motor cortex region of both HP and HP-LID rats, total beta (12–30 Hz) band activity was enhanced. Injection of levodopa decreased beta band activity in HP and HP-LID rats. These results support the concept that cortical inputs to the BG may be important for the generation and amplification of the beta oscillatory activity in PD or may even be cortically generated (Magill et al. 2001; McCarthy et al. 2011; Pavlides et al. 2012). Injection of levodopa decreased beta band in HP-LID dykinetic rats more pronounced than in HP rats, leading to a significant difference between these groups. This is in line with clinical studies in PD patients demonstrating that in advanced PD with peak-dose dyskinesias in the “on” levodopa condition beta band activity is markedly reduced (Weinberger et al. 2006, 2012). Further, experimental studies showed that in free moving rats with dyskinesias (HP-LID), beta activity is more reduced by levodopa compared to denovo HP rats (Alam et al. 2014). When differentiating between low (12–19 Hz) and high (19–30 Hz) beta band frequencies, injection of levodopa profoundly decreases the low beta activity only in HP-LID groups, whereas levodopa treatment reduced high beta activity in both HP and HP-LID groups. Recently, it has been proposed that the low beta band shows a greater decrease in power than the high beta component in response to dopaminergic treatment (Lopez-Azcarate et al. 2010; Priori et al. 2004).
Changes in oscillatory activity are thought to be correlates of abnormal neuronal processing in movement disorders. Further, neural spiking activity is likely transiently coupled to the field potentials in a rhythmic or non-oscillatory fashion and allows insight into how altered firing pattern in different nuclei relate to changes in oscillatory activity throughout the basal ganglia network (Fries et al. 2007; Kayser et al. 2009). In the present study, the coherence of EPN spike and MCx field potentials showed enhanced beta activity in both HP and HP-LID rats when using the whole range of beta frequency band (12–30 Hz), however, without difference between groups. This differs from our previous studies using the 6-OHDA rat model of PD, where we observed a higher beta band activity in HP rats than in HP-LID rats (Alam et al. 2014). Interestingly, however, we observed a shift of high (19–30 Hz) to low (12–19 Hz) range of beta in the EPN spikes and MCx coherence between HP and HP-LID groups, which was also observed for the phase relation analysis. High beta range oscillatory activity in the EPN may therefore be associated with drug-naïve PD, whereas enhanced low beta range activity may be a correlate of changes in neuronal activity that parallels the behavioral development of dyskinesias after chronic injection of levodopa.
The role of high versus low oscillatory beta band activity is not entirely clear. In humans it has been suggested that low beta modulations are specific for action observation, whereas high beta modulations have been related to the action scene (Marceglia et al. 2009). The rigid-akinetic parkinsonian state has been associated with an increase in high range beta power (20–30 Hz) in M1 motor cortex or STN (Crowell et al. 2012; Shimamoto et al. 2013). In line with this, the power and coherence of high beta (22–32 Hz) oscillations in the cerebral cortex and STN of awake free moving rats was enhanced in the 6-OHDA rat model of PD (Sharott et al. 2005; Li et al. 2012). On the other hand, several clinical studies described a predominant peak within the low beta band (12–20 Hz) in the off levodopa state, together with a smaller peak in the high beta (20–30 Hz) band (Lopez-Azcarate et al. 2010; Thompson et al. 2014). The low beta peak disappeared after levodopa application or was greatly reduced during movement, whereas the high beta peak remained at similar power (Thompson et al. 2014). It should be noted, however, that after levodopa injection, both high and low beta activities of HP and HP-LID rats decreased to the level of control rats, making it difficult to explain the pathophysiology of LID by these parameter changes.
In patients with PD, it has been shown that oscillatory activity in the theta frequency band was higher after administration of levodopa, i.e., in the ‘On’ dyskinesia condition, than in patients in the ‘Off’ medication state (Alonso-Frech et al. 2006). In line with this, the theta oscillatory activity recorded in different basal ganglia regions was significantly more pronounced in 6-OHDA-lesioned animals with LIDs than in drug-naïve 6-OHDA-lesioned rats (Alam et al. 2014; Meissner et al. 2006). In our present study, theta band activity in the MCx was enhanced in HP, but not in HP-LID rats. Injection of levodopa increased oscillatory theta band activity only HP rats and decreased in HP-LID rats. However, analysis of the coherence and phase lock ratio of theta frequency spike trains of MCx-ECoG showed that although oscillatory theta band activity was somewhat higher in HP-LID rats than in drug-naïve HP rats, levodopa injection only enhanced theta band activity in HP rats, but reduced this measure in HP-LID rats.
It should be noted that all recordings in the present study were made in the urethane anaesthetized condition, and therefore, the genesis of increased theta spike-MCx coherence or phase-locked ratio after treatment of levodopa occurred without the sensory motor feedback of involuntary movements. In particular, theta band activity has been shown to depend on the presence or absence of the abnormal dystonic posture or the phasic movements and may therefore be more pronounced in the non-anesthetized state (Brazhnik et al. 2012; Lemaire et al. 2012).
The pathophysiological mechanisms underlying the development of dyskinesias still need further clarification. While we did not find differences of single-unit activity in the EPN of drug-naïve HP and HP-LID rats, the coupling of neural spiking activity to the MCx oscillatory activity differed between HP and HP-LID.
Further research is needed to investigate whether the interaction between BG activity and cortical processing would indeed be relevant for the occurrence of dyskinesias.
References
Alam M, Heissler HE, Schwabe K, Krauss JK (2012) Deep brain stimulation of the pedunculopontine tegmental nucleus modulates neuronal hyperactivity and enhanced beta oscillatory activity of the subthalamic nucleus in the rat 6-hydroxydopamine model. Exp Neurol 233:233–242. doi:10.1016/j.expneurol.2011.10.006
Alam M, Capelle HH, Schwabe K, Krauss JK (2014) Effect of deep brain stimulation on levodopa-induced dyskinesias and striatal oscillatory local field potentials in a rat model of Parkinson’s disease. Brain Stimul 7:13–20. doi:10.1016/j.brs.2013.09.001
Alonso-Frech F, Zamarbide I, Alegre M et al (2006) Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 129:1748–1757. doi:10.1093/brain/awl103
Benhamou L, Cohen D (2014) Electrophysiological characterization of entopeduncular nucleus neurons in anesthetized and freely moving rats. Front Syst Neurosci 8:7. doi:10.3389/fnsys.2014.00007
Boraud T, Bezard E, Guehl D et al (1998) Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res 787:157–160
Brazhnik E, Cruz AV, Avila I et al (2012) State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci 32:7869–7880. doi:10.1523/JNEUROSCI.0943-12.2012
Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18:357–363. doi:10.1002/mds.10358
Chen CC, Litvak V, Gilbertson T et al (2007) Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp Neurol 205:214–221. doi:10.1016/j.expneurol.2007.01.027
Crowell AL, Ryapolova-Webb ES, Ostrem JL et al (2012) Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain 135:615–630. doi:10.1093/brain/awr332
Filion M, Tremblay L (1991) Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547:142–151
Fries P, Nikolić D, Singer W (2007) The gamma cycle. Trends Neurosci 30:309–316. doi:10.1016/j.tins.2007.05.005
Halliday DM, Rosenberg JR, Amjad AM et al (1995) A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64:237–278
Hassani OK, Mouroux M, Feger J (1996) Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience 72:105–115
Hollerman JR, Grace AA (1992) Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res 590:291–299
Hutchison WD, Lozano AM, Tasker RR et al (1997) Identification and characterization of neurons with tremor-frequency activity in human globus pallidus. Exp Brain Res 113:557–563
Kayser C, Montemurro MA, Logothetis NK, Panzeri S (2009) Spike-phase coding boosts and stabilizes information carried by spatial and temporal spike patterns. Neuron 61:597–608. doi:10.1016/j.neuron.2009.01.008
Kuhn AA, Kupsch A, Schneider GH, Brown P (2006) Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 23:1956–1960. doi:10.1111/j.1460-9568.2006.04717.x
Kuhn AA, Brucke C, Schneider GH et al (2008) Increased beta activity in dystonia patients after drug-induced dopamine deficiency. Exp Neurol 214:140–143. doi:10.1016/j.expneurol.2008.07.023
Labarre D, Meissner W, Boraud T (2008) Measure of the regularity of events in stochastic point processes, application to neuron activity analysis. In: Acoustics, speech and signal processing, 2008. {ICASSP} 2008. {IEEE} International Conference on, pp 489–492
Lemaire N, Hernandez LF, Hu D et al (2012) Effects of dopamine depletion on LFP oscillations in striatum are task- and learning-dependent and selectively reversed by L-DOPA. Proc Natl Acad Sci U S A 109:18126–18131. doi:10.1073/pnas.1216403109
Levy R, Dostrovsky JO, Lang AE et al (2001) Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson’s disease. J Neurophysiol 86:249–260
Li Q, Ke Y, Chan DC et al (2012) Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76:1030–1041. doi:10.1016/j.neuron.2012.09.032
Lindemann C, Alam M, Krauss JK, Schwabe K (2013) Neuronal activity in the medial associative-limbic and lateral motor part of the rat subthalamic nucleus and the effect of 6-hydroxydopamine-induced lesions of the dorsolateral striatum. J Comp Neurol 521:3226–3240. doi:10.1002/cne.23342
Lopez-Azcarate J, Tainta M, Rodriguez-Oroz MC et al (2010) Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci 30:6667–6677. doi:10.1523/jneurosci.5459-09.2010
Lourens MAJ, Meijer HGE, Contarino MF et al (2013) Functional neuronal activity and connectivity within the subthalamic nucleus in Parkinson’s disease. Clin Neurophysiol 124:967–981. doi:10.1016/j.clinph.2012.10.018
Lozano AM, Lang AE, Levy R et al (2000) Neuronal recordings in Parkinson’s disease patients with dyskinesias induced by apomorphine. Ann Neurol 47:S141–S146
Lundblad M, Andersson M, Winkler C et al (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Magill PJ, Bolam JP, Bevan MD (2001) Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience 106:313–330
Marceglia S, Foffani G, Bianchi AM et al (2006) Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease. J Physiol 571:579–591. doi:10.1113/jphysiol.2005.100271
Marceglia S, Bianchi AM, Baselli G et al (2007) Interaction between rhythms in the human basal ganglia: application of bispectral analysis to local field potentials. IEEE Trans Neural Syst Rehabil Eng 15:483–492. doi:10.1109/tnsre.2007.907893
Marceglia S, Fiorio M, Foffani G et al (2009) Modulation of beta oscillations in the subthalamic area during action observation in Parkinson’s disease. Neuroscience 161:1027–1036. doi:10.1016/j.neuroscience.2009.04.018
Marin C, Aguilar E, Bonastre M (2008) Effect of locus coeruleus denervation on levodopa-induced motor fluctuations in hemiparkinsonian rats. J Neural Transm 115:1133–1139. doi:10.1007/s00702-008-0060-5
Marin C, Aguilar E, Mengod G et al (2009) Effects of early vs. late initiation of levodopa treatment in hemiparkinsonian rats. Eur J Neurosci 30:823–832. doi:10.1111/j.1460-9568.2009.06877.x
McCarthy MM, Moore-Kochlacs C, Gu X et al (2011) Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc Natl Acad Sci U S A 108:11620–11625. doi:10.1073/pnas.1107748108
Meissner W, Ravenscroft P, Reese R et al (2006) Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol Dis 22:586–598. doi:10.1016/j.nbd.2006.01.009
Merello M, Lees AJ, Balej J et al (1999) GPi firing rate modification during beginning-of-dose motor deterioration following acute administration of apomorphine. Mov Disord 14:481–483
Ni ZG, Bouali-Benazzouz R, Gao DM et al (2001) Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA-induced dopamine depletion in rats. Brain Res 899:142–147
Obeso JA, Rodriguez-Oroz MC, Rodriguez M et al (2000) Pathophysiology of levodopa-induced dyskinesias in Parkinson’s disease: problems with the current model. Ann Neurol 47:S22–S32 discussion S32–S34
Obeso JA, Rodriguez-Oroz MC, Javier Blesa F, Guridi J (2006) The globus pallidus pars externa and Parkinson’s disease. Ready for prime time? Exp Neurol 202:1–7. doi:10.1016/j.expneurol.2006.07.004
Papa SM, Desimone R, Fiorani M, Oldfield EH (1999) Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann Neurol 46:732–738
Pavlides A, Hogan SJ, Bogacz R (2012) Improved conditions for the generation of beta oscillations in the subthalamic nucleus–globus pallidus network. Eur J Neurosci 36:2229–2239. doi:10.1111/j.1460-9568.2012.08105.x
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press Inc, San Diego
Picconi B, Pisani A, Barone I et al (2005) Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology 26:779–783. doi:10.1016/j.neuro.2005.02.002
Priori A, Foffani G, Pesenti A et al (2004) Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol 189:369–379. doi:10.1016/j.expneurol.2004.06.001
Ray NJ, Jenkinson N, Wang S et al (2008) Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol 213:108–113. doi:10.1016/j.expneurol.2008.05.008
Rodriguez-Oroz MC, Rodriguez M, Guridi J et al (2001) The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain 124:1777–1790
Rodriguez-Oroz MC, Lopez-Azcarate J, Garcia-Garcia D et al (2011) Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 134:36–49. doi:10.1093/brain/awq301
Rumpel R, Alam M, Klein A et al (2013) Neuronal firing activity and gene expression changes in the subthalamic nucleus after transplantation of dopamine neurons in hemiparkinsonian rats. Neurobiol Dis 59:230–243
Sharott A, Magill PJ, Harnack D et al (2005) Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci 21:1413–1422. doi:10.1111/j.1460-9568.2005.03973.x
Shimamoto SA, Ryapolova-Webb ES, Ostrem JL et al (2013) Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson’s disease. J Neurosci 33:7220–7233. doi:10.1523/jneurosci.4676-12.2013
Thompson JA, Lanctin D, Ince NF, Abosch A (2014) Clinical implications of local field potentials for understanding and treating movement disorders. Stereotact Funct Neurosurg 92:251–263. doi:10.1159/000364913
Von Wrangel C, Schwabe K, John N et al (2015) The rotenone-induced rat model of Parkinson’s disease: behavioral and electrophysiological findings. Behav Brain Res 279:52–61. doi:10.1016/j.bbr.2014.11.002
Weinberger M, Mahant N, Hutchison WD et al (2006) Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol 96:3248–3256. doi:10.1152/jn.00697.2006
Weinberger M, Hutchison WD, Alavi M et al (2012) Oscillatory activity in the globus pallidus internus: comparison between Parkinson’s disease and dystonia. Clin Neurophysiol 123:358–368. doi:10.1016/j.clinph.2011.07.029
Wichmann T, Dostrovsky JO (2011) Pathological basal ganglia activity in movement disorders. Neuroscience 198:232–244. doi:10.1016/j.neuroscience.2011.06.048
Wichmann T, Bergman H, DeLong MR (1994) The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 72:521–530
Acknowledgments
The authors are thankful to China Scholarship Council (CNC), for their student fellowship to X. Jin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jin, X., Schwabe, K., Krauss, J.K. et al. Coherence of neuronal firing of the entopeduncular nucleus with motor cortex oscillatory activity in the 6-OHDA rat model of Parkinson’s disease with levodopa-induced dyskinesias. Exp Brain Res 234, 1105–1118 (2016). https://doi.org/10.1007/s00221-015-4532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4532-1