Abstract
Successful adaptation to novel sensorimotor contexts critically depends on efficient sensory processing and integration mechanisms, particularly those required to combine visual and proprioceptive inputs. If the basal ganglia are a critical part of specialized circuits that adapt motor behavior to new sensorimotor contexts, then patients who are suffering from basal ganglia dysfunction, as in Parkinson’s disease should show sensorimotor learning impairments. However, this issue has been under-explored. We tested the ability of 8 patients with Parkinson’s disease (PD), off medication, ten healthy elderly subjects and ten healthy young adults to reach to a remembered 3D location presented in an immersive virtual environment. A multi-phase learning paradigm was used having four conditions: baseline, initial learning, reversal learning and aftereffect. In initial learning, the computer altered the position of a simulated arm endpoint used for movement feedback by shifting its apparent location diagonally, requiring thereby both horizontal and vertical compensations. This visual distortion forced subjects to learn new coordinations between what they saw in the virtual environment and the actual position of their limbs, which they had to derive from proprioceptive information (or efference copy). In reversal learning, the sign of the distortion was reversed. Both elderly subjects and PD patients showed learning phase-dependent difficulties. First, elderly controls were slower than young subjects when learning both dimensions of the initial biaxial discordance. However, their performance improved during reversal learning and as a result elderly and young controls showed similar adaptation rates during reversal learning. Second, in striking contrast to healthy elderly subjects, PD patients were more profoundly impaired during the reversal phase of learning. PD patients were able to learn the initial biaxial discordance but were on average slower than age-matched controls in adapting to the horizontal component of the biaxial discordance. More importantly, when the biaxial discordance was reversed, PD patients were unable to make appropriate movement corrections. Therefore, they showed significantly degraded learning indices relative to age-matched controls for both dimensions of the biaxial discordance. Together, these results suggest that the ability to adapt to a sudden biaxial visuomotor discordance applied in three-dimensional space declines in normal aging and Parkinson disease. Furthermore, the presence of learning rate differences in the PD patients relative to age-matched controls supports an important contribution of basal ganglia-related circuits in learning novel visuomotor coordinations, particularly those in which subjects must learn to adapt to sensorimotor contingencies that were reversed from those just learned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our capacity to learn novel arbitrary associations between vision and action allows for efficient interactions with the world. For instance, the precision with which we manipulate a novel tool or drive a new car depends greatly on our ability to learn and adjust movements to altered external conditions. A common approach to studying how novel sensorimotor associations are formed involves perturbing the relationship between the movement and its sensory consequences, either by altering the relation between the actual and visually presented hand positions (visual distortions), or by changing the relationship between applied forces and resulting hand displacements (mechanical distortions).
In recent years, such adaptation learning has been intensively studied using anatomical, imaging and psychophysical approaches (Imamizu et al. 2000; Ghilardi et al. 2000; Krebs et al. 1998, 2001; Houk and Wise 1995; Grafton et al., 1995; Alexander et al. 1994; Hoover and Strick 1993; Doyon et al. 2003). An increasing body of evidence suggests a substantial contribution of the basal ganglia in sensorimotor learning (Rauch et al. 1997; Krebs et al. 1998; Shadmehr and Holcomb 1999; Graybiel 2004). Of particular interest, a number of recent studies have provided insights about the specific sensorimotor contexts requiring the greatest participation of the basal ganglia (Shadmehr and Holcomb 1999; Krebs et al. 1998, 2001; Contreras-Vidal and Buch 2003; Krakauer et al. 2004). For example, Krebs et al. (1998) examined how patterns of regional cerebral blood flow (rCBF) changed as a function of learning phase when subjects reached to visual targets in a force field. They found a significant contribution of the corticostriatal loop during early implicit motor learning, whereas late implicit motor learning was associated with a large increase in the corticocerebellar loops. However, when the force field was reversed, both the corticostriatal and the corticocerebellar loops showed a significant change in rCBF. These observations provide evidence that basal ganglia are more involved at the beginning of the acquisition process (early exposure) or when a novel situation is imposed (reversal learning). These conclusions were corroborated by Shadmehr and Holcomb (1999) who used a similar force-field task with PET. Likewise, using fMRI, Seidler et al. (2006) found initial basal ganglia engagement when subjects adapted to visuomotor rotations. However, other studies have failed to show such initial basal ganglia activation (Inoue et al. 2000), have found no initial but only late basal ganglia activation (Graydon et al. 2005), or have found initial basal ganglia engagement in gain adaptation only (Krakauer et al. 2004).
If the basal ganglia are a critical part of specialized circuits that adapt motor behavior to new sensorimotor contexts, then patients who are suffering from basal ganglia dysfunction, as in Parkinson’s disease should show sensorimotor learning impairments. Successful adaptation to novel sensorimotor contexts critically depends on efficient sensory processing and integration mechanisms, particularly those required to combine visual and proprioceptive inputs. Studies of adaptation learning in PD patients have also led to sometimes conflicting results, with some studies showing mild or no sensorimotor learning impairments, whereas others reported marked learning deficits (Stern et al. 1988; Fucetola and Smith 1997; Contreras-Vidal et al. 2002; Teulings et al. 2002; Contreras-Vidal and Buch 2003; Fernandez-Ruiz et al. 2003; Krebs et al. 2001). Moreover, very few studies have examined the specific sensorimotor learning phases within which PD patients show deficits. To further investigate the specific effects of Parkinson disease and normal aging on visuomotor adaptation-learning abilities, we compared the pointing accuracy of young controls, elderly controls and PD patients in a novel visuomotor multistage learning task.
Finally, almost all previous studies have analyzed movements restricted to a single plane or have presented visual feedback in a different plane from that in which the movements were performed. Such requirements can alter movement accuracy compared to conditions of unconstrained movements with feedback presented in the same space as the movements (Messier and Kalaska 1997; Desmurget et al. 1997). In the present experiment, we used a three-dimensional virtual immersive environment to dissociate visual from proprioceptive information. The position of a simulated arm endpoint used for movement feedback was altered by shifting its apparent location diagonally, requiring thereby both horizontal and vertical compensations. No study has yet examined the ability of PD patients to compensate for a visual distortion during natural unconstrained movements within three-dimensional space. This becomes particularly important since reaching in the vertical dimension requires compensation for gravity, a dimension that requires the most dependence on processing proprioception. Since this sense is impaired in PD patients (e.g., Maschke et al. 2003), it is predicted that PD patients should show difficulties in a sensorimotor learning task in which novel visuomotor relationships are imposed. To further test the nature of adaptation learning in PD patients and to examine whether it is globally impaired or selectively deficient, we used a four phase learning paradigm. This paradigm comprised baseline performance, initial learning, reversal learning (in which the sign of the distortion in initial learning was reversed), and aftereffect (baseline performance again). The comparison of pointing errors made during the baseline before exposure to the visual distortion to those made in initial learning will allow the dissociation of any difficulty PD patients have in utilizing and integrating proprioceptive information from deficits in learning the novel visuomotor relationships. Importantly, the reversal phase of learning required subjects to switch from one newly learned motor pattern to a different motor pattern, since subjects were exposed to opposite biaxial discordances in quick succession. If the basal ganglia play a critical role in facilitating learning of novel sensorimotor contexts, then Parkinsonian subjects should show impairments in initial and reversal learning, perhaps with the greatest impairments during the reversal learning phase.
Methods
Subjects
Ten neurologically normal young adults (mean age = 27), eight PD subjects (mean age = 71; range 61–79 years) and ten age-comparable healthy controls (mean age = 68.5; range 63–78 years) participated in this study.Footnote 1 There was no significant difference in age between elderly healthy subjects and PD patients (t = –1.056, P > 0.05). All subjects were right-handed individuals. The PD subjects were evaluated by a movement disorders specialist at the time of testing and were found to have mild to moderate Parkinson’s disease (Hoehn and Yahr Stages II and III; Hoehn and Yahr 1967), and showed motor scores ranging from 25.5 to 48 on the United Parkinson’s Disease Rating Scale (UPDRS, Fahn and Elton 1987) (see Table 1). Parkinsonian subjects were also evaluated with neuropsychological tests. PD subjects showing signs of dementia or depression as revealed by a battery of tests that included the Mini-Mental Test (cut-off score <25/30) and Beck Depression Inventory (cut-off score >10) were excluded from the experiments. PD subjects were studied the morning before taking their daily anti-parkinsonian medication, so that they were at least 12 h off medication. Subjects were informed about the general nature of the study and signed an institutionally approved consent form. However, no information was provided about the specific nature of the visual distortion applied in the VR world.
Virtual reality environment
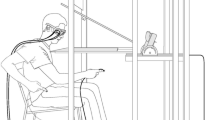
Software routines were developed to present subjects with a VR visual world, to graphically simulate the motions of their arms, and to calibrate the VR world using movements sensing devices and an SGI Octane/sse workstation. To view the VR world, the subject wore a head-mounted display unit (Virtual Technologies, Inc. V–8 headset) that provided immersive stereo visual input. The arm’s position and orientation was monitored by signals from “Flock of Birds” electromagnetic sensors (Ascension Technology, Inc.) positioned on the right shoulder, wrist, and index fingertip, and on the head-mounted display.
General experimental procedure
Subjects were seated with their backs resting on the back of a straight-back chair. The VR world was adapted to each participant by a calibration procedure. First, the position of the left eye and the inter-pupillary distance were computed by appropriate placement of Flock of Birds markers and a software routine. This allowed computation of the viewpoint, which was defined to be the point directly between the two eyes, and allowed creation of a coherent immersive stereo visual input. Second, the recorded position of the marker placed on the headset was translated to the position of the viewpoint, so that rotations of the head produced rotations of the virtual world centered about the viewpoint. Finally, the length of the subject’s arm from shoulder to wrist was measured to normalize across subjects the placement of the target (a green sphere) in the virtual world at a distance reachable without full arm extension and close to subject’s midline at eye level.
The experiment consisted of a series of conditions. The first was a “familiarization” condition in which participants executed ten movements in the VR world in the absence of any perturbations and with continuous visual feedback in the form of a stick finger representing the subject’s index displayed in VR. The start position of each movement was indicated with a Velcro marker (7 mm × 7 mm) placed on the right thigh of participant 10 cm from the knee. For a starting trial, subjects were asked to point in front of them at eye level in the VR environment. No target was displayed in the VR world for this first trial. After each movement, a 3D reconstruction of the subject’s hand trajectory was shown simultaneously with the target location (Fig. 1a). Also, the subject’s finger movement was replayed. That is, subjects could see the displacement of the stick finger moving along the trajectory path, thus providing dynamic visual information about the previous trial.
Subjects were then required to point to the spatial position that would minimize the distance between the target and their trajectory endpoint, and to bring back their arm to the initial position. Subjects were encouraged to make straight and uncorrected movements at natural speed. The “go” signal for the subject to initiate movements was given verbally by the experimenter to insure that subjects were ready to execute their movements. There was no instruction to initiate responses rapidly, which might have influenced reaction time.
The second condition consisted of “baseline” trials during which subjects performed twelve movements in absence of any perturbations. In contrast to the familiarization block, visual feedback was removed during the movements, thereby forcing participants to rely only on the visual feedback (trajectory and target) shown after each trial to maintain and/or improve their accuracy level. The third condition consists of the “initial learning”. Subjects performed twenty trials while exposed to a biaxial visuomotor discordance. The same visual feedback mode (trajectory and target) was used as in the baseline condition; however this perturbation shifted the trajectory used for movement feedback diagonally, 10 cm to the right and 10 cm higher than the veridical feedback provided in the Baseline condition, requiring thereby both horizontal and vertical compensations. Thus, this visual distortion forced subjects to learn new coordinations between what they see in VR and the actual position of their arm, which they must derive from proprioceptive information since no vision of the arm was given during the movement.
The fourth condition was “reversal learning”. This condition required the learning of the reverse biaxial discordance; the trajectory endpoint was now shifted 10 cm to the left and 10 cm lower than the veridical feedback provided in the baseline condition. Fifteen trials were performed during the reversal learning condition.
In the fifth condition or “aftereffect”, participants performed ten movements in the undistorted VR world as in baseline.
Kinematic recordings and data analysis
As mentioned previously, the arm’s position and orientation was monitored by signals from “Flock of Birds” electromagnetic sensors (Ascension Technology, Inc.) positioned on the right shoulder, elbow, wrist, and fingertip, and on the head-mounted display. The position of each marker was sampled at 100 Hz. The position series were then digitally low-pass filtered using a Butterworth filter with a cutoff frequency of 8 Hz. Movement onset was defined as 5% of peak velocity of the index fingertip. After the hand accelerated from rest, and continued until the velocity returned to near zero at the end of the outward movement, where the path reversed direction and began returning toward the subject’s body. The point of path reversal was determined by a minimum in tangential velocity and/or by visually determining the spatial reversal of the trajectory, i.e., the first time the handpath changed direction and returned toward the subject’s body. This reversal point was defined to be movement offset. The movement paths that corresponded to the outward movements toward the target were selected for further analysis. All trajectories were visualized in 3D and could be rotated, translated, scaled, and viewport mapped in real-time for interactive analysis (Poizner et al. 1998).
Performance indices and statistics
Since the distortion of the VR environment was restricted to the frontal plane, constant and absolute horizontal and vertical pointing errors were analyzed. Constant horizontal and vertical errors were calculated as the deviation between the coordinates of the target and those of index finger endpoint in the horizontal (lateral direction ‘x’) and vertical (vertical direction ‘z’) dimensions, respectively. Absolute horizontal and vertical errors were absolute values of the constant horizontal and vertical errors respectively.
Constant errors (signed errors) can cancel each other out when averaged across trials and subjects, should some errors be positive and others negative, thereby reducing the magnitude of the average errors. Therefore, absolute errors were used to indicate the size of errors, whereas the constant horizontal and vertical errors indicated the spatial location of the hand relative to the target in all task phases.
The standard deviations of constant horizontal and vertical errors obtained for each subject for the baseline condition and the last five trials of initial learning, reversal learning and aftereffect were used as variability indexes to test for group differences in trial-to-trial variability. This allowed us to test how subject groups differed in their ability to attain a ‘stable’ level of accuracy by the end of all task phases.Footnote 2
Baseline
The control subjects and PD patients could easily “touch” the targets with close to zero horizontal and vertical constant error in VR after 5–10 trials of training in the familiarization condition. However, in the baseline condition, after the removal of visual feedback of hand displacement during the movement, accuracy and inter-trial variability increased for the first baseline trials before the performance stabilized. For this reason, only the eight last trials of baseline condition were analyzed in this study.
To test whether control subjects and PD patients showed similar levels of accuracy during the baseline condition, separate two factor analyses of variance (group × trial) were performed on the constant horizontal and vertical errors of each subject over the last eight trials. Further, to test for group difference in trial-to-trial variability, single factor analyses of variance were performed on the standard deviation of horizontal and vertical errors made by each subject during baseline trials. For the two analyses mentioned above as well as for all variance analyses used in this study a significance level of 0.05 was used. Post-hoc pairwise comparisons across groups were then performed with a Newman–Keuls test. For these post-hoc analyses, we reduced the probability of type I error by dividing the original alpha level (0.05) by the number of planned comparisons. Thus, given that we compared young controls to elderly controls and older controls to PD patients, an alpha level of 0.025 was used for these analyses.
Learning conditions
In order to test if all three subject groups made a similar constant horizontal and vertical error when first exposed to both initial and reversal visuomotor biaxial discordances a single factor ANOVA was performed on the first trial of exposure to each visual distortion.
To test for difference in learning rates exhibited by each subject group, separate two factor analyses of variance (group × trial) were performed on the constant and absolute horizontal and vertical errors obtained during both initial and reversal learning for each subject of each group.
To further determine how subject groups differed in their ability to learn the biaxial visuomotor discordances, we computed an adaptation magnitude score. This was calculated as the difference between the constant horizontal and vertical errors obtained when first exposed to each visual distortion (trial 1) and the average value of the last five trials of exposure to each visual distortion.2
Aftereffect
To further evaluate adaptation to the reversed visuomotor biaxial discordance, an aftereffect test was performed separately on horizontal and vertical constant errors. Comparisons between the average baseline constant errors and the constant error made when the discordance was first removed was tested using t-tests for independent samples for both the horizontal and vertical constant errors for each subject group. Also, to assess any fatigue effect, i.e., whether subjects were able to return to their baseline level of accuracy by the end of aftereffect, we compared the average baseline constant errors (last 5 trials) with the average aftereffect constant errors (last 5 trials) using t tests for independent samples, for both the horizontal and vertical constant errors for each subject group.
Results
PD patients showed learning deficits along both the vertical and the horizontal dimensions of the biaxial discordance
To investigate how exposure to the sequential biaxial discordances influenced the accuracy and learning rate of controls and PD patients, separate ANOVAs were performed on the horizontal and vertical dimensions of the endpoint positions of subjects for all task phases. This allowed the evaluation of the possible difference in the time course and magnitude of adaptation to the vertical and horizontal dimensions of the biaxial discordances.
Accuracy along the horizontal dimension
Figure 2a and b shows constant and absolute reaching errors made along the horizontal dimension during the 12 trials of the baseline (pre-exposure phase), during initial learning, learning of the reversed discordance and aftereffect conditions for the three subject groups. In the baseline condition, PD patients as well as both groups of control subjects made very small horizontal constant errors that were of similar magnitude, with mean constant errors ranging from −1.08 cm to 1.21 cm. Moreover, all groups showed a stable level of horizontal accuracy across baseline trials. There was no significant difference in the magnitude of horizontal constant errors both among groups (F (2,25) = 0.50; P > 0.05) and between trials (F (7,175) = 0.54; P > 0.05). However, the magnitude of trial-to-trial variability significantly differed between groups (F (2,25) = 7.97; P < 0.05). Post-hoc tests indicated that young and elderly controls displayed similar levels of intertrial variability along the horizontal dimension (P > 0.05), whereas intertrial variability was significantly higher in the PD subjects compared to elderly control subjects (P < 0.025).
a Mean constant horizontal pointing errors for each of the three groups of subjects for all task phases as a function of trial number. Positive horizontal constant errors correspond to movement endpoints to the right of the target, whereas negative horizontal constant errors correspond to movement endpoints to the left of the target. Error bars represent standard errors of the means (SE). b Mean absolute horizontal pointing errors for each of the three groups of subjects for all task phases as a function of trial number
When the initial biaxial discordance was suddenly and unexpectedly introduced, control subjects and PD patients made a large horizontal constant error of similar size to the imposed visuomotor discordance (Fig. 2a). Although this initial error appeared, on average, slightly larger for PD patients than controls, this between group difference did not reach statistical significance (F (2,25) = 2.54; P > 0.05).
For young controls, this initial horizontal error declined rapidly. During early exposure trials, young controls made large compensatory movements, i.e., movements of large amplitude that either undershot or overshot the horizontal position of the virtual target. As a result, the mean horizontal constant error across subjects was close to zero after only 1 trial of interaction with the visuomotor discordance (Fig. 2a). In contrast, although elderly controls and PD patients also improved with training, they compensated for a smaller proportion of the distortion in each movement and thus exhibited a slower decrease in the magnitude of constant horizontal errors (Fig. 2a). However, the average horizontal errors made by elderly and young controls showed great overlap from intermediate to late exposure trials, whereas those of PD patients were systematically larger than both control groups during all trials of exposure to the initial visuomotor discordance. Accordingly, there was a significant main effect of both group (F (2,25) = 8.58; P < 0.05) and trials (F (19,475) = 13.87; P < 0.05), as well as a significant group by trial interaction (F (38,475) = 2.64; P < 0.05). Post-hoc comparisons across groups revealed that when constant horizontal errors were pooled over all exposure trials to the initial discordance, there was no significant difference between young and elderly subjects (P > 0.05), but there was a significant difference between elderly controls and PD patients (P < 0.025). To exclude the possibility that the observed significant difference between elderly controls and PD patients is due to intersubject differences in the first exposure trial to the initial discordance, the analysis was subsequently performed on constant horizontal errors normalized for each subject to the magnitude of the horizontal error that subject made when first exposed to the initial discordance. For these normalized horizontal errors, the difference between elderly controls and PD patients fell below the corrected significance level (P = 0.035). However, consistent with the observed between group differences in the initial rate of adaptation to the horizontal component of the visuomotor biaxial discordance, the group by trial interaction was still significant (F (38,475) = 2.38; P < 0.05, Fig. 2).
To further evaluate these between group differences, we analyzed the horizontal constant errors made during the first five exposure trials to the initial discordance. As expected, there was both a main effect of group (F (2,25) = 11.80; P < 0.05) and a main effect of trial (F (4,100) = 13.05; P < 0.05) as well as a significant group by trial interaction (F (8,100) = 2.48; P < 0.05). During early exposure trials, young subjects made significantly smaller horizontal constant errors than elderly controls (P < 0.025), whereas elderly controls and PD patients made horizontal errors of similar magnitude (P > 0.05).
The results were similar when applied on absolute horizontal errors. There were main effects of group (F (2,25) = 6.13; P < 0.05) and trial (F (19,475) = 21.07; P < 0.05), and the group by trial interaction was significant (F(38,475) = 2.19; P < 0.05). As was the case for the normalized constant horizontal errors, the difference between elderly controls and PD patients did not reach significance. Further, the comparison of horizontal absolute errors made during the first five trials of exposure revealed main effects of group (F (2,25) = 6.63; P < 0.05) and trial (F (4,100) = 11.42; P < 0.05) and a significant group by trial interaction (F (8,100) = 2.19; P < 0.05). However, in contrast to horizontal constant errors, the difference between the horizontal absolute errors made by elderly and young controls during early exposure to the initial discordance did not reach significance. These results indicate that while the size of horizontal errors (absolute) made by elderly and young controls were similar during early exposure to the initial discordance, young controls showed less of a spatial bias in their movement endpoints around the target, and as a result, had smaller constant horizontal errors.
However, the adaptation magnitude score for the horizontal component of the initial biaxial discordance was similar in all three groups (F (2,25) = 0.02; P > 0.05). Further, elderly controls and PD patients were able to stabilize their performance by the end of exposure to the initial discordance as revealed by their similar level of trial-to-trial variability along the horizontal dimension during late exposure trials (F (2,25) = 2.86; P > 0.05).2
These results confirmed the patterns seen in Fig. 2a and b that elderly controls and PD patients were able to adapt their movements to the horizontal component of the novel visuomotor arrangement. However, they did so less efficiently than young controls and therefore, needed more trials to reach asymptotic performance.
Consistent with both their similar adaptation magnitude to the horizontal component of the initial biaxial discordance and their comparable trial-to-trial variability level during late exposure trials to the initial discordance, all three subject groups showed an average constant horizontal error of similar magnitude when the visual perturbation was first reversed (F (2,25) = 0.94; P > 0.05, Fig. 2a). Note that the magnitude of the horizontal error made for this first exposure trial represents the additive effect of the size of the imposed visual distortion and the aftereffect of initial learning.
During reversal learning, both young and elderly controls largely compensated for their initial horizontal error during early exposure trials and thereafter returned to their baseline level of horizontal accuracy (Fig. 2a, b). In contrast, PD patients reduced their horizontal errors more slowly and continued to produce larger horizontal errors than both control groups during intermediate and late exposure trials to the reverse discordance. As shown in Fig. 2a and b, these larger horizontal errors were associated with large between subject variability during all reversal learning exposure trials. In accord with these observations, there was a significant main effect of both group (F (2,25) = 6.88; P < 0.05) and trials (F (14,350) = 21.38; P < 0.05) as well as a significant group by trial interaction (F (28,350) = 1.90; P < 0.05) on horizontal constant errors. Post-hoc comparisons across groups confirmed that there was no significant difference between young and elderly controls (P > 0.05) whereas the difference between PD patients and elderly controls was marginally significant during reversal learning (P < 0.027).
Furthermore, examination of trial-to-trial variability during reversal learning revealed a large increase in oscillatory behavior in PD patients. Indeed, PD patients were unable to stabilize their performance by the end of exposure to the reversed discordance such that the average horizontal variable errors of PD patients (6.18 cm) was nearly three times greater than those of both young (1.19 cm) and elderly controls (2.21 cm) during late reversal learning trials. Accordingly, there was a significant difference in horizontal variable errors between groups for late exposure trials to the reverse discordance (F (2,25) = 5.97; P < 0.05). Young and elderly controls displayed similar level of intertrial variability (P > 0.05), whereas PD patients showed significantly larger intertrial variability than age-matched controls (P < 0.025).
The oscillatory behavior of some PD subjects was so pronounced that on some trials they made large errors in both positive and negative directions during all trials of exposure to the reverse discordance. As a result, horizontal constant errors canceled each other out when averaged across subjects and therefore largely reduced the magnitude of horizontal constant errors shown in Fig. 2a. Thus, for reversal learning, the significantly degraded performance of PD patients is better captured by the observation of absolute horizontal errors (Fig. 2b). Accordingly, the analysis of variance on absolute horizontal errors revealed a main effect of both group (F (2,25) = 20.60; P < 0.05) and trials (F (14,350) = 41.67; P < 0.05) and the group by trial interaction was significant (F (28,350) = 2.24; P < 0.05). Further, post hoc tests confirmed that PD patients made significantly greater absolute horizontal errors than elderly controls (P < 0.025) during the reversal phase of learning.
Consistent with the results mentioned above, there was a significant main effect of group on the adaptation magnitude score for the reversal visuomotor discordance (F (2,25) = 4.26; P < 0.05). Young and elderly controls showed similar adaptation magnitude score (P > 0.05). However, likely due to the large amount of intertrial and intersubject variability in the PD patients group, the difference between elderly controls and PD patients was only marginally significant at the corrected alpha level (P = 0.028). This difference was, however, highly significant when computed on absolute horizontal errors (P < 0.025). Further, PD patients displayed post-exposure shifts (aftereffects) when computed in terms of signed horizontal errors. However, in striking contrast to the control subject groups who showed degradation in accuracy similar in magnitude to the horizontal bias (10 cm) when the reversed discordance was first removed, PD patients showed an absolute horizontal error of similar magnitude to those made during late exposure trials to the reversed discordance (Fig. 2b).
Accuracy along the vertical dimension
All three subject groups displayed a stable level of constant vertical errors during baseline. However, PD patients showed a pronounced systematic downward shift of movement endpoints (Fig. 3a). There was a significant difference between groups (F (2,25) = 8.38; P < 0.05) but no overall difference across trials (F (7,175) = 0.48; P > 0.05) in the magnitude of the constant vertical errors. Young and elderly controls made constant vertical errors of similar magnitude (P > 0.05), whereas those of PD patients were significantly larger than those of age-matched controls (P < 0.025).
a Mean constant vertical pointing errors for each of the three groups of subjects for all task phases as a function of trial number. Unfilled triangles represent the mean constant vertical errors after subtracting the baseline downward bias of each PD subject. Positive vertical constant errors correspond to movement endpoints above the target, whereas negative vertical constant errors correspond to movement endpoints below the target. Error bars represent standard errors of the means (SE). b Mean absolute vertical pointing errors for each of the three groups of subjects for all task phases as a function of trial number
Consequently, young and elderly controls made an initial large vertical error of similar magnitude when the visuomotor discordance was introduced (Fig. 3a). As for the horizontal dimension, young controls quickly adapted their movements to the vertical component of the initial biaxial discordance and thus rapidly reduced their initial vertical error, while elderly controls reduced these errors more gradually (Fig. 3a). PD patients, however, made a much smaller initial vertical error than control subjects and thereafter slightly decreased the magnitude of their vertical errors throughout exposure to the initial discordance. As a result, the three subject groups displayed a similar vertical accuracy level during intermediate and late exposure trials.
The vertical component of the biaxial discordance moved the apparent position of the simulated arm endpoint used for movement feedback higher than the actual hand position, thereby forcing subjects to point lower than the target location presented in the virtual environment. Since PD patients were already pointing lower during the baseline condition, there was no need for large compensations along the vertical axis. Therefore, there was no evidence of learning along the vertical dimension in PD patients. Indeed, this simplified the learning of the initial biaxial discordance in PD patients as they were not required to produce large movement adjustments along the horizontal and vertical axes simultaneously.
The above mentioned results were confirmed by the analysis of variance. For constant vertical errors in initial learning, there was no main effect of group (F (2,25) = 2.72; P > 0.05) but a significant effect of trials (F (19,475) = 8.67; P < 0.05) and a significant group by trial interaction (F (38,475) = 2.13; P < 0.05). In a similar manner as for the horizontal dimension, we further analyzed the group by trial interaction by applying the ANOVA to the first 5 trials following introduction of the visuomotor distortion. There was a main effect of both group (F (2,25) = 6.23; P < 0.05) and trials (F (4,100) = 8.47; P < 0.05) and a significant group by trial interaction (F (8,100) = 3.59; P < 0.05). Post-hoc tests confirmed that elderly controls made larger constant vertical errors than young controls during early exposure trials (P < 0.025). Additionally, consistent with the smaller initial vertical errors made by PD patients, their constant vertical errors were significantly smaller than those of age-matched controls during early exposure trials of initial learning (P < 0.025). Similar results were found when ANOVA were applied on absolute vertical errors. There was no main effect of group (F (2,25) = 3.35; P > 0.05) but a significant effect of trials (F (19,475) = 9.05; P < 0.05) as well as a significant group by trial interaction (F (38,475) = 2.29; P < 0.05).
Furthermore, in accord with these findings, there was a significant between group difference in the adaptation magnitude score for the vertical component of the initial discordance (F (2,25) = 7.39; P < 0.05). Young and elderly controls showed a similar level of adaptation (P > 0.05), while PD patients displayed significantly smaller adaptation magnitude (P < 0.025).
During reversal learning, both young and elderly controls showed an initial rapid reduction in vertical errors, followed by a more progressive decline throughout intermediate and late exposure trials (Fig. 3a). Therefore, although their initial vertical error was very large (20 cm), both control groups easily made the required compensations bringing their final vertical errors on average to 1–2.5 cm during late exposure trials. In striking contrast, PD patients only slightly moved their hand higher along the vertical axis across learning trials, and therefore, did not completely compensated for the reverse discordance. As a result, they showed a constant vertical error that was on average nearly four times greater than that of both young and elderly controls, even at the end of the reversal condition (Fig. 3a). Analysis of variance on constant vertical errors revealed both a main effect of group (F (2,25) = 56.50; P < 0.05) and trials (F (14,350) = 17.25; P < 0.05) as well as a significant group by trial interaction (F (28,350) = 1.73; P < 0.05). Post-hoc tests showed a significant difference between PD patients and age-matched controls (P < 0.025), but no difference between young and elderly controls (P > 0.05). These effects were also found when absolute vertical errors were analyzed. There was a main effect of group (F (2,25) = 51.86; P < 0.05) and trials (F (14,350) = 27.24; P < 0.05) and a significant interaction (F (28,350) = 2.33; P < 0.05).
Furthermore, there was a significant group difference in the adaptation magnitude score (F (2,25) = 17.42; P < 0.05). Again, young and elderly controls showed similar adaptation levels (P > 0.05), while PD patients displayed significantly smaller adaptation magnitude than age-matched controls (P < 0.025) during reversal learning. The marked impairment in PD patient’s reversal learning was also evidenced by an absence of an aftereffect. Following removal of the reverse discordance, both control groups showed a large deterioration in their pointing accuracy along the vertical dimension. As a result, the constant vertical error made when the reverse discordance was first removed was significantly larger than the average constant vertical error made during baseline for both young (t = −14.16; P < 0.05) and elderly (t = −7.80; P < 0.05) controls. By contrast, the vertical constant error made by PD patients when the reverse discordance was first removed was similar than the average constant vertical error made during baseline (t = −0.51; P > 0.05). Further, PD patients maintained this level of accuracy until the end of aftereffect. As a result, there was no significant difference between the average constant vertical error made during the last five trials of baseline and the average constant vertical error made during the last five trials of aftereffect (t = −1.04, P > 0.05). The observation that PD patients showed a similar level of accuracy along the vertical axis at the end of the first (baseline) and the last task phase (aftereffect) suggests that fatigue was not a major contributor of the observed degradation in performance of PD patients during reversal learning.
In contrast to initial learning, the reverse discordance shifted the apparent hand location lower than the actual hand position, thereby forcing subjects to point higher than the target location presented in the virtual environment. Therefore, instead of reducing the magnitude of the downward shift exhibited by PD patients, as was the case during initial learning, the implementation of the reverse discordance would increase the magnitude of the downward shift.
To test whether the large baseline vertical bias of the PD patients is the major contributor to the much slower adaptation rates of PD patients observed along the vertical dimension, the mean constant vertical error made by each PD subject during the baseline was subtracted from the vertical bias made by the subject for each trial for reversal learning. Subsequently, an analysis of variance was performed on these ‘corrected’ constant vertical errors values. This analysis revealed that even after subtracting these baseline downward shifts, there was still a significant between group difference in the overall magnitude of vertical constant errors made during reversal learning (F (2,25) = 15.70; P < 0.05; Fig. 3a). Importantly, constant vertical errors made by PD patients were still significantly larger than those of age-matched controls (P < 0.025). Similar results were obtained when this subtraction analysis was performed on absolute vertical errors. There was a significant main effect of group (F (2,25) = 12.91; P < 0.05; Fig. 3b) with absolute vertical errors made by PD patients being significantly larger than those of elderly controls (P < 0.025). These finding indicates that the impaired ability of the PD patients to learn the vertical component of the reversal discordance was not entirely due to performance factors such as those related to moving the arm against gravitational forces acting along the vertical axis.
Discussion
To investigate the effect of Parkinson’s disease and normal aging on adaptation learning, we compared the pointing accuracy and learning rates of young controls, elderly controls and PD subjects in a novel visuomotor multistage learning task. Subjects were required to adapt to an initial sudden biaxial discordance between the actual hand displacement and one visually-simulated in a three-dimensional virtual environment. They then had to adapt to the reversed visuomotor discordance in quick succession. Since the basal ganglia are known to be important for sensorimotor learning (Graybiel 2005; Barnes et al. 2005), and since learning new visuomotor mappings depends on efficient use of proprioceptive information which is impaired in Parkinson Disease (Schneider et al. 1986; Jobst et al. 1997; Zia et al. 2000; Klockgether et al. 1995; Contreras-Vidal and Gold 2004; Adamovich et al. 2001), we predicted that PD patients would show difficulty in learning new sensorimotor mappings. In a previous study, a multistage force field learning task was used to examine how specific learning phases may differentially impact the ability of PD subjects to learn to adapt their movements to an imposed force field (Krebs et al. 2001). In that study, the learning rate of PD subjects and age-matched controls was evaluated during early motor learning of an initial force field, during late motor learning and during the learning of a force field of opposite direction. The learning rate of PD patients was less than that of control subjects in all phases. However, the greatest deterioration in the learning indices of the PD patients was found during reversal learning. Based on this prior work, we predicted that PD patients would show more severe deficits in the reversal phase of learning than in the initial learning of a visuomotor biaxial discordance.
The results showed learning phase-dependent difficulties in both aged subjects and PD patients. First, elderly controls were slower than young subjects when learning both dimensions of the initial biaxial discordance. However, their performance improved during reversal learning and as a result, elderly and young controls showed similar adaptation rates during reversal learning. Second, in striking contrast to healthy elderly subjects and consistent with our predictions, PD patients were more profoundly impaired during the reversal phase of learning. PD patients were able to learn the initial visuomotor bias but were on average slower than age-matched controls in adapting to the horizontal component of the biaxial discordance. This difference, however, did not reach the corrected significance level likely due to the large amount of intersubject variability. More importantly, when the biaxial discordance was reversed, PD patients were unable to make appropriate movement corrections. Consequently, they showed slower adaptation rates and smaller adaptation scores relative to age-matched controls for both dimensions of the biaxial discordance. Together, these results suggest that the ability to adapt to a sudden biaxial visuomotor discordance applied in three-dimensional space declines in normal aging and in Parkinson disease. Furthermore, the presence of learning rate differences in the PD patients relative to age-matched controls supports an important contribution of basal ganglia to adaptation learning, particularly when the task requires a rapid reconfiguration of newly learned visuomotor coordinations.
PD subjects are impaired when required to reconfigure visuomotor mappings
Initial learning
When the initial biaxial discordance was first introduced, young controls immediately made large movement compensations in the appropriate direction, whereas some elderly controls and PD patients moved in the erroneous direction which increased their spatial errors instead of reducing it (Fig. 2a). This inappropriate behavior was more frequent in the PD group which may account, at least in part, for the observed slower learning rate along the horizontal dimension. Nonetheless, PD patients displayed similar adaptation scores as age-matched controls. These observations suggest that during initial learning, PD patients were able to improve their accuracy with training but were less efficient than age-matched controls at using knowledge of result (KR) about their spatial errors to select and scale appropriate movement compensations for the imposed visual perturbation.
The vertical component of the initial biaxial distortion moved the apparent position of the simulated arm endpoint used for movement feedback higher than the actual hand position, thereby forcing subjects to point lower than the target location presented in the VR world. Since PD patients were already pointing lower than the target during the baseline condition, the imposed vertical error could not be detected so that there was no need for PD patients to produce large movement adaptations along the vertical axis. Thus, the accurate vertical components of PD patients’s movements during initial learning are likely due to their baseline vertical bias. Accordingly, and in contrast to elderly controls, no learning curve was observed along the vertical dimension for PD patients.
Given this downward shift in movement endpoints, the learning of the initial biaxial discordance was simplified for PD patients, as it eliminated the requirement to simultaneously adapt their movements to bias applied along two dimensions of the 3D space. The performance of PD patients usually degrades when task complexity increases or when multiple motor acts must be simultaneously produced (Benecke et al. 1986; Jackson et al. 2000; Poizner et al. 2000; Pessiglione et al. 2003). Therefore, the extent to which PD patients are impaired during initial learning of a complex novel visuomotor coordination in absence of vision during 3D movements may have been underestimated in this study. Nevertheless, the observation that PD patients were eventually able to learn the horizontal component of the biaxial discordance is in general agreement with studies showing that the adaptation-learning abilities are not completely compromised in PD patients (Stern et al. 1988; Fucetola and Smith 1997; Contreras-Vidal et al. 2002; Teulings et al. 2002; Fernandez-Ruiz et al. 2003; Contreras-Vidal and Buch 2003). The larger constant horizontal errors made by PD patients during exposure to the initial biaxial distortion reflect modest impairments in the ability of PD patients to adapt their movements to a new initial mapping between visual information and motor responses.
Reversal learning
In striking contrast to initial learning, differences between PD patients and age-matched controls were marked during exposure to the reverse discordance. Indeed, PD patients were unable to efficiently use KR from previous trials to implement large and appropriate compensations to the imposed errors. As a result, both their horizontal and vertical errors were larger than those of elderly controls during all reversal learning exposure trials. Consequently, PD patients showed a smaller adaptation magnitude score compared to age-matched controls. Together, these results provide strong evidence for reduced learning in PD patients when confronted with the reversed visual distortion.
In contrast to initial learning, the reverse discordance shifted the apparent hand location lower than the actual hand position. This forced subjects to point higher than the target location presented in the virtual environment. Therefore, one may ask whether fatigue may have had more impact on the reversal than the initial phase of learning, as the reversal phase required more force to lift the hand higher. However, a number of observations are inconsistent with the interpretation of fatigue being a primary factor. First, as stated above, the degraded performance of PD patients during reversal learning was found along both the vertical dimension, where subject had to lift their arm against gravity and along the horizontal dimension where there is no increase in force requirements. This suggests that the degraded performance of PD patients during reversal learning is, at least in part, independent from the force required to reach the target. Second, consistent with the results of the present study, two previous studies have shown that the magnitude of the downward shift in movement endpoints exhibited by PD patients relative to elderly controls was fairly constant across target elevations (Poizner et al. 1998; Adamovich et al. 2001). That is, the vertical biases were similar whether PD patients pointed to the central target (shoulder elevation) or to the most elevated target (25 cm higher, similar in elevation to the reversal target in this study). Therefore, in contrast to what would be expected from fatigue related to arm movements performed against gravity, PD patient’s undershooting did not increase with increase in the vertical distance to the target. Further, based on this previous observation, we performed a subtraction analysis to test whether the baseline vertical bias of the PD patients is a major contributor to the much slower adaptation rates of PD patients observed along the vertical dimension. We subtracted the mean constant vertical error made by each PD subject during the baseline from the constant vertical error made for each trial during reversal learning. This analysis revealed that even after subtracting out the systematic downward shift PD patients showed during baseline, PD patients still pointed significantly lower than age-matched controls during reversal learning (Fig. 3a). For these reasons and because PD patients were not presenting any joint limitations that would have prevent them from pointing at the reversal target elevation, we view the impaired ability of PD patients to learn the vertical component of the reversal discordance as a learning difficulty not accounted for by fatigue due to moving against the gravitational forces acting along the vertical axis.
Several studies have reported systematic hypometria in PD (Flowers 1976; Klockgether and Dichgans 1994). Therefore, it might be possible that the deficits observed during reversal learning were due to target undershooting. However, results of the present study make this explanation unlikely. The vertical distance between the initial position of the hand (on the right thigh) and the target location was greater during reversal learning than initial learning. In contrast, the horizontal distance between the initial position of the hand and the target was greater during initial learning than during reversal learning. Despite these distance differences relative to start location, PD patients were impaired in their ability to consistently reach the target location along both the vertical and the horizontal dimensions during the reversal phase of learning. Further, results from previous studies of 3D reaching movements to the same target locations in PD patients with similar severity levels are not consistent with the target undershooting explanation. In the study by Poizner et al. (1998) and in the present study, movements were initiated from a horizontal start position. Therefore, the general direction of the movements during pointing was upward. As a result, hypometric movements would predict a downward bias. In contrast, in the study by Adamovich et al. (2001), movements toward the same target spatial locations were initiated from a vertical position such that hypometric movements would predict an upward bias. Instead, a systematic depression in endpoint elevation was observed. Further and as mentioned previously, this depression in endpoint elevation did not show any significant increase as a function of the vertical distance to the target in both studies (Poizner et al. 1998; Adamovich et al. 2001). Therefore, it appears most likely that the observation of a downward shift in three different studies with different PD subjects, using different initial arm postures, reflects inappropriate movement scaling along the vertical dimension.
Sensory processing and integration are important factors that can accelerate or retard the effective learning of novel visuomotor coordinations. Therefore, one may ask to what degree the observed learning deficits may in fact be due to impaired sensory processing. It has been known for some time that PD patients have certain visual-spatial deficits, among them a lack of precise perception of the visual-vertical. Proctor et al. (1964), for example, showed that PD patients had impaired judgment of visual-vertical under conditions of body tilt. Likewise, Azulay et al. (2002) found that PD patients showed larger errors than controls when adjusting a rod to visual-vertical when the rod was inside a tilted frame. Moreover, Lee et al. (2001, 2002) report that PD patients with primarily left-sided symptoms show both rightward and downward biases in coding left and upper visual space respectively, deficits akin to visual neglect. Therefore, one possibility is that reversal learning deficits of PD patients, i.e., greater spatial errors when pointing at the upper right target, are due to impaired perception of visual space. However, we feel that this explanation is unlikely since roughly half of the patients tested in the present study were more affected on each side (Table 1). Further, some investigators have found that PD patients show normal perception of visual-vertical (e.g., Bronstein et al. 1996). More telling however, is that 3D reaching studies using similar targets to those in the present experiment demonstrate that visual perceptual deficits could not account for the pattern of reaching performance. For example, Adamovich et al. (2001) showed that PD patients had significant elevation errors when reaching to either actual or remembered targets when they could not see their arms. However, when an illuminated LED was placed on the fingertip, so that vision of the arm endpoint was visible throughout the movement, the PD patients’s elevation errors normalized despite the fact that subjects were pointing to remembered targets. Thus, the initial visual perception of target elevation had to be intact (at least within the accuracy constraints of these reaching experiments) since providing vision of the fingertip during movements normalized the elevation errors (see also Keijsers et al. 2005 who found that providing PD patients with vision of the fingertip and an illuminated reference frame normalized variable errors for reaches to remembered 3D targets). Numerous studies are now indicating that PD patients have deficient proprioception or sensorimotor integration mechanisms (Schneider et al. 1986; Jobst et al. 1997; Zia et al. 2000; Klockgether et al. 1995; Adamovich et al. 2001; Maschke et al. 2003). Accurate positioning of the hand in vertical space challenges proprioceptive processing to a greater degree than does positioning in the horizontal dimension, since vertical movements require compensation for gravitational forces. Thus we feel that along the vertical dimension, altered proprioceptive or sensorimotor integration processing appeared to have interfered with the ability of PD patients to perform accurate movements, as reflected by the large and constant downward bias present in all task phases.
The sudden introduction of the opposite distortion after subjects adapted to the initial biaxial bias induced a substantial error. Indeed, there was a double error signal in reversal learning compared to the initial learning, since once subjects had learned to point down and to the left in initial learning, they then had to learn to point up and to the right in reversal learning. In face of this novel situation, subjects not only had to compensate for that large visuomotor error, but were required to reconfigure a newly learned visuomotor mapping. Simulation studies of basal ganglia function show that unlearning a previous response set is a separate process from learning a new one and has a differential learning rate (Berns and Sejnowski 1998). All of these requirements represent a great challenge and may explain the marked degradation of PD patient’s performance when the initial distortion was reversed.
In this complex reversal condition in which subjects must both reconfigure their visuomotor mapping and simultaneously compensate for the horizontal and vertical components of the biaxial discordance, PD patients appeared to have adopted a decomposition strategy. Along the horizontal dimension, they implemented large and inappropriate movement changes from trial to trial (oscillations). Along the vertical dimension, however, they had both a relatively stable level of constant vertical errors and a similar level of trial-to-trial variability during late exposure trials as both control groups, despite vertical errors that were four times greater then those of both control groups. These results suggest that PD patients were repetitively pointing at similar, although inappropriate spatial locations. Perseveration behavior has been previously reported in Parkinson’s disease (Ebersbach et al. 1994; Stoffers et al. 2001). Further, a number of studies have suggested that PD patients are impaired when required to modify their behavioral response in face of changing contingencies both in the cognitive and the motor domains. For instance, Haaland et al. (1997) studied tracking performance using three different speeds. PD patients showed slower learning rates during experiments with a randomized design but normal rates with a blocked design. These findings suggested that PD patients have more ‘rigid’ motor programs and cannot rapidly switch performance ‘set’ (Cools et al. 1984; Richards et al. 1993, Cronin-Golomb et al. 1994, Hayes et al. 1998, Monchi et al. 2004). Likewise, Vakil and Herishanu-Naaman (1998) found that PD patients showed difficulties with learning motor sequencing when required to switch from one motor program to the next. Further, in a previous study, we examined in which sensorimotor learning phase PD patients’ deficits are more apparent in a multi-stage force-field learning task (Krebs et al. 2001). As previously mentioned, the greatest deterioration in learning indices of the PD patients was in reversal learning, a phase which required a rapid reconfiguration of sensorimotor response. These results, together with those of the present study, suggest that the basal ganglia might be a key center for context switching.
However, it is also possible that the impairments that PD patients show when confronted with the reversed visuomotor mapping are not the expression of their general inability to switch set. Rather, their markedly deteriorated performance may reflect profound difficulties in implementing appropriate compensations when large visual errors are imposed. It has been recently proposed that adaptation to gradual and sudden new visuomotor mappings engage two distinct and complementary learning processes (Florian et al. 1997; Malfait and Ostry 2004; Robertson and Miall 1999; Contreras-Vidal and Buch 2003). Adapting to gradual changes in visual feedback involve small movement corrections that require small reconfigurations of the visuomotor map. By contrast, a sudden large perturbation in visual feedback requires a search in visuomotor space to minimize errors that are more likely consciously detected by subjects. In support of distinct learning processes, the ability of young controls to reconfigure visuomotor mappings in gradual versus sudden experimental contexts led to different pattern of results both for learning a visual distortion and for learning a dynamic one (Florian et al. 1997; Malfait and Ostry 2004). Although, no study compared adaptation to gradual and sudden distortions in Parkinson Disease, Contreras-Vidal and Buch (2003) reported marked deficits in PD patients when learning a sudden large visual distortion created by a 90° cursor rotation. They concluded that the observed deficits reflect the critical role of the basal ganglia in tasks that are initially effortful, such as when adapting to a large sudden distortion (Contreras-Vidal and Buch 2003). This interpretation is in agreement with results of a recent study examining PD patient’s ability to correct for movement errors induced by target jumps of different sizes (Desmurget et al. 2004). In that study, PD patients were easily able to compensate for small subliminal target jumps but their performance significantly deteriorated when large consciously detected target jumps were imposed. Along the same lines as the adaptation studies mentioned above, Desmurget et al. (2004) suggested that corrections for these two types of errors rely on distinct processes and that basal ganglia circuits are more critically involved in correcting large errors.
Given that this study and all previous studies of sensorimotor adaptation to sequential perturbations used opposite distortions which double the error signal, it remains unclear whether both the marked impairments of PD patients during reversal learning, and the observed greater involvement of basal ganglia during the reversal phase of learning, reflect mainly a major contribution of basal ganglia in context switching or an important contribution of basal ganglia in correcting for large visual errors. Future studies in which the size and the order of visual distortions are systematically manipulated are needed to isolate the participation of basal ganglia in these two important and complementary aspects of adaptive motor control. Nevertheless, this study provides the first evaluation of the effect of PD and normal aging on the ability to adapt natural movements performed in three-dimensional space. The presence of sensorimotor learning deficits in PD itself is a controversial issue. Our results that PD patients were able to learn the initial visual distortion and showed marked degradation in performance relative to age-matched controls during the reversal phase of learning indicates that PD produces selective deficits in visuomotor learning when the task is complex involving either a large visual distortion or when subjects must learn to adapt to reversed visual distortions in quick succession. Further, this study extends previous findings of impaired sensorimotor learning in PD and supports imaging studies showing that the basal ganglia are involved when subjects must learn novel visuomotor associations sequentially presented (Shadmehr and Holcomb 1999; Krebs et al. 1998; Krakauer et al. 2004).
In the context of the present study, however, both adapting to the initial smaller visual distortion and to the reversed larger visual distortion involved the selection and elaboration of appropriate motor responses. Thus, the somewhat slower adaptation rate observed during initial learning and the markedly deteriorated performance of PD patients during reversal learning may both involve, as suggested by Shadmehr and Holcomb (1999) and Krebs et al. (2001), the expression of a key role of basal ganglia in the selection of appropriate motor programs. Such selection would involve the learning of a new motor response in a novel visuomotor context, and, in the case of reversal learning, would involve as well the suppression of a just learned but now inappropriate motor response (Jueptner et al. 1996; Schmidt 1998; Kropotow and Etlinger 1999; Brooks 2000).
PD patients exhibited a large downward vertical bias
Accurate reaching in the vertical dimension requires compensation for gravity. Therefore, it is most dependent on proprioceptive information. Since several studies have reported impaired proprioceptive processing in Parkinson disease (Schneider et al. 1986; Jobst et al. 1997; Zia et al. 2000), the systematic downward shift of movement endpoints exhibited by PD patients in all task phases may likely represent their inability to utilize proprioceptive inputs to compensate for gravitational force. Two previous studies of three-dimensional reaching movements support this view (Poizner et al. 1998; Adamovich et al. 2001).
Visuomotor learning in normal aging
In the present study, healthy elderly subjects showed an overall slower adaptation rate to the initial biaxial distortions compared to young controls. As mentioned in the introduction, the effect of normal aging on visuomotor learning is controversial (Fernandez-Ruiz et al. 2000; Bock and Schneider 2002; Buch et al. 2003). As is the case for PD, studies find either a slower adaptation rate or a smaller aftereffect from one learning paradigm to another suggesting that the influence of aging on visuomotor learning is context dependent. Recently, Buch et al. (2003) suggested that much of the variability in the patterns of results could be explained by the nature of the perturbation used in the different studies. They tested the ability of aged subjects to reconfigure their visuomotor mapping when exposed both to a gradual and a sudden visual distortion. Elderly subjects showed reduced performance indices in both visuomotor conditions. When exposed to the gradual distortion, they produced smaller aftereffects, whereas in the sudden distortion they exhibited more slowly decreasing learning curves (Buch et al. 2003). These results were interpreted as the manifestation of two distinct learning deficits. The smaller aftereffect was suggested to reflect reduced implicit learning, whereas the slower adaptation rate was considered the expression of cognitive factors interfering with rapid selection of an appropriate strategy to reduce large visual errors (Buch et al. 2003). This interpretation is consistent with previous studies using dual task paradigms suggesting that adaptation learning requires a higher proportion of the available computational resources in elderly subjects relative to young controls (Bock and Schneider 2002). The finding of the present study that aged subjects made greater errors than young controls during early exposure to the initial biaxial distortion before normalizing their performance supports these interpretations. Interestingly, however, when aged subjects were suddenly confronted with the reverse discordance and had to correct for large visual errors that were of double size, unlike PD subjects their adaptation rates were not significantly different from young controls for either dimension of the biaxial discordance. Therefore, although aged subjects were slower learners than young controls during initial learning, possibly due to reduced strategic processes, they were able to learn general strategies to reconfigure their visuomotor mapping and to apply them to improve their reversal learning, an ability referred as ‘learning to learn’ (Bock and Schneider 2001, 2002). The results of the present study are consistent with these previous studies in the context of a multistage learning task involving two opposite visual distortions presented in quick succession.
The capacity to flexibly adapt movements to novel environmental contexts is central to normal everyday human behavior. By using a multistage visuomotor learning task, this study provided the first evaluation of the selective effect of Parkinson Disease and normal aging on the ability to adapt natural movements performed in three-dimensional space. Results showed that adaptation-learning abilities decline in both aging and Parkinson disease. However, the more strongly impaired performance of PD patients when confronted with a reversed visuomotor distortion suggests that Parkinson’s disease produces selective deficits rather than a profound generalized impairment in the ability to learn novel visuomotor coordinations. The present findings are consistent with the recent hypothesis that dopamine depletion results in a loss of functional segregation within basal ganglia circuits which may interfere with a number of motor and cognitive abilities including movement selection and context switching (Pessiglione et al. 2005).
Notes
An eleventh elderly subject was run, but his data were excluded since analysis revealed that his spatial errors fell well outside those of all other elderly control subjects; they were greater than two standard deviations from the mean
The number of trials tested is different for the initial learning (20 trials) and the reversal learning phases (15 trials). To compare more directly performance indices between the two learning phases, we also performed statistical analyses on the learning phases using only the first 15 trials. For instance, we evaluated the variability indexes and the adaptation magnitude scores on trials 10–15 of initial learning as was done for reversal learning. Similar results were obtained as when using all 20 trials of initial learning. The adaptation score for the horizontal component of the initial biaxial discordance was similar in all three subject groups (F (2,25) = 0.285; P > 0.05) as was the level of trial-to-trial variability (F (2,25) = 0.012; P > 0.05). Also, there was a significant between group difference in the adaptation magnitude score for the vertical component of the initial discordance (F (2,25) = 8.49; P < 0.05). Young and elderly controls showed a similar level of adaptation (P > 0.05), while PD patients showed significantly smaller adaptation magnitude score (P < 0.025). In a similar manner, we performed the ANOVAs on constant errors for trials 1–15 of initial learning. Again, these analyses confirmed results found when trials 1–20 were analyzed
References
Adamovich SV, Berkinblit MB, Henning W, Sage J, Poizner H. (2001) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 1027–1041
Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11:420–431
Azulay JP, Mesure S, Amblard B, Pouget J (2002) Increased visual dependence in Parkinson’s disease. Percept Mot Skills 95(3 Pt 2):1106–1114
Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM (2005) Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437(7062):1158–1161
Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1986) Performance of simultaneous movements in patients with Parkinson’s disease. Brain Aug 109(Pt 4):739–57
Berns SB, Sejnowski TJ (1998) A computational model of how the basal ganglia produce sequences. J Cogn Neurosci 10:108–121
Bock O, Schneider S (2001) Acquisition of a sensorimotor skill in younger and older adults. Acta Physiol Pharmacol Bulg 26(1–2):89–92
Bock O, Schneider S (2002) Sensorimotor adaptation in young and elderly humans. Neurosci Biobehav Rev 26:761–767
Bronstein AM, Yardley L, Moore AP, Cleeves L (1996) Visually and posturally mediated tilt illusion in Parkinson's disease and in labyrinthine defective subjects. Neurology 47(3):651–656
Brooks DJ (2000) Imaging basal ganglia function. J Anat 196:543–554
Buch ER, Young S, Contreras-Vidal JL (2003) Visuomotor adaptation in normal aging. Learn Mem 10:55–63
Contreras-Vidal JL, Buch ER (2003) Effects of Parkinson’s deasese on visuomotor adaptation. Exp Brain Res 150:25–32
Contreras-Vidal JL, Gold DR (2004) Dynamic estimation of hand position is abnormal in Parkinson’s disease. Parkinsonism Relat Disord 10(8):501–506
Contreras-Vidal JL, Teulings HL, Stelmach GE, Adler CH (2002) Adaptation to changes in vertical display gain during handwriting in Parkinson’s disease patients, elderly and young controls. Parkinsonism Relat Disord 9:77–84
Cools AR, Van Den Bercken JH, Horstink MW, Van Spaendonck KP, Berger HJ (1984) Cognitive and motor shifting aptitude disorder in Parkinson’s disease. J Neurol Neurosurg Psychiatry 47(5):443–453
Cronin-Golomb A, Corkin S, Growdon JH (1994) Impaired problem solving in Parkinson’s disease: impact of a set- shifting deficit. Neuropsychologia 32:579–593
Desmurget M, Jordan M, Prablanc C, Jeannerod M (1997) Constrained and unconstrained movements involve different control strategies. J Neurophysiol Mar 77(3):1644–1650
Desmurget M, Gaveau P, Vindras RS, Turner E, Broussolle S Thobois (2004) On-line motor control in patients with Parkinson’s disease. Brain 127(Pt 8):1755–1773 Epub Jun 23
Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-strial and cortico cerebellar systems to motor skill learning. Neuropsychologia 41:252–262
Ebersbach G, Hattig H, Schelosky L, Wissel J, Poewe W (1994) Perseverative motor behaviour in Parkinson’s disease. Neuropsychologia 32(7):799–804
Fahn S, Elton RL (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D Goldstein M (eds) Recent developments in Parkinson’s disease. Macmillan:29304, New York
Fernandez-Ruiz J, Hall C, Vergara P, Diaz R (2000) Prism adaptation in normal aging: Slower adaptation rate and larger after effect. Brain Res Cogn Brain Res 9:223–226
Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Mischner J, Nunez L, Drucker-Colin R, Ochoa A, Alonso ME (2003) Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task 18:689–694
Florian A, Kagerer, Contreras-Vidal JL, Stelmach GE (1997) Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res Jul 115(3):557–561
Flowers K (1976) Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain 99:269–310
Fucetola R, Smith MC (1997) Distorted visual feedback effects on drawing in Parkinson’s disease. Acta Psychol (Amst) 95:255–266
Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F (2000) Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res 876:112–113
Grafton ST, Hazeltine E, Ivry R (1995) Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7:497–510
Graybiel AM (2004) Network-level neuroplasticiy in cortico-basal ganglia pathways, Parkinsonism Rel Disord 10:293–296
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15(6):638–644
Graydon FX, Friston KJ, Thomas CG, Brooks VB, Menon RS (2005) Learning-related fMRI activation associated with a rotational visuo-motor transformation. Cogn Brain Res 22:373–383
Haaland KY, Harrington DL, O’Brien S, Hermanowicz N (1997) Cognitive-motor learning in Parkinson’s disease. Neuropsychology 11:180–186
Hayes AE, Davidson MC, Keele SW, Rafal RD (1998) Toward a functional analysis of the basal ganglia. J Cogn Neurosci 10:178–198
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Hoover JE, Strick PL (1993) Multiple output channels in the basal ganglia. Science 259:819–821
Houk JC, Wise SP (1995) Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex 5:95–110
Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B et al (2000) Human cerellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195
Inoue K, Kawashima R, Satoh K, Kinomura S, Sugiura M, Goto R, Ito M, Fukuda H (2000) A PET study of visuomotor learning under optical rotation. Neuroimage 11:505–516
Jackson GM, Jackson SR, Hindle JV (2000) The control of bimanual reach-to-grasp movements in hemiparkinsonian patients. Exp Brain Res 132:390–398
Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ (1997) Sensory perception in Parkinson disease. Arch Neurol 54:450–454
Jueptner M, Jenkins IH, Brooks DJ, Frackowiak RS, Passingham RE (1996) The sensory guidance of movement: a comparison of the cerebellum and basal ganglia. Exp Brain Res 112:462–474
Keijsers NL, Admiraal MA, Cools AR, Bloem BR, Gielen CC (2005) Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. Eur J Neurosci 21(1):239–248
Klockgether T, Dichgans J (1994) Visual control of arm movements in Parkinson's disease. Mov Disord 9(1):48–56
Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J (1995) A defect of kinesthesia in Parkinson’s disease. Mov Disord 10:460–465
Krakauer JW, Ghilardi MD, Mentis M, Barnes A, Veytsman, Eidelberg D, Ghez C (2004) Differential cortical and subcortical activations in learning rotations and gains for reaching: a pet study. J Neurophysiol 91:924–933
Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM (1998) Robot aided functional imaging: application to a motor learning study. Hum Brain Mapp 6:59–72
Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H (2001) Procedural motor learning in Parkinson’s disease. Exp Brain Res 141:425–437
Kropotow JD, Etlinger SC (1999) Selection of actions in the basal ganglia-thalamocortical circuits: review and model. Int J Psychophysiol 31:197–217
Lee AC, Harris JP, Atkinson EA, Fowler MS (2001) Evidence from a line bisection task for visuospatial neglect in Left Hemiparkinson’s disease. Vis Res 41:2677–2686
Lee AC, Harris JP, Atkinson EA, Nithi K, Fowler MS (2002) Dopamine and the representation of the upper visual field: evidence from vertical bisection errors in unilateral Parkinson’s disease. Neuropsychologia 40:2023–2029
Malfait N, Ostry DJ (2004) Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24(37):8084–8089
Maschke M, Gomez CM, Tuite PJ, Konczak J (2003) Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126:2312–2322
Messier J, Kalaska JF (1997) Differential effect of task conditions on errors of direction and extent of reaching movements. Exp Brain Res 115:469–478
Monchi O, Petrides M, Doyon J, Postuma RV, Worsley K, Dagher A (2004) Neural bases of set-Shifting deficits in Parkinson’s disease. J Neurosci 24(3):702–710
Pessiglione M, Guehl D, Agid Y, Hirsch EC, Feger J, Tremblay L (2003) Impairment of context-adapted movement selection in a primate model of presymptomatic Parkinson’s disease. Brain 126(Pt 6):1392–1408
Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L (2005) Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 25(6):1523–1531
Poizner H, Fookson O, Berkinglit MB, Hening W, Feldman G, Adamovich SV (1998) Pointing to remembered targets in 3D space in Parkinson’s disease. Motor Control 2:251–277
Poizner H, Feldman AG, Levin MF, Berkinblit MB, Hening WA, Patel A, Adamovich SV (2000) The timing of arm-trunk coordination is deficient and vision-dependent in Parkinson’s patients during reaching movements. Exp Brain Res 133(3):279–292
Proctor F, Riklan M, Cooper IS, Teuber H-L (1964) Judgment of visual and postural vertical by Parkinsonian patients. Neurology 14:282–293
Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD et al (1997) Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp 5:124–132
Richards M, Cote LJ, Stern Y (1993) Executive function in Parkinson’s Disease: set-shifting or set-maintenance? J Clin Exp Neuropsychol 15 2:266–279
Robertson EM, Miall RC (1999) Visuomotor adaptation during inactivation of the dentate nucleus; NeuroReport 10(5):1029–1034
Schmidt WJ (1998) Dopamine-glutamate interactions in the basal ganglia. Amino Acids 14:5–10
Schneider JS, Diamond SG, Markham CH (1986) Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol 19:275–282
Seidler RD, Noll DC, Chintalapati P (2006) Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res [Epub ahead of print]
Shadmehr R, Holcomb HH (1999) Inhibitory control of competing motor memories. Exp Brain Res 126:235–251
Stern Y, Mayeux R, Hermann A, Rosen J (1988) Prism adaptation in Parkinson’s disease. J Neurol Neuroseurg Psychiatry 51:1584–1587
Stoffers D, Berendse HW, Deijen JB, Wolters EC (2001) Motor perseveration is an early sign of Parkinson’s disease. Neurology 57:2111–2113
Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH (2002) Adaptation of handwriting size under distorted visual feedback in patients witn parkinson’s disease and elderly and young controls. J Neurol Neurosurg Psychiatry 72:315–324
Vakil E, Herishanu-Naaman S (1998) Declarative and procedural learning in Parkinson’s disease patients having tremor or bradykinesia as the predominant symptom. Cortex 34:611–620
Zia S, Cody F, O’Boyle D (2000) Joint position sense is impaired by Parkinson’s disease. Ann Neurol 47:218–228
Acknowledgments
This research was supported in part by an FRSQ and CIHR Postdoctoral Fellowship to JM and by NIH Grant 7 R01 NS36449 to UCSD (HP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messier, J., Adamovich, S., Jack, D. et al. Visuomotor learning in immersive 3D virtual reality in Parkinson’s disease and in aging. Exp Brain Res 179, 457–474 (2007). https://doi.org/10.1007/s00221-006-0802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0802-2