Abstract
Tiotropium bromide (TIO) is a long-acting bronchodilator used in the treatment of chronic obstructive pulmonary disease (COPD) and asthma. Specifically, it is used to prevent patients from worsening breathing difficulties. In this study, a new TIO-imprinted electrochemical sensor was designed to detect TIO in serum and pharmaceutical samples. Methacryloyl-L-histidine-cobalt(II) [MAH-Co(II)] has been used as a metal-chelating monomer for synthesizing selective molecularly imprinted polymer (MIP). MIP film has been developed on glassy carbon electrodes using MAH-Co(II) as the functional monomer, 2-hydroxyethyl methacrylate (HEMA) as the basic monomer, and ethylene glycol dimethacrylate (EGDMA) as the cross-linker in the photopolymerization method. The surface characterization of the developed MAH-Co(II)@MIP/GCE electrochemical sensor was done using scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). Also, the electrochemical behavior of the sensor was provided by differential pulse voltammetry (DPV), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) techniques. Under optimized experimental conditions, the linearity range was in the range of 10–100 fM, and the limit of detection (LOD) and limit of quantitation (LOQ) values were calculated as 2.73 fM and 9.75 fM, respectively. The MAH-Co(II)@MIP/GCE sensor was used to precisely determine TIO in capsule and commercial serum samples. The results demonstrated that the MIP could specifically recognize TIO compared to structurally related drugs and could be reliably applied to the direct determination of drugs from real samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tiotropium bromide (TIO) is an anticholinergic bronchodilator that shows its mechanism of action by binding to muscarinic receptors. The most significant feature of TIO is the long-lasting activity of bronchodilatation (24 h), and it is widely used in chronic obstructive pulmonary disease (COPD) [1]. Thanks to this feature, it can be administered only once daily, increasing patient compliance. However, TIO is available in the dry inhalation powder form, and systematic bioavailability can be at very low levels [2]. Therefore, it is important to develop a highly sensitive sensor that can reach the low limit of detection (LOD) values and be applicable for biological and pharmaceutical samples to determine TIO. Even though TIO is a strong therapeutic agent for COPD treatment, the studies available in the literature for TIO analysis are limited and mostly based on combined chromatographic methods [2,3,4,5,6,7]. Chromatographic methods are frequently used in drug analysis, especially as combined methods, and they are very useful for the separation of multiple analytes simultaneously. However, the major drawbacks such as high cost, consuming a high amount of chemicals, producing lots of waste, and requiring expert personnel lead researchers to develop alternative analytical methods [8].
Electrochemistry is one of the critical methods in drug analysis; therefore, electrochemical sensors have always been highly preferable due to their great sensitivity, easy application, versatility, rapid results, less sample consumption, and greener analysis options. Various modification strategies such as nanomaterials, ionic liquids, and polymers are used to improve the performance of electrochemical sensors. Molecular imprinting, which possesses selective recognition sites for the target molecule created in the polymer matrix, is a promising technique used to obtain a recognizing layer on electrochemical sensors due to its high selectivity [9,10,11,12,13,14,15].
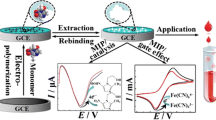
In this study, a novel sensor was developed by integrating MIP technology with electrochemistry. The obtained sensor offers enhanced selectivity and sensitivity. To create the selective recognizing layer via a semi-covalent imprinting approach, MAH-Co(II) was used as a metal-chelating functional monomer to increase the selectivity of the sensor for the target molecule due to the high affinity of the imidazole ring against Co(II) ions. The interactions of biological molecules with metal ions tend to be quite specific [16]. Co(II) ions can form stable complexes with electron-donating groups, such as imidazole, thiol, and indole residues, and may coordinate molecules containing O, N, and S by ion–dipole interactions. Therefore, as a transition metal, we used Co(II) ions for the specific interaction of the binding residue of the template molecule, since the specific thiophene group of the TIO exhibits strong reversible interactions with immobilized Co(II) ions. Cobalt ions were utilized to coordinate functional monomers with the template by creating a metal-chelating bridge. Therefore, MAH-Co(II) appears to be a potential selective ligand for the selective recognition of drug molecules in the molecular imprinting strategy. Besides this strategy to increase selectivity, the porosity of the polymeric film formed on the glassy carbon electrode (GCE) surface was enhanced by using poly(vinyl alcohol) (PVA) as a sacrificial pore-making agent. Photopolymerization with UV irradiation was used during sensor preparation, whereas the electrochemical measurements were performed indirectly using 5 mM [Fe(CN)6]3−/4− solution as the redox probe.
To the best of our knowledge, this study is the first electrochemical sensor that fully evaluates the determination of TIO in biological and pharmaceutical samples with great sensitivity, high selectivity, stability, and accuracy.
Experimental
Reagents and chemicals
Drug substance tiotropium bromide (99.9%) and drug product (Brontio®) capsules were provided from DEVA Holding A.S. (Istanbul, Turkey). All reagents including ascorbic acid (≥ 99.0%), dopamine, acetic acid (99.0%), sodium hydroxide (> 97.0%), acetone (99.5%), methanol (99.9%), acetonitrile (99.9%), sodium sulfate (99.0%), potassium chloride (≥ 99.0%), commercial human serum, ethylene glycol dimethacrylate (EGDMA; ≥ 98.0%), 2-hydroxyethyl methacrylate (HEMA; ≥ 99.0%), 3-(trimethoxysilyl)propyl methacrylate (TMSPMA; ≥ 97.0%), 2-hydroxy-2-methylpropiophenone (≥ 97.0%), potassium ferricyanide (99.0%), and potassium ferrocyanide (≥ 99.0%) were purchased from Sigma-Aldrich.
The materials used in the experiments were used directly without any pretreatment. A 10 mM standard stock solution of TIO in methanol and a 5 mM solution of [Fe(CN)6]3−/4− (1:1) in 0.1 mM KCl were prepared weekly and sonicated in an ultrasonic bath for 10 min. All solutions were prepared using ultrapure water (with resistivity not less than 18 MΩ cm at 25 °C) and stored in a refrigerator at 4 °C.
Apparatus
All electrochemical measurements such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS) were carried out using a potentiostat/galvanostat system (AUTOLAB) operated by the NOVA 2.1.5 software. Electrochemical experiments were conducted using a conventional electrochemical cell, which consisted of an Ag/AgCl reference electrode, a platinum wire used as the auxiliary electrode, and the MIP/NIP-modified glassy carbon electrode (GCE; Ø = 3.0 mm) as the working electrode. Ohaus Instruments (Shanghai, China) precision balance was utilized to weigh the required amount of chemicals. Thermo-Shaker (Biosan TS-100) was used in numerous phases throughout the MIP-based sensor preparation. Ultrasonic bath (J.P. Selecta, Barcelona, Spain), vortex mixer (ISOLAB Laborgeräte GmbH, Germany), and incubator (EN 055/120, Nüve, Turkey) were the other pieces of equipment used throughout the studies.
Synthesis of MAH-Co(II) complex
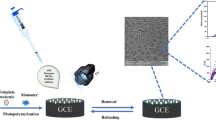
In the first step, N-methacryloyl-L-histidine (MAH) was synthesized according to the method proposed by Hur et al. [17]: 1-(methacryloyl)-1,2,3-benzotriazole (MA-Bt; 5.52 mmol) solution in 1,4-dioxane was added dropwise into an aqueous solution of L-histidine (5.52 mmol in 1.0 M aq. NaOH) and stirring was continued at 25 °C for 30 min. The solvent, 1,4-dioxane, was evaporated in vacuo. The residue was diluted with water, and the resulting mixture was extracted with ethyl acetate (3 × 50 mL) to separate 1H-benzotriazole. Then, the aqueous mixture solution was neutralized with 10% HCl and evaporated in vacuo. The aqueous solution was acidified to pH 6–7 and then removed by rotary evaporation; finally, the remaining solid was crystalized to afford amino acid–based functional monomer (MAH). In the second step, MAH (0.223 g, 1.0 mmol) monomer was dissolved in 10 mL of ethanol and then treated with cobalt nitrate Co(NO3)2.6H2O (0.290 g, 1.0 mmol). This solution was stirred at room temperature for 3 h min until turning clear red solution. The ethanol was then removed on a rotary evaporator to yield a red solid. MAH–Co(II) complex was recrystallized from ethanol/acetonitrile to give a pale red crystalline solid (Fig. 1).
Preparation of MIP and NIP-based sensors via photopolymerization
Before the photopolymerization method, GCE was sonicated in a solution containing methanol and double distilled water (1:1, v/v) for 15 min, and then the electrode surface was polished with alumina slurry. After washing with double distilled water, it was dried at room temperature. The photopolymerization mixture was prepared by vortexing HEMA (basic monomer, 50 µL), EGDMA (cross-linker, 10 µL), MAH-Co(II) (metal-chelating functional monomer, 10 µL), TIO (template molecule, 10 µL), and PVA solution (10 µL, 25 mg/mL in deionized (DI) water) in an Eppendorf tube. After adding the photoinitiator (2-hydroxy-2-methylpropiophenone, 1 µL) into the stock monomer solution, an aliquot (0.25 µL) was dropped onto the electrode surface before starting photopolymerization being carried out at room temperature under UV irradiation (100 W, 365 nm) for 10 min. To expose selective imprinted cavities, the template TIO molecules were removed from the polymeric film formed on the GCE surface using an acetic acid solution (10 M) via incubating in ThermoShaker at 650 rpm for 15 min. The most important point in the sensor preparation process, the non-imprinted polymer (NIP), was prepared by the same procedure but without TIO molecules, and the performance of the sensor was verified. For comparison purposes, the non-imprinted version was also prepared by following the same experimental procedure except for adding TIO. Electrochemical measurements of the fabricated sensor were performed with CV and DPV using 5 mM [Fe(CN)6]3−/4− solution as the redox probe.
Application of inhalation capsule and commercial serum sample
The practical applicability of the sensor was confirmed with five capsules, each containing approximately 18.0 µg of TIO drug substance. First, the full and empty capsules were weighed. The amount equivalent to 1 µM TIO was taken and dissolved in pure methanol, sonicated for 15 min, and filtered through filter paper. Measurement solutions were prepared by taking the calculated amounts from the drug product solution and the intermediate solution. Recovery experiments were carried out after adding a known amount of pure drug substance to the drug product (capsule) solution to prove the accuracy of the method. The amount of active substance was calculated from the calibration curve of the regression equation.
To calculate the recovery from serum samples, commercial human serum (3.6 mL) stored in a deep freeze at − 20 °C was diluted with 1.0 mL of TIO solution and 5.4 mL of acetonitrile in a test tube. The solution was centrifuged at 5000 rpm for 20 min to precipitate protein residues in the test tube. After the supernatant was separated, necessary dilutions were made and used for calibration and recovery studies. Three repeatable measurements were taken from all measurements, and the relative standard deviation values were calculated.
Results and discussion
Surface characterization of the developed sensor
FTIR/ATR spectroscopy performed in the range of 500–4000 cm−1 was utilized to confirm the functional group of the developed sensor surface. Figure 2A shows the main characteristic band of polymeric material at around 3400 cm−1 which reveals a dominant band attributed to -OH stretching. Furthermore, the developed sensor displayed specific bands at 2920 cm−1 (C-H stretching) and 1710 cm−1 (carbonyl stretching vibration of ester groups), which exhibited the presence of the basic monomer HEMA in the polymeric material. Furthermore, the characteristic -NH bands almost matched up for functional monomer [MAH-Co(II)] in the polymeric network. These results confirmed that the functional MAH-Co(II) complex was successfully incorporated into MIP and NIP sensors. The surface morphology of the fabricated sensor was observed by scanning electron microscopy (SEM) in Fig. 2B and C. The image of the MIP and NIP sensors showed that the synthesized MIP and NIP had a rough and porous morphology because the sacrificial poremaker, PVA, provided the porous morphology with interconnected pores to allow easy access to the recognition sites of the template molecule as well as giving an opportunity to improve sensor sensitivity.
Electrochemical characterization of MAH-Co(II)@MIP/GCE sensor
Electrochemical characterization of the sensor explains the electrochemical, conductive, and oxido-reductive processes on the electrode surface. For this purpose, CV and electrochemical impedance spectroscopy (EIS) methods were used on MAH-Co(II)@MIP/GCE sensor. A [Fe(CN)6]3−/4− solution (5 mM) was used as the redox marker during all CV and EIS measurements.
Firstly, the CV voltammograms obtained before polymerization, after polymerization, after removal, and after rebinding of TIO were evaluated (Fig. 3A). Figure 3A shows that the CV peak of [Fe(CN)6]3−/4− solution on the bare GCE surface reached the highest value. After polymerization, the polymeric film formation on the GCE surface obstructed the electron transfer. Therefore, the peak of [Fe(CN)6]3−/4− solution cannot be obtained. The removal process of the TIO molecules from polymer provides cavities on the surface, and reduction–oxidation of the [Fe(CN)6]3−/4− solution can be observed again. After rebinding of TIO, the obtained peaks are lower than the bare GCE and after removal but lower than after polymerization due to the closure of the cavities on the polymeric film.
The same processes were also evaluated with EIS measurements. EIS explains the changes in the charge transfer resistance (Rct) with obtained Nyquist plots. The results of the EIS measurements are given in Fig. 3B. On the bare GCE surface, electron transfer can occur easily, and the Rct value is very low (127.45 Ω). After polymerization, the presence of the polymeric film on the GCE surface increases the Rct value that reaches the highest value (6966 Ω) due to the obstructed electron transfer. The removal of TIO molecules resulted in a decrease in the Rct value (301.72 Ω), indicating the formation/exposing out of cavities. On the other hand, as the last characterization step, after rebinding Rct value (720.02 Ω) increases, confirming the binding of TIO molecules to the specific cavities on the polymer.
Optimization of significant parameters
Photopolymerization time
During the MAH-Co(II)@MIP/GCE sensor preparation, the polymerization process was carried out using UV irradiation at 365 nm. In this case, photopolymerization time should be optimized to ensure a stable polymer on the GCE surface. For this purpose, after dropping 0.25 μL of polymerization solution (0.25 μL) onto the electrode surface, GCE was kept under the UV lamp for 3, 5, 7, 10, and 15 min. When the obtained current values were compared (Fig. 4A), it can be seen that at 7 min, the lowest current value was measured, and after that, it stayed almost the same. This result indicates that the longer the time, the better the surface is coated with a polymer and the lower the [Fe(CN)6]3−/4− peak. Therefore, considering that the sensor preparation time is not too long, 7 min was chosen as the optimum polymerization time.
Dropping volume
The volume of the polymerization solution dropped onto the GCE surface is another significant parameter because it directly affects the polymeric film thickness and polymerization time. If there is an excessive or insufficient amount of solution, polymerization cannot occur properly. To choose the optimum volume, five different monomer volumes, 0.25, 0.5, 1, 1.5, and 2 μL, were tested (Fig. 4B). As it was in photopolymerization time optimization, the peak current values obtained after polymerization were evaluated, and 1 μL was chosen as the optimum amount. Thus, during sensor preparations, 1 μL of photopolymerization solution was used in further experiments.
Monomer/template ratio
Among all the parameters affecting the formation of a stable and effective polymer, the monomer:template ratio has significant importance. Because it is directly associated with the interactions between monomer and template molecule. Figure 4C shows the effect of various molar ratios of monomer/template (1:1, 2:1, 3:1, 4:1, and 5:1) on the difference between peak currents obtained after removal and after polymerization (ΔI1). As a result, the molar ratio of 1:1, which provides the most efficient and stable polymer after removal and polymerization, was chosen as the optimum value.
Removal solution and time
During the MIP preparation process, removing the template molecule ensures the formation of specific cavities for the binding of the analyte. Hence, it is critical to select the most suitable removal solution and time during the optimization. At this step, acetic acid solution and its mixtures with distilled water (1:1), methanol (1:1), acetonitrile (1:1), and acetone (1:1) were tested by incubating the MAH-Co(II)@MIP/GCE sensor for 15 min using ThermoShaker. When the ΔI1 values were examined (Fig. 4D), acetic acid was chosen as the removal solution. As the next step, acetic acid was assessed for the optimum removal time MAH-Co(II)@MIP/GCE sensor was incubated in the acetic acid solution for 5, 10, 15, 20, and 25 min using the ThermoShaker. As it can be seen in Fig. 4E, the best result was acquired at 15 min, and it was selected for the removal time.
Rebinding time
The sensor’s analytical performance and response time are associated with the rebinding process. Therefore, the incubation time for rebinding was studied for 3, 5, 7, 10, and 15 min (Fig. 4F). When the difference between peak currents after rebinding and after removal (ΔI2) is evaluated, it can be seen that after reaching the highest value at 5 min, ΔI2 stays almost the same. Hence, 5 min was chosen as the rebinding time to ensure a stable and efficient binding.
Analytical validation of MAH-Co(II)@MIP/GCE sensor
TIO was determined after rebinding the different concentrations of TIO solutions in the linear concentration range between 1 × 10−14 and 1 − 10−13 M on MAH-Co(II)@MIP/GCE sensor. Obtained results were evaluated for the analytical validation of the MAH-Co(II)@MIP/GCE sensor. Different TIO concentrations were plotted versus the calculated ΔI2 values to obtain the linear calibration graph (Fig. 5A). The regression equation of this calibration graph was found as of ΔΙ(μΑ) = 2.77 × 1014C (M) + 10.085 (R2 = 0.996), confirming the linear relationship between ΔI2 values and TIO concentration (Table 1). Using the regression data, LOD (LOD = 3 s/m) and LOQ (LOQ = 10 s/m) values were calculated as 2.11 × 10−15 M and 7.04 × 10−15 M, respectively. In Fig. 5B, DPV voltammograms show the decrease in the peak current of the redox probe with the rebinding of increasing concentrations of TIO.
The same concentration range was also evaluated with the NIP-based sensor. The acquired results showed that there was no linear relationship between TIO concentration and ΔI values on the NIP-based sensor indicating the selectivity of the MAH-Co(II)@MIP/GCE sensor.
Application of MAH-Co(II)@MIP/GCE sensor in pharmaceutical dosage form and biological sample
The developed MAH-Co(II)@MIP/GCE sensor was successfully applied to the spiked commercial human serum and inhalation capsule preparation with high accuracy. Determination of TIO was performed in the spiked serum samples in the concentration range of 1 × 10−14 M and 1−10−13 M. The peak current responses showed linearity in this range with the regression equation of ΔΙ(μΑ) = 2.86 × 1014C (M) + 11.723 (R2 = 0.999) (Fig. 5C). Very low LOD and LOQ values (2.93 × 10−15 M and 9.75 × 10−15 M) were calculated, demonstrating the high sensitivity of the sensor [18]. The acquired regression data is summarized in Table 1. The obtained DPV voltammograms after rebinding the different TIO concentrations in the serum sample are given in Fig. 5D. The recovery analysis resulted in a recovery% value of 101.97%, confirming the accuracy of the MAH-Co(II)@MIP/GCE sensor (Table 2). Furthermore, the accuracy and applicability of the MAH-Co(II)@MIP/GCE sensor were also demonstrated with the recovery analysis of the inhalation capsule dosage form. The recovery result was found as 99.71% (Table 2).
Selectivity
The selectivity (k) value is calculated using structurally and chemically identical compounds to show the sensor’s affinity toward TIO. The k value of the proposed sensor was investigated using DPV. After rebinding for 0.05 pM TIO, oxitropium bromide (OXI), ipratropium bromide (IPR), glycopyronium bromide (GLY), and aclidinium bromide (ACL), the ratio of ΔIp values of the template to competitor molecule was calculated. Additionally, the relative selectivity (k’) value was calculated to demonstrate the obtained selectivity by molecular imprinting (Table 3). k’ value should be higher than 1.0 to indicate that the developed sensor has higher affinity and selectivity for a target molecule. According to the results, it was found that the developed MAH-Co(II)@MIP/GCE sensor was more selective for TIO than other similar pharmacological compounds.
Interference study
Another application of selectivity, which is one of the critical parameters of an analytical method, is interference studies. Various interfering agents are widely used in biological fluids, such as K+, Cl+, Na+, SO42−, dopamine (DA), and ascorbic acid (AA). For the selectivity test, 0.05 pM TIO was used, and no significant effect on the peak current of TIO was observed, although there were 10 times more interference agents. Table 4 summarizes the percent RSD values (≥ 1.50), and the relative errors obtained. The results showed that interference materials did not affect the analysis performance of the MAH-Co(II)@MIP/GCE sensor.
Stability
The stability of the MAH-Co(II)@MIP/GCE sensor was investigated over a 10-day period. The MIP-modified electrode was kept in a desiccator for 10 days. While the stability of the electrode surface was 91.93% in the first 3 days, it decreased to 89.80% on the 5th day, 84.75% on the 7th day, and 79.19% on the 10th day. The results revealed that the sensor had acceptable stability, retaining 91.93% of its original value in the first three days.
Comparison with other methods
Various methods have been developed to determine TIO when the literature is examined in detail. Clearly, the developed approach is equivalent to these methods in terms of detection limit, linear range, and recovery results. In addition, the developed sensor has several advantages compared to other methods, such as fast analysis times, low cost, and environmental friendliness. The MAH-Co(II)@MIP/GCE sensor exhibits precise, fast, and simple detection for a comparison study of real samples. An overview of the TIO assay methods is reported in Table 5.
Conclusion
In this study, for the first time, a simple, low-cost, highly sensitive, and selective electrochemical MIP-based sensor was developed using a synthesized functional monomer to detect TIO. The developed sensor was provided by the photopolymerization method using MAH-Co(II) monomer. Electrochemical characterization of the fabricated MIP-based sensor was performed using CV and EIS techniques. Under optimum conditions, the developed MIP sensor showed very low LOD (2.93 fM) and LOQ (9.75 fM) values in the 10–100 fM linear range. MAH-Co(II)@MIP/GCE sensor exhibited excellent selectivity for TIO over molecules with similar structures such as OXI, IPR, GLY, and ACL. It was applied to real samples such as commercial serum and capsule, and the obtained recovery values were found to be 101.97% and 99.71%, respectively. In addition, the RSD value obtained as a result of reproducibility studies was found below 1.70% (n = 3) for both standard and commercial serum solutions. The fast response time, low cost, low sample consumption, high sensitivity, extremely low interference, and good stability proved that this sensor was successfully applied for TIO determination.
References
Yehia AM, Abo-Elhoda SE, Hassan NY, Badawey AM. Experimental validation of a computationally-designed tiotropium membrane sensor. New J Chem. 2018;42:16354–61. https://doi.org/10.1039/C8NJ03507E.
Chi J, Li F, Jenkins R. Ultrasensitive sub-pg/ml determination of tiotropium bromide in human plasma by 2D-UHPLC-MS/MS: challenges and solutions. Bioanalysis. 2016;8:385–95. https://doi.org/10.4155/bio.15.256.
Zayed S, Fouad F, Belal F. A simple and economic chromatographic method for simultaneous determination of six bronchodilator drugs in pharmaceutical dosage forms. J Iran Chem Soc. 2021;18:1251–9. https://doi.org/10.1007/s13738-020-02107-6.
Elkady EF, Tammam MH, Elmaaty AA. Development and validation of RP-HPLC method for simultaneous estimation of tiotropium bromide, formoterol fumarate, and olodaterol HCl in bulk and metered dose aerosols: application to olodaterol HCl forced degradation study and degradation kinetics. Chromatographia. 2017;80:1749–60. https://doi.org/10.1007/s10337-017-3413-0.
Joung SK, Kang SY, Ma KN, Kim SA, Cho K, La S, Lee HJ. Bioanalytical validation for the determination of fluticasone propionate, salmeterol and tiotropium in human plasma at the sub pg/mL level using UPLC/MS/MS. Drug Metab Pharmacokinet. 2017;32:S27. https://doi.org/10.1016/j.dmpk.2016.10.128.
Ding L, Tan W, Zhang Y, Shen J, Zhang Z. Sensitive HPLC-ESI-MS method for the determination of tiotropium in human plasma. J Chromatogr Sci. 2008;46:445–9. https://doi.org/10.1093/chromsci/46.5.445.
Wang J, Jiang Y, Wang Y, Li H, Fawcett JP, Gu J. Highly sensitive assay for tiotropium, a quaternary ammonium, in human plasma by high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1755–8. https://doi.org/10.1002/rcm.
Sarvestani MRJ, Madrakian T, Afkhami A. Developed electrochemical sensors for the determination of beta-blockers: a comprehensive review. J Electroanal Chem. 2021;899:115666. https://doi.org/10.1016/j.jelechem.2021.115666.
Çorman ME, Cetinkaya A, Ozcelikay G, Özgür E, Atici EB, Uzun L, Ozkan SA. A porous molecularly imprinted nanofilm for selective and sensitive sensing of an anticancer drug ruxolitinib. Anal Chim Acta. 2021; 1187.https://doi.org/10.1016/j.aca.2021.339143.
Kaya SI, Cetinkaya A, Ozcelikay G, Çorman ME, Karakaya M, BellurAtici E, Ozkan SA. Computational design and fabrication of a highly selective and sensitive molecularly imprinted electrochemical sensor for the detection of enzalutamide. J Electroanal Chem. 2022;907:116030. https://doi.org/10.1016/j.jelechem.2022.116030.
Cetinkaya A, Kaya SI, Çorman ME, Karakaya M, BellurAtici E, Ozkan SA. A highly sensitive and selective electrochemical sensor based on computer-aided design of molecularly imprinted polymer for the determination of leflunomide. Microchem J. 2022;179:107496. https://doi.org/10.1016/j.microc.2022.107496.
Armutcu C, Özgür E, Çorman ME, Uzun L. Interface imprinted polymers with well-oriented recognition sites for selective purification of hemoglobin. Colloids Surfaces B Biointerfaces. 2021; 197.https://doi.org/10.1016/j.colsurfb.2020.111435.
Armutcu C, Tartan Ç, Özgür E, Nemutlu E, Uzun L. Phosphate anion ımprinted cryogel cartridges for selective preconcentration of phosphorylated amino acids from protein lysate: an alternative sorbent for proteome analyses. ChemistrySelect. 2020;5:11730–6. https://doi.org/10.1002/slct.202001959.
Cetinkaya A, Kaya SI, Ozcelikay G, Atici EB, Ozkan SA. A molecularly imprinted electrochemical sensor based on highly selective and an ultra-trace assay of anti-cancer drug axitinib in its dosage form and biological samples. Talanta. 2021;233:122569. https://doi.org/10.1016/j.talanta.2021.122569.
Cetinkaya A, Yıldız E, Kaya SI, Çorman ME, Uzun L, Ozkan SA. A green synthesis route to develop molecularly imprinted electrochemical sensor for selective detection of vancomycin from aqueous and serum samples. Green Anal Chem. 2022;2:100017. https://doi.org/10.1016/j.greeac.2022.100017.
Odabaşi M, Say R, Denizli A. Molecular imprinted particles for lysozyme purification. Mater Sci Eng C. 2007;27:90–9. https://doi.org/10.1016/j.msec.2006.03.002.
Hur D, Ekti SF, Say R. N-acylbenzotriazole mediated synthesis of some methacrylamido amino acids. Lett Org Chem. 2007;4:585–7. https://doi.org/10.2174/157017807782795556.
Ozkan SA, Kauffmann J-M, Zuman P. Electroanalysis in biomedical and pharmaceutical sciences. In: Scholz F (ed). Springer; 2015. https://doi.org/10.1007/978-3-662-47138-8.
Srinivasu K, Venkateswara Rao J, Appala Raju N, Mukkanti K. Simultaneous RP-HPLC method for the estimation of formoterol fumarate and tiotropium bromide in pharmaceutical dosage forms. Asian J Chem. 2010;22:3943–8.
Bhoomaiah B, Jayasree A. Simultaneous quantification of olodaterol and tiotropium bromide by high-performance liquid chromatography. Asian J Chem. 2017;29:145–8. https://doi.org/10.14233/ajchem.2017.20164.
Funding
Ahmet Cetinkaya received financial support from the Council of Higher Education 100/2000 (YOK) under the special 100/2000 scholarship program and the Scientific and Technological Research Council of Turkey (TUBITAK) under the BIDEB/2211-A Ph.D. and ARDEB/1004 Ph.D. Scholarship Programmes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No human or animal subjects were used in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cetinkaya, A., Kaya, S.I., Atici, E.B. et al. A semi-covalent molecularly imprinted electrochemical sensor for rapid and selective detection of tiotropium bromide. Anal Bioanal Chem 414, 8023–8033 (2022). https://doi.org/10.1007/s00216-022-04335-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04335-6