Abstract
In this study, carbonyldiimidazole was used to bond maltose-modified oligopeptides (Ala-Glu-Ala-Glu-Ala-Lys-Ala-Lys) to the surface of silica spheres for hydrophilic interaction liquid chromatography (HILIC). Attenuated total reflectance-Fourier transform infrared spectroscopy, elemental analysis, X-ray photoelectron spectroscopy, thermogravimetric analysis, BET technique, and water contact angle measurement results confirmed the successful immobilization of the obtained material. Compared with the conventional method for preparing carbohydrate stationary phases, this method involves simpler steps and less time-consuming processes. The experimental results proved that the retention mechanism of the maltose-based HILIC column matched the typical HILIC retention mechanism. The column showed high separation efficiency and stability toward the separation of polar compounds such as amino acids, bases, nucleosides, water-soluble vitamins, and salicylic acid and its analogs. The column achieved high selectivity toward oligosaccharide separation. In addition, this efficient analysis demonstrates the applicability of the as-prepared material in the field of food inspection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of glycoproteomics, metabolomics, and food and environment monitoring fields, highly polar and ionic compounds have become important research materials in biology and chemistry [1]. It is difficult to separate these compounds on reverse-phase liquid chromatography (RPLC), thereby limiting their applications. Although normal-phase liquid chromatography (NPLC) can separate these substances, this method is typically ineffective because of defects caused by the effects of the environment on peak trailing and retention time. Moreover, the mobile phase for NPLC is less soluble than the most hydrophilic samples [2]. HILIC can overcome the limitations of RPLC and serves as a good alternative to NPLC toward the effective separation of various highly polar compounds [3,4,5,6,7,8]. The use of water and water-soluble organic solvents as mobile phases can improve the dissolution of samples in the mobile phase [9] and allow suitable retention for various highly polar samples. In addition, a high proportion of the organic phase increases the flow rate, reduces the retention time, and effectively improves the detection sensitivity and speed of the instrument [10,11,12]; HILIC is also compatible with mass spectrometry. Thus, this analytical technique has received widespread attention. However, owing to the diverse and complex analytical requirements of various samples, the need for high chromatographic performance of the stationary phase is gradually increasing. There is no standard research system for determining the retention mechanism of a new hydrophilic chromatographic stationary phase. Therefore, it is important to explore and develop high-water-affinity stationary-phase materials with novel structures and excellent performances.
Almost all HILIC stationary phases have been modified with high-polarity groups on the surface of a substrate. The polarity of the functional groups determines the separation performance of the stationary phases in hydrophilic and highly polar samples, which directly affects the application and development of HILIC [13, 14]. There are many types of HILIC stationary phases with different functional groups, such as amino, poly(succinimide), cyano, glycol, zwitterion, and glycosyl groups. Carbohydrates possess abundant unique polyhydroxy groups and possess high polarity. New carbohydrate-based HILIC stationary phases have been recently explored and developed. Guo et al. used the click reaction to prepare a β-cyclodextrin-type HILIC stationary phase for the first time [15]. This motivated Liang’s research group to use azido-alkynyl and sulfhydryl-alkenyl click reactions to prepare maltose-, glucose-, and β-cyclodextrin-modified HILIC stationary phases, which have a strong separation effect on small molecules, such as adenosine, amino acids, and sugars [16]. Huang et al. also used the click reaction to prepare chitosan-modified spherical-particle-based HILIC stationary phases to separate carbohydrates, nucleosides, and amino acid compounds; this column achieved a strong separation effect [17]. However, surface bonding in click chemistry requires the addition of azide or alkynyl groups on the sugar unit, which is difficult to achieve in polysaccharides because of their low solubility in organic solvents. The preparation process of this method is time consuming; therefore, a simpler and more effective method of polysaccharide immobilization is needed to prepare the HILIC stationary phase.
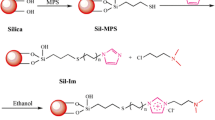
To this end, herein, carbonyldiimidazole (CDI) was used as a cross-linking agent, and AEK8-maltose was immobilized on the silica surface to prepare a maltose-based HILIC stationary phase (Scheme 1). This method for stationary phase preparation is simpler than those used for the development of conventional saccharide stationary phases and does not involve time-consuming processes. In the HILIC mode, the effects of different mobile phase compositions, salt concentrations, pH values, and temperatures on the solute retention behavior were investigated. The results confirmed that the retention mechanism of the maltose-based HILIC column matched that of the typical HILIC column. The column prepared using the as-synthesized stationary phase exhibits high separation efficiency and stability for separating polar compounds, such as amino acids, bases, water-soluble vitamins, and organic acids, and achieves high selectivity toward oligosaccharide separation.
Materials and methods
Materials and reagents
Silica gel, CDI, VPP, VB2, VB7, VB1, VC, salicylamide, salicylic acid, trans-cinnamic acid, acetylsalicylic acid, salicylic acid, N,N-dimethylformamide (DMF), methanol, and acetonitrile were purchased from the Shanghai Chemical Reagents Company (China). 3,5-Dihydroxybenzoic acid, lysine, arginine, glutamic acid, aspartic acid, ribose, glucose, sucrose, melezitose, and trifluoroacetic acid were obtained from Sigma-Aldrich. Deionized water was purified using Milli-Q water prepared in the laboratory and all other chemicals were of analytical grade. All participants provided written informed consent.
Characterization techniques
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was performed using a Tensor-27 infrared spectrometer (Bruker, Billerica, MA) with a wedged germanium crystal as an attenuated total reflectance accessory. Transmission electron microscopy (TEM) images were obtained using a JEM-2100 microscope (JEOL, Japan). Thermogravimetric analysis (TGA) was performed using a SACS Q600SDT thermogravimetric analyzer (USA). N2 adsorption surface areas were measured by the BET technique on an ASPS 2020 M analyzer (USA). X-ray photoelectron spectroscopy (XPS) was performed using an Axis Ultra X-ray photoelectron spectrometer (Kratos Analytical Ltd., Manchester, UK) with an Al X-ray source operating at 150 W (15 kV, 10 mA).
Synthesis of AEK8-maltose
The Fmoc-Ala-Glu(OtBu)-Ala-Glu(OtBu)-Ala-Lys(BOC)-MBHA resin was obtained by extending the peptide chain from the C-end to N-end according to the sequence AEAEAKAK (Ala-Glu-Ala-Glu-Lys-Ala-Lys) on a Rink Amide-MBHA resin by solid-phase synthesis. Maltose and sodium cyanoborohydrin were added to the as-synthesized oligopeptide and methanol was used as a solvent for the reflux reaction at 80 ℃, followed by suction filtration and washing. Then, a mixture of trifluoroacetic acid (TFA), triisopropylsilane (TIS), and water was used as the cutting solution for the residue protective groups of amino acid in the oligopeptide. AEK8-maltose was separated from the resin at room temperature, and suction filtration was performed on it. The filtrate was precipitated using ice-cold ether. After centrifugation, the precipitate was dialyzed and freeze-dried to obtain maltose-modified oligopeptide (AEK8-maltose).
Preparation of maltose-type silica gel stationary phase
A total of 1.0 g of silica gel activated with 10% HCl and 1.17 g of CDI were mixed in 10 mL of a 70% methanol aqueous solution. The solution was refluxed for 5 h at 25 ℃, washed with water and methanol for several times, and dried at 80 ℃. Next, 1.0 g of the as-prepared CDI-activated silica gel was mixed with 6 mL of 15 mg/mL AEK8-maltose DMF solution at 40 ℃ for 5 h. The mixture was washed with DMF and methanol for several times and dried at 60 ℃ to obtain AEK8-maltose-modified silica gel (SiO2-AEK8-maltose).
Column packing
SiO2-AEK8-maltose was ultrasonically dispersed in chromatographic methanol, and the dispersion was transferred to a homogenization tube using chromatographic methanol as a replacement liquid. This system was maintained in a column packer at 35 MPa for 40 min, after which the nitrogen valve in the column packer was closed. When the pressure dropped to 0 MPa, the column was packed to obtain a 50 × 4.6 mm chromatographic column. The bare SiO2 sphere column was prepared using the same method and specifications. After loading the column, it was activated with chromatographic methanol for 2 h prior to use.
Separation and application of maltose-based HILIC column
To explore the performance of the maltose-based HILIC stationary phase, experiments were performed to separate several common polar compounds, such as nucleosides, bases, amino acids, water-soluble vitamins, salicylic acid and its analogs, and oligosaccharides. The separation performance of the maltose-based HILIC column was compared with that of a self-made bare SiO2 sphere chromatographic column under same conditions.
Reproducibility and stability test
Three batches of simultaneously prepared SiO2-AEK8-maltose were loaded onto three chromatographic columns (50 mm × 4.6 mm). The samples of VPP, VB2, VB7, VB1, salicylamide, salicylic acid, and salicylide were analyzed under the mobile phase of 85% ACN-20 mM NH4AcO at a pH 4.0. Then, the stability of the chromatographic behavior of the stationary phase was investigated and the same column was injected with all samples for 1, 5, 15, 30, 45, 60, and 75 days to analyze the changes in the retention behavior.
Results and discussion
Characterization of SiO2-AEK8-maltose
The morphology of silica was analyzed by TEM. From the TEM images shown in Fig. 1a and b, no apparent changes in the silica spheres, before and after modification, can be observed. It may be because the functionalized AEK8-maltose molecule was too small to be observed. To investigate the success of AEK8-maltose modification on silica spheres, Fourier transform infrared spectroscopy (FT-IR) spectroscopy was performed for the functional groups present on the surface of SiO2 and SiO2-AEK8-maltose. As shown in Fig. 1c, the strong absorption peaks at 1100 and 805 cm−1 correspond to the symmetric and asymmetric stretching vibrations of Si–O-Si, respectively. After modifying the silica spheres with AEK8-maltose, the stretching vibration absorption peak of CH2 appeared at 2900 cm−1, and the absorption peaks of amide I and amide II appeared at 1640 and 1560 cm−1, respectively. The characteristic absorption peak of sugar appeared at 3600 cm−1. These results indicated the successful preparation of SiO2-AEK8-maltose. Furthermore, the average pore size of the prepared stationary phase was approximately 12.4 nm, and the BET surface area was approximately 312 m2/g (Fig. 1e). The TGA of bare SiO2 spheres and SiO2-AEK8-maltose was also analyzed (Fig. 1f). The endothermic mass loss, caused by the loss of water, was observed to be approximately 2.0% over the 0–100 °C range. At 200–600 °C, a mass loss of 2% (from 12.39 to 12.14 g) was observed due to the breaking and dehydration of the silicon–oxygen bond in the silica gel. However, the mass of SiO2-AEK8-maltose decreased from 9.54 to 9.34 g over 0–100 °C owing to the loss of water, resulting in a mass loss of 2.1%. At 200–600 °C, the mass of SiO2-AEK8-maltose decreased to 8.53 g, resulting in a mass loss of 8.4%. These observations preliminarily showed that the loss of AEK8-maltose on the silica surface was about 6.4%, indicating that the new stationary phase was modified with 6.4% wt. of AEK8-maltose to bare SiO2 spheres.
To further confirm that AEK8-maltose was successfully modified on the surface of the silica spheres, the composition of SiO2 and SiO2-AEK8-maltose was analyzed by XPS. As shown in Fig. 1d, the XP spectra of both materials exhibit four peaks: O 1 s, C 1 s, Si 2 s, and Si 2p. The XP spectra of the AEK8-maltose-modified silica spheres had a new N 1 s peak, while the peak corresponding to N in the amino group of the peptide was only present, confirming that AEK8-maltose was successfully modified on the surface of the silica spheres. At the same time, the relative content of each element was calculated from the spectrum; the results are summarized in Table 1. Elemental analysis (EA) was performed before and after modifying the silica spheres, as shown in Table 2. The contents of N, C, and H in the modified silica spheres were significantly increased, while AEK8-maltose contained only N (the trace amount of N in the bare SiO2 spheres came from the air), indicating that AEK8-maltose and the silica spheres were successfully bonded. According to the calculation formula for surface bonding [18], the binding amount of AEK8-maltose on the surface of the maltose-based HILIC stationary phase was 0.806 μmol/m2.
Here, %X is the percentage increase in carbon or nitrogen in the bonded support determined by EA, AM is the atomic mass of carbon or nitrogen, MW is the molecular weight of the species bonded to the silica surface, n is the number of carbon or nitrogen atoms present in the bonded species, and S is the specific surface area of the silica support in meters squared per gram.
After coating SiO2 and SiO2-AEK8-maltose on the glass sheet, the water contact angle (WCA) of the coating was measured. As shown in Table 3, the measured contact angle of the SiO2-AEK8-maltose coating was larger than that of the SiO2 coating, indicating that AEK8-maltose was successfully modified on the surface of the silica spheres.
Influence of water content in the mobile phase
The performance of HILIC is typically analyzed on the basis of the effect of the water content in the mobile phase on solute retention. If the increase in the water content weakens the retention, it is the HILIC mode. The organic phase of the HILIC mode mainly contains acetonitrile and methanol. Methanol is a polar protic solvent that can easily form hydrogen bonds with the stationary phase and competitively adsorbs the solutes, reducing solute retention. Although acetonitrile is more toxic, it does not react with the sample, and the column pressure of acetonitrile as the organic phase is the lowest, which prolongs the service life of the instrument and the chromatographic column. Therefore, acetonitrile was selected as the organic phase for this experiment. The effect of adjusting the proportion of acetonitrile (5–96%) in the mobile phase on the retention factor (k) of salicylic acid and its analogs and water-soluble vitamins is shown in Fig. 2a. The overall retention behaviors of the two analytes were similar. In the ACN content range of 5–70%, water-soluble vitamins, salicylic acid, and their analogs are almost not retained; in the high ACN range of the mobile phase, especially when ACN > 85%, k increases sharply. This shows that the maltose-based HILIC stationary phase had strong hydrophilicity, and the retention mechanism of the two analytes on the maltose-based HILIC column was mainly hydrophilic. Therefore, when the column was used to separate polar compounds, the ACN content in the mobile phase was selected within the range of 75–100%.
Separation performance of the maltose-based HILIC column
To investigate the performance of the maltose-modified column toward the separation of polar compounds, experiments were performed to separate acids, bases, nucleosides, water-soluble vitamins, salicylic acid and its analogs, and oligosaccharides. This performance was compared with that of the bare SiO2 sphere column.
Separation of amino acids
The experiment was performed under optimized chromatographic conditions. The mobile phase was 75% acetonitrile/20 mM KH2PO4, the pH was 3.50, and the UV detector wavelength was 190 nm. Using amino acids as solutes, the separation effects of the four amino acids on the maltose-based HILIC and bare SiO2 sphere columns were compared under the same chromatographic conditions. As shown in Fig. 3a and b, the four analytes could be separated well within 5 min on the maltose-based HILIC column, and the peak shape is better than that obtained for the bare SiO2 sphere column. The separation effect on the bare silica sphere column was weak, indicating that the maltose-based HILIC column can effectively separate amino acids.
Chromatograms of four amino acids on (a) maltose and (b) bare SiO2 sphere columns (1—lysine, 2—arginine, 3—glutamic acid, 4—aspartic acid). Chromatograms of nucleic acid bases and nucleosides on (c) maltose and (d) bare SiO2 sphere columns (1—naphthalene, 2—uracil, 3—adenosine, 4—adenine, 5—cytosine, 6—cytidine, 7—guanosine). Chromatograms of water-soluble vitamins on (e) maltose and (f) bare SiO2 sphere columns (1—VPP, 2—VB2, 3 -VB7, 4—VB1, 5—VC)
Separation of bases and nucleosides
Naphthalene was used as a reference to separate the bases and nucleosides with gradient elution. The optimized chromatographic conditions were as follows: 0–5 min: 97% ACN, 5–15 min: 97–80% ACN, 20 mM ammonium acetate, pH 4.0 gradient, and 0.6 mL/min flow rate. Under the same chromatogram conditions, the separation effects of these substances on maltose-based HILIC and bare SiO2 sphere columns were compared. The results indicate that the maltose-based HILIC column allowed for a stronger separation effect than the bare SiO2 sphere column, as represented in Fig. 3c and d, respectively.
Separation of water-soluble vitamins
To further investigate the separation effect of the maltose-based HILIC column, five water-soluble vitamins were separated. The optimized chromatographic conditions were as follows: 85% acetonitrile/20 mM ammonium acetate and 0.8% acetic acid, pH 4.0 mobile phase; 210 nm detection wavelength; and 0.6 mL/min flow rate. The separation effects of five water-soluble vitamins on the maltose-based HILIC and bare SiO2 sphere columns under the same chromatographic conditions were compared. As shown in Fig. 3e and f, the five analytes achieved good separation within 8 min on the maltose-based HILIC column, while the bare SiO2 sphere column could not separate them.
Separation of salicylic acid and its analogs
The optimized chromatographic conditions were as follows: 92% acetonitrile/20 mM ammonium acetate and 0.8% acetic acid, pH 4.0 mobile phase; 200 nm detection wavelength; and 0.6 mL/min flow rate. Salicylic acid and its analogs were used as analytes to investigate the maltose-based HILIC column. The separation effects of the same mixed sample on the maltose-based HILIC and bare SiO2 sphere columns under the same separation conditions were compared. As shown in Fig. 4a and b, the six analytes achieved were more effectively separated within 10 min on the maltose-based HILIC column than on bare SiO2 sphere column. This result suggested that the maltose-based HILIC column effectively separated salicylic acid and its analogs.
Chromatograms of salicylic acid and its analogs on (a) maltose and (b) bare SiO2 sphere columns (1—salicylamide, 2—salicylic acid, 3—trans-cinnamic acid, 4—acetylsalicylic acid, 5—salicylic acid, 6—3,5-dihydroxybenzoic acid). Chromatograms of oligosaccharide on (c) maltose and (d) bare SiO2 sphere columns (1—ribose, 2—glucose, 3—sucrose, 4—melezitose). (e) and (f) Practical application toward the separation of melamine in infant milk on maltose columns
Separation of oligosaccharides
The maltose-based HILIC column was used to separate oligosaccharides. The optimized chromatographic conditions were as follows: 75% acetonitrile/water mobile phase; 35 ℃ column temperature; 350 kappa N2 pressure; 50 ℃ drift tube temperature; one filter; and 5 × 16 gain value. Under the same conditions, the separation effects of the same mixed sample on a maltose-based HILIC column and a bare SiO2 sphere column were compared. As shown in Fig. 4c and d, six analytes achieved baseline separation within 4 min on the maltose-based HILIC column, while the silica sphere column could not separate the analytes, indicating that the maltose-based HILIC column was suitable for the separation of oligosaccharides.
Practical application
Owing to its high nitrogen content, melamine can be illegally added to infant milk formulas. In this study, the applicability of the maltose-based HILIC column was verified by analyzing melamine (Fig. 4e, f). With the mobile phase of 75/25 (v/v) ACN/water, flow rate of 1.0 mL/min, and detection wavelength of 254 nm, the standard melamine retention was approximately 4 min (Fig. 4e, 1). The main components of infant milk were eluted within approximately 2 min (Fig. 4e, 2) causing no interference in the detection of melamine (Fig. 4f). This efficient analysis demonstrates the applicability of the as-prepared material in the field of food inspection.
Reproducibility and stability test
The reproducibility of different batches of the maltose-based HILIC stationary phase was investigated. Using the mobile phase of 85% ACN-20 mM NH4AcO, pH 4.0, the retention times of water-soluble vitamins and salicylic acid compounds on the stationary phase of different batches were monitored. As shown in Table 4, the retention times of the four analytes were almost the same in these batches, indicating good inter-column reproducibility. In addition, the stability of the chromatographic behavior of the stationary phase was investigated. As shown in Fig. 2b, after 75 days of the continuous use of the same maltose-based HILIC column, the retention times of different analytes did not change, indicating that the maltose-based HILIC stationary phase had high stability.
Conclusions
In this study, AEK8-maltose was bonded with silica spheres using CDI as a cross-linking agent to prepare a maltose-based HILIC stationary phase. The steps involved in this method are simple. In the HILIC mode, the effects of the compositions, salt concentrations, pH values, and temperatures of different mobile phases on the solute retention behavior were investigated. The experimental results indicated that the retention mechanism of the maltase-based HILIC stationary phase matched the typical HILIC retention mechanism. The maltose-based HILIC column showed a strong separation effect toward polar compounds such as amino acids, bases, and nucleosides, water-soluble vitamins, salicylic acid and its analogs, and oligosaccharides. In addition, the maltose-based HILIC column exhibited high reproducibility, high stability, and a long service life.
References
Periat A, Krull IS, Guillarme D. Applications of hydrophilic interaction chromatography to amino acids, peptides, and proteins. J Sep Sci. 2015;38(3):357–67. https://doi.org/10.1002/jssc.201400969.
Dell’aversano C, Hess P, Quilliam MA. Hydrophilic interaction liquid chromatography mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J Chromatogr A. 2005;1081(2):190–201. https://doi.org/10.1016/j.chroma.2005.05.056.
Paczkowska M, Mizera M, Tężyk A, Zalewski P, Dzitko J, Cielecka-Piontek J. Hydrophilic interaction chromatography (HILIC) for the determination of cetirizine dihydrochloride. Arab J Chem. 2019;12(8):4204–11. https://doi.org/10.1016/j.arabjc.2016.05.012.
Zborníková E, Knejzlík Z, Hauryliuk V, Krásný L, Rejman D. Analysis of nucleotide pools in bacteria using HPLC-MS in HILIC mode. Talanta. 2019;205:120161. https://doi.org/10.1016/j.talanta.2019.120161.
Mathon C, Larive K. Separation of ten phosphorylated mono-and disaccharides using HILIC and ion-pairing interactions. Anal Chimica Acta. 2017;972:102–10. https://doi.org/10.1016/j.aca.2017.03.029.
Shao W, Liu J, Yang K, Liang Y, Weng Y, Li S, Liang Z, Zhang L, Zhang Y. Hydrogen-bond interaction assisted branched copolymer HILIC material for separation and N-glycopeptides enrichment. Talanta. 2016;158:361–7. https://doi.org/10.1016/j.talanta.2016.05.034.
Rampler E, Schoeny H, Mitic M, Schwaiger M, Koellensperger G. Simultaneous non-polar and polar lipid analysis by on-line combination of HILIC, RP and high resolution MS. Analyst. 2018;143(5):1250–8. https://doi.org/10.1039/C7AN01984J.
Fan F, Wang L, Li Y, Wang X, Lu X, Guo Y. A novel process for the preparation of Cys-Si-NIPAM as a stationary phase of hydrophilic interaction liquid chromatography(HILIC). Talanta. 2020;218:121154. https://doi.org/10.1016/j.talanta.2020.121154.
Pack BW, Risley DS. Evaluation of a monolithic silica column operated in the hydrophilic interaction chromatography mode with evaporative light scattering detection for the separation and detection of counter-ions. J Chromatogr A. 2005;1073(1–2):269–75. https://doi.org/10.1016/j.chroma.2004.09.061.
Tipke I, Bücker L, Middelstaedt J, Winterhalter P, Lubienski M, Beuerle T. HILIC HPLC-ESI-MS/MS identification and quantification of the alkaloids from the genus Equisetum. Phytochem Anal. 2019;30(6):669–378. https://doi.org/10.1002/pca.2840.
Tsochatzis E, Papageorgiou M, Kalogiannis S. Validation of a HILIC UHPLC-MS/MS method for amino acid profiling in triticum species wheat flours. Foods. 2019;8(10):514. https://doi.org/10.3390/foods8100514.
Bento-Silva A, Gonçalves L, Mecha E, Pereira F, Patto M, Bronze R. An improved HILIC HPLC-MS/MS method for the determination of β-ODAP and its α isomer in Lathyrus sativus. Molecules. 2019;24(17):3043. https://doi.org/10.3390/molecules24173043.
Molnarova K, Kozlík P. Comparison of different HILIC stationary phases in the separation of hemopexin and immunoglobulin G glycopeptides and their isomers. Molecules. 2020;25(20):4655. https://doi.org/10.3390/molecules25204655.
Zhang J, Yang W, Li S, Yao S, Qi P, Yang Z, Feng Z, Hou J, Cai L, Yang M, Wu W, Guo D. An intelligentized strategy for endogenous small molecules characterization and quality evaluation of earthworm from two geographic origins by ultra-high performance HILIC/QTOF MS and Progenesis QI. Anal Bioanal Chem. 2016;408:3881–90. https://doi.org/10.1007/s00216-016-9482-3.
Guo Z, Lei A, Liang X. Click chemistry: a new facile and efficient strategy for preparation of functionalized HPLC packings. Chem Commun. 2006;43:4512–4. https://doi.org/10.1039/B610733H.
Guo Z, Jin Y, Liang T. Synthesis, chromatographic evaluation and hydrophilic interaction/reversed-phase mixed-mode behavior of a “Click β-cyclodextrin” stationary phase. J Chromatogr A. 2009;1216(2):257–63. https://doi.org/10.1016/j.chroma.2008.11.071.
Huang H, Jin Y, Xue M. A novel click chitooligosaccharide for hydrophilic interaction liquid chromatography. Chem Commun. 2009;45:6973–5. https://doi.org/10.1039/B911680J.
Kibbey CE, Meyerhoff ME. Preparation and characterization of covalently bound tetraphenylporphyrin-silica gel stationary phases for reversed-phase and anion-exchange chromatography. Anal Chem. 1993;65(17):2189–96. https://doi.org/10.1021/ac00065a005.
Funding
This work was supported by the National Key R&D Program of China (2019YFB2103000), the Natural Science Foundation Project of Shaanxi Province (No. 2020JZ-24), and the Fundamental Research Funds for the Central Universities (GK201801006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, H., Zhang, L. Maltose-functionalized HILIC stationary phase silica gel based on self-assembled oligopeptides and its application for the separation of polar compounds. Anal Bioanal Chem 414, 3917–3925 (2022). https://doi.org/10.1007/s00216-022-04036-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04036-0