Abstract
There is an urgent need for the rapid and simultaneous detection of multiple analytes present in a sample matrix. Here, a multiplex immunochromatographic test (multi-ICT) was developed that successfully allowed for the rapid and simultaneous detection of four major nitrofuran metabolites, i.e., 3-amino-2-oxazolidinone (AOZ), semicarbazide (SEM), 3-amino-5-methylmorpholino-2-oxazolidinone (AMOZ), and 1-aminohydantoin (AHD), in fish samples. Four different antigens were separately immobilized in four test lines on a nitrocellulose membrane. Goat anti-mouse immunoglobulin (IgG) was used as a control. Sensitive and specific monoclonal antibodies (mAbs) that recognize the corresponding antigens were selected for the assay, and no cross-reactivity between the antibodies in the detection assay was observed. The free analytes in samples or standards were pre-incubated with freeze-dried mAb–gold conjugates to improve the sensitivity of the detection assay. The multi-ICT detection was accomplished in less than 15 min by the naked eye. The cutoff values for the strip test were 0.5 ng/mL for AOZ and 0.75 ng/mL for AHD, SEM, and AMOZ, which were all below the maximum residue levels set by the European Union and China. A high degree of consistency was observed between the multi-ICT method and commercially available enzyme-linked immunosorbent assay (ELISA) kits using spiked, incurred, and “blind” fish samples, indicating the accuracy, reproducibility, and reliability of the novel test strip. This newly developed multi-ICT strip assay is suitable for the rapid and high-throughput screening of four nitrofuran metabolites in fish samples on-site, with no treatment or devices required.

A multiplex immunochromatographic test (multi-ICT) was developed that successfully allowed for the rapid and simultaneous detection of four major nitrofuran metabolites (AOZ, SEM, AMOZ, and AHD) in fish samples
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrofurans containing furaltadone (FTD), furazolidone (FZD), nitrofurantoin (NFT), and nitrofurazone (NFZ) are a class of broad-spectrum antibiotics that contain a 5-nitrofuran ring and various substituents in the 2-position and are widely used in food animal production as growth promoters and for disease prevention and treatment [1,2,3]. Shortly after being absorbed into the body, nitrofurans are highly unstable and are metabolized very rapidly in vivo: FTD, FZD, NZF, and NFT are rapidly metabolized to 3-amino-5-morpholinomethyl-2-oxazolidone (AMOZ), 3-amino-2-oxazolidone (AOZ), semicarbazide (SEM), and 1-aminohydantoin (AHD), respectively [4, 5]. Therefore, the parental drug form itself does not normally remain in the meat products of treated animals, and only these metabolites are detectable as tissue-bound residues [6].
Nitrofurans and their metabolites exhibit significant toxicity in humans, which is manifested via their carcinogenic, mutagenic, and teratogenic effects [7,8,9]. For this reason, nitrofurans were banned from use in food-producing animals in the European Union (EU) in 1995 [10]. Strict prohibitions on the use of nitrofurans in animal husbandry were also put into place in the USA [11] and China [12]. In accordance with European Directive 96/23/EC [13] and Decision 657/2002/EC [14], a definitive minimum required performance limit (MRPL) of 1 μg/kg was set for these drugs (markers = metabolites of nitrofurans) in March of 2003 [15].
Various methods have been introduced for the determination of nitrofuran drugs, including liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI/MS/MS) [8], liquid chromatography/mass spectrometry (LC–MS) [16], and liquid chromatography/tandem mass spectrometry (LC–MS/MS) [6, 17]. These analytical methods have many quantitative advantages, including precision and accuracy [18, 19]. However, despite the high sensitivity and specificity that can be achieved using these methods, their limitations should not be ignored. For example, the procedures are time-consuming and require expensive equipment, well-trained personnel, a long operating time, and tedious sample preparation and can only be performed in laboratories. Enzyme-linked immunosorbent assay (ELISA)-based methods, which are an important alternative method for the detection of small molecules, are widely used in screening approaches for nitrofuran metabolites [2, 20,21,22]. A drawback of analyzing all four metabolites using ELISA is four separate plates would be required for testing [23]. Thus, the special instruments required for these detection processes make such methods unsuitable for on-site and simultaneous detection of various contaminants.
As a food product may be contaminated with multiple nitrofuran drugs, a single detection method is insufficient to meet the current requirements. Instrument-free methods to detect multiple residues make possible the simultaneous detection of multiple analytes present in a sample matrix [24]. Antibody-based immunochromatographic tests (ICTs) have been developed in response to the need for rapid screening methods to monitor food safety [25, 26]. Some studies have reported the successful detection of multiple chemicals using this assay [27,28,29,30,31]. ICTs, which are based on a colloidal gold nanoparticle (AuNP)–antibody probe, are regarded as another new immunosensor and have become a well-established and accepted point-of-care testing technique [32, 33]. Compared to other approaches, ICTs have many advantages, such as no requirement for expensive instrumentation, simple operation, short detection time, and low cost. Additionally, there is no need for trained personnel or a specific apparatus for high-throughput on-site determination by ICTs.
In the present study, a multi-ICT based on different antibodies was developed for the simultaneous and high-throughput detection of AMOZ, AOZ, SEM, and AHD within single samples. The accuracy of the ICT was further compared with that of a standard ELISA kit using fish samples collected in the field. The advantages of this newly developed instrument-free method allow for the simultaneous detection of multiple nitrofuran analytes present in the same sample matrix.
Materials and methods
Reagents and apparatus
AOZ and AMOZ were purchased from WITEGA Laboratorien Berlin-Adlershof GmbH (Berlin, Germany). AHD was purchased from DaRui Shanghai Industrial Co., Ltd. (Shanghai, China). 4-Nitrobenzaldehyde (4-NBA) was obtained from Heng Ye Zhong Yuan Chemical Industrial Co., Ltd. (Beijing, China). 4-Carboxybenzaldehyde (4-CBA) was obtained from Accela ChemBio Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from Sigma Chemical Company (USA). N-Dimethylformamide (DMF), N-hydroxysuccinimide (NHS) dicyclohexylcarbodiimide (DCC), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and dimethyl sulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Hydrogen tetrachloroaurate hydrate (HAuCl4), trisodium citrate, and goat anti-mouse immunoglobulin (IgG) were purchased from Sigma Chemical Company (USA). All solvents, chemicals, and salts used in this study were of analytical grade. Nitrophenyl derivatives (NPAHD, NPAOZ, NPSEM, and NPAMOZ); conjugated coating antigens (3-{[(3-carboxyphenyl)-methylene]-amino}-2-oxazolidinone (CPAOZ)–BSA, 4-{[5-(morpholinomethyl)-2-oxooxazolidin-3-ylimino]methyl}benzoic acid (CPAMOZ)–BSA, 3[(3-carboxyphenyl)-methylene]-hydrazinecarboxamide (CPSEM)–BSA, and 1-[(4-carbo-benzylidene)-amino]-imidazolidin-2,4-dione (CPAHD)–jeffamine–BSA); and specific monoclonal antibodies (mAbs) that recognize AOZ, SEM, AMOZ, or AHD were prepared in our laboratory [2, 21, 34]. Commercial ELISA kits for AMOZ and AHD were purchased from R-Biopharm AG (Darmstadt, Germany); the kit for AOZ was purchased from R&D Diagnostics-Biotechnology (Italy), and the kit for SEM was purchased from Huaan Magnech Bio-Tech Co. (Beijing, China). The fish samples used for detection were purchased from a local supermarket. An XYZ Biostrip Dispenser (Bio-Dot, Irvine, CA, USA) was used, and the strip cutting model CM 4000 Cutter (Bio-Dot, Irvine, CA, USA) was employed. The sample pad (glass fiber membranes), nitrocellulose (NC) high-flow-plus membranes, absorbent pads, and polyvinylchloride backing material were purchased from Millipore Corporation (Shanghai, China). Solutions were prepared using Milli-Q18 Ω water (Millipore Purification System).
Synthesis of coating antigens
The compounds CPAMOZ, CPAOZ, CPSEM, and CPAHD were derivatized from AMOZ, AOZ, SEM, and AHD with 4-CBA, respectively, according to a previously published method [35]. Next, CPAOZ, CPAMOZ, and CPSEM were conjugated to the carrier protein BSA via an active ester method. The carboxylic acid on CPAOZ, CPAMOZ, or CPSEM was activated with DCC and NHS to produce an active ester, which was then reacted with the amine groups on BSA to form an amide bond. Briefly, a mixture of 0.1 mM CPAOZ (or CPAMOZ or CPSEM), 0.1 mM NHS, and 0.1 mM DCC in 1.0 mL dehydrated dimethyl formamide (DMF) was stirred for 6 h at RT and centrifuged at 2500×g for 10 min. The supernatant was added very slowly with stirring to BSA (100 mg) in 5 mL phosphate-buffered saline (PBS, pH 7.4) solution (a mixing solution of 2 mL DMF and 3 mL PBS) and stirred overnight at 4°C. The mixture was dialyzed against PBS for 3 days (two buffer changes per day). The final conjugates were CPAOZ–BSA, CPAMOZ–BSA, and CPSEM–BSA.
CPAHD was coupled to jeffamine–BSA according to the method described by Wang [2]. First, 20 mg BSA was dissolved in 2 mL of 0.05 M 2-(N-morpholino)ethanesulfonic acid (MES) buffer (0.05 M MES, 0.5 M NaCl, pH 4.7) solution and activated by the addition of EDC (5 mg) and NHS (3 mg). Next, 15 μL jeffamine was added and stirred gently at RT for 4 h. The conjugates were then dialyzed against 0.01 M PBS (pH 7.4) for 3 days. CPAHD activated with DCC and NHS was coupled to the jeffamine–BSA complex and allowed to react overnight at RT to form CPAHD–jeffamine–BSA.
Characterization of mAbs
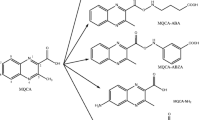
In the commonly used method to analyze these metabolites (AMOZ, AOZ, SEM, and AHD), they are first released by mild acid hydrolysis from tissue proteins, which is usually followed by derivatization with nitrobenzaldehyde (NBA) to increase their molecular mass prior to detection. In this study, the nitrophenyl-derivatized metabolites (NPAHD, NPAOZ, NPSEM, and NPAMOZ) were used as inhibitors in the screening of positive hybridoma cells during monoclonal antibody preparation. These metabolites act as marker residues for the detection of the illegal use of nitrofurans. The structures of the metabolites and nitrophenyl-derivatized metabolites of these four nitrofurans are shown in Table 1.
All monoclonal antibodies (mAbs) produced in our laboratory were purified with a protein G immunoaffinity column according to the manufacturer’s instructions (GE Healthcare). A competitive chemiluminescent ELISA (dcCLELISA) that is similar to conventional protocols was developed to detect each mAb: high-binding white opaque 96-well plates were coated (100 μL/well) with hapten–BSA complex in 0.05 M carbonate buffer (CB) for 2 h at 37 °C. The plates were then washed three times with 0.01 M PBS containing 0.05% Tween-20 (PBST) and blocked with 0.05 M CB containing 0.2% w/v gelatin for 2 h at 37 °C. After washing once more, 50 μL purified antibody dissolved in antibody diluent (0.01 M PBS containing 0.05% v/v Tween-20 and 10% fetal bovine serum) and 50 μL of the indicated concentrations of NP-analytes (NPAOZ, NPSEM, NPAMOZ, or NPAHD) in PBS were added to each well, and the plates were incubated at 37 °C for 30 min. Plates were washed three times, and 100 μL of 4000-fold-diluted goat anti-mouse IgG–horseradish peroxidase (HRP) was added to each well and incubated for 1 h at 37 °C. After washing, 100 μL of the prepared chemiluminescence substrate mixture was added to each well. Chemiluminescence was measured on a fluorescence microplate reader within 10 min of substrate addition.
Preparation of mAb–AuNP conjugates
The gold nanoparticles (AuNPs) were prepared in our laboratory as previously described [32], with some modifications. Briefly, 100 mL of 0.01% HAuCl4 solution was brought to a boil with stirring, and 3 mL of 1% trisodium citrate solution (w/v) was added under constant stirring. The reaction solution changed to the color of red wine within approximately 1 min and was kept boiling for an additional 5 min. The pH value of the colloidal gold solution and the dosage of each purified mAb were optimized. The optimal pH was adjusted with 0.2 M K2CO3. An aliquot (10 μL) of serially diluted mAb was taken from each dilution and was mixed gently with 100 μL of colloidal gold solution and incubated for 15 min at RT. Then, 0.1 mL of 10% (w/v) NaCl solution was added and incubated for 10 min at RT. The change in color of colloidal gold to claret red was observed and also detected on a microplate reader. The minimum protein concentration that had no effect on the claret red color of colloidal gold was noted. The optimal concentration of purified mAb chosen for conjugation to colloidal gold should increase by 20%.
The mAb–AuNP conjugates were prepared in the following steps. Approximately 1 mL (1.0 mg/mL) of purified anti-AOZ mAb was mixed gently under constant stirring with 100 mL of colloidal gold solution prepared as described above and incubated for 30 min at RT. BSA was added to a final concentration of 1% (w/v) to stabilize and block the nonreactive sites. After 1 h, the mixture was centrifuged twice at 8000×g for 20 min, and the conjugated pellet was suspended in 0.01 M PBS containing 1% (w/v) BSA, 0.3% (v/v) Tween-20, 0.9% (w/v) NaCl, and 0.05% (w/v) sodium azide and was stored at 4 °C. The anti-CPAMOZ, anti-CPSEM, and anti-jeffamine–CPAHD mAb conjugates were prepared in the same manner under individually optimized conditions. The average particle diameter of the obtained colloidal gold was measured on a transmission electron microscope (TEM) and a UV–vis spectrometer.
Preparation of the test strip
The composition of the test strip is shown in Fig. 1. The sample pad, NC membrane, and absorbent pad were assembled sequentially onto a plastic backing sheet. Briefly, four types of antigens and the goat anti-mouse IgG antibody were separately used as capture reagents and microsprayed at 1 μL/cm onto the four test lines and one control line situated 4 mm apart on the NC membrane (25 mm × 300 mm; Millipore, Bedford, MA, USA) using Quanti 3000 Biojets attached to an XYZ Biostrip Dispenser (Bio-Dot, Irvine, CA, USA). After drying for 3 h at 37 °C, the membrane was sealed in a plastic bag and stored under dry conditions at RT. The sample pad was impregnated with a buffer solution (0.2% BSA and 0.2% Tween-20 in 0.01 M PBS) and air-dried overnight before use. The absorption pad, made of 100% pure cellulose fiber, was applied immediately without pretreatment. These components were assembled sequentially with a 1- to 2-mm overlap on the support card; one end of the sample pad overlapped with the NC membrane, and the other end was attached to the absorption pad. This assembly was then cut into 4-mm-wide strips using a CM4000 Cutter (Bio-Dot, Irvine, CA, USA). The optimal volumetric proportions of the four mAb–AuNP conjugates were taken and mixed in microtiters and freeze-dried with a vacuum freeze dryer. The strips and freeze-dried mAb–AuNP conjugate mixtures were housed in a plastic cassette with silica desiccant gel and stored under dry conditions until use.
Detection procedure and principle of multi-ICT strip detection
As shown in Fig. 2A, detection was initially performed by adding a 200-μL aliquot of sample solution to the freeze-dried mAb–AuNP conjugate mixtures and reacted at RT for 5 min. This pre-incubation step ensured that the mAb–AuNP conjugates could completely react with each of the four analytes (if present) in the sample. Next, a strip was placed into the reaction solution in each microtiter well. The solution permeated the sample pad and moved along the NC membrane via capillary action and aggregated on the test and control lines due to the antigen–antibody reaction. The results were observable within 15 min by the naked eye. The principle of the multi-ICT is illustrated in Fig. 2B. The assay is based on the competitive/inhibitory format. The density of the red band was inversely proportional to the concentration of analyte in the sample. As showed in Fig. 2B, if no analyte was present in the sample solution, the four mAb–AuNP conjugates migrated into the NC membrane and bound to the coating antigens fixed on the four T lines, forming four red lines, respectively. In the positive sample, which contained different concentrations of the four analytes, mAb–AuNP conjugated with the corresponding free analyte, forming the mAb–AuNP–analyte complex, leading to a decrease in the amount of mAb–AuNP binding to the antigen fixed on the T lines. If the concentration of the analyte exceeded a certain value, the mAb–AuNP–analyte complexes did not bind to the corresponding coating antigen, and no red bands would form on the test line. The C line should always emerge; in cases where no line was evident at the control position, the test was considered invalid.
Specificity, sensitivity, and stability of the multi-ICT strip
In this study, a multiplex ICT strip method was established for the simultaneous detection of AMOZ, AOZ, SEM, and AHD animals in fish tissues. The AMOZ, AOZ, SEM, and AHD residues were derivatized into NPAMOZ, NPAOZ, NPSEM, and NPAHD, respectively, for detection using the multiplex ICT strip method developed herein. The concentration of the NP–analyte was determined and converted into an analyte concentration using the following formula: C analyte = (M analyte/M NPanalyte) × C NPanalyte, where C NPanalyte is the concentration of NPAMOZ, NPAOZ, NPSEM, or NPAHD detected in the sample; M analyte is the molecular weight of AMOZ, AOZ, SEM, or AHD; M NPanalyte is the molecular weight of NPAMOZ, NPAOZ NPSEM, or NPAHD; and C analyte is the concentration of AMOZ, AOZ, SEM, or AHD calculated from the formula used to detect samples.
The sensitivity of the multi-ICT strip was evaluated by determining a series of diluted standard NP–analyte solutions. The concentration of the NP–analyte was converted to the analyte concentration so that the corresponding concentration of each analyte ranged from 0.125 to 1 ng/mL. The serially diluted standard solution was added to the microtiter wells with the mAb–AuNP conjugate mixtures and was detected by ICT in triplicate. The concentration that produced no red color on the test line was defined as the cutoff value.
The specificity of the strip test was determined by individually testing the parent nitrofuran compounds (FTD, FZD, NFT, and NFZ) and other drugs (norfloxacin, enrofloxacin, quinocetone, oxytetracycline, chloramphenicol, diethylstilbestrol, and leucomalachite green) at high concentrations. The specificity of the strip sensor was also evaluated by testing each analyte separately to exclude false-positive results. The NP–analytes (NPAMOZ, NPAOZ, NPSEM, and NPAHD) representing the four analytes (AMOZ, AOZ, SEM, and AHD) were mixed for the simultaneous detection analysis. Each test was repeated ten times at each concentration.
To estimate the stability of the multi-ICT strip, several freeze-dried mAb–AuNP conjugate mixtures were stored for 3, 6, 9, and 12 months at RT and at 4 °C. The stored strips were re-examined for specificity and sensitivity.
Analysis of fish samples using the multi-ICT strip and commercial ELISA kits
Fish samples were used to estimate the applicability of the multiplex strip. The selected fish sample was confirmed using HPLC and was demonstrated to be a blank sample. For fortified samples, fillets (skin removed) from control fish were homogenized in a blender. Fish muscle homogenates (2 g) were fortified with AMOZ, AOZ, SEM, and AHD at 0, 0.5, 1.0, and 2.0 μg/kg, respectively. The analytes were subsequently derived to NP–analytes (NPAOZ, NPSEM, NPMOZ, and NPAHD) and extracted according to a method previously described by Pimpitak [36] with minor modifications. Briefly, 2 g homogenized fish muscle was placed into a 50-mL centrifuge tube. Next, 4 mL of H2O, 0.5 mL of 1 M HCl, and 100 μL of 40 mM 4-NBA in DMSO were added successively to the homogenized samples. Each sample was thoroughly mixed and incubated for 3 h in a water bath at 55 °C. After cooling to RT, 5 mL of 0.1 M K2HPO4, 0.4 mL of 1 M NaOH, and 6 mL of ethyl acetate were added to the sample, shaken vigorously (30 s), and centrifuged at 3500×g for 10 min at RT. The upper ethyl acetate layer (3 mL) was transferred to glass tubes and evaporated by heating to dryness at 45 °C under nitrogen. The resulting residues were dissolved in 2 mL of a 1:1 (v/v) mixture of hexane and 0.1 M PBS at pH 7.4. The buffer phase, containing the derivative, was separated by centrifugation at 3000×g for 10 min and collected for ICT determination.
Incurred residues in fish muscle were generated from a previous study on the depletion of tissue-bound metabolites of nitrofuran drugs. Fish were orally dosed with parent FZD, FTD, NFT, or NFZ at 2 mg/kg body weight and were sampled at intervals for up to 15 days after dosing. Samples selected for the present study provided a range of levels as appropriate to the evaluation. Homogenates were stored at −80 °C until analysis.
Furthermore, 50 fish samples with unknown concentrations of these compounds were sourced from retail outlets in Shanghai. The fortified samples, incurred samples, and 50 “blind” samples were all assayed using the multiplex strip sensor and the commercial ELISA kits.
Results and discussion
Preparation of mAb–AuNP conjugates
The size and shape of AuNP are related to the stability of the colloidal gold probe. TEM images revealed the colloidal gold particles to be round and homogeneous in size distribution, with an average size of 15 nm (Fig. 3A). The mAb–AuNP conjugates were formed through electrostatic interactions between the antibodies and the surfaces of the charged particles. The pH value of the reaction system plays a key role in the process of mAb–AuNP conjugation because the solution pH determines the charge and stability of the conjugate. The optimal pH value for conjugation was between 7.0 and 9.0, which was slightly higher than the isoelectric point (pI) of mAb, making it easier to conjugate with AuNPs and more stable. Our results indicated that the final pH values for the conjugated systems (100 mL) were 8.5 for anti-CPAOZ, 8.0 for anti-CPSEM, 8.0 for anti-CPAMOZ, and 8.5 for anti-CPAHD mAbs, respectively.
The concentrations of the mAbs were also optimized to achieve the required visibility and the best sensitivity. An appropriate dose of mAbs was important to the sensitivity and activity of mAb–AuNP conjugation. Our results indicated that the optimal mAb concentrations of the four mAb–AuNP conjugates were 8 μg/mL for anti-CPAOZ, 10 μg/mL for anti-CPSEM, 9 μg/mL for anti-CPAMOZ, and 8 μg/mL for anti-CPAHD mAbs. In addition to the antibody adsorbed onto the surfaces of the AuNPs, their size increased, and the absorbance peak shifted. The UV–vis spectra of the AuNPs displayed maximum absorbance at 519 nm, while the maximum absorbances of the four mAb–AuNP conjugates occurred at wavelengths of 524 nm (AuNP–anti-CPAOZ mAb conjugate), 525 nm (AuNP–anti-CPAHD mAb conjugate and AuNP–anti-CPSEM mAb conjugate), or 526 nm (AuNP–anti-CPAMOZ mAb conjugate) (Fig. 3B), indicating successful preparation of the mAb–AuNP conjugates.
Optimization of the strip
As the sensitivity of the multi-ICT strips is dependent on the color intensities of the T and C lines, the performance of the strip is primarily affected by the antibody sensitivity and the concentrations of mAb–AuNP conjugates and coating antigens. The high sensitivity of the antibody is the most decisive factor. Here, the 50% inhibitory concentration (IC50) values of the four competitors were determined with dcCLELISA. Our results indicate that these antibodies were highly sensitive (IC50 values of 0.206 ng/mL for NPAOZ, 0.27 ng/mL for NPSEM, 0.289 ng/mL for NPAMOZ, and 0.365 ng/mL for NPAHD).
To obtain better sensitivity, optimization experiments were used to determine the optimal immunoreagent concentrations and dilution factors of the mAb–AuNP conjugates. These were selected based on a clear color appearing in the test line in the negative control, while the test line was completely inhibited by a low inhibitor concentration, which was around the minimum required performance level of 1 ng/g identified [37].
Antibody–AuNP solutions for each mAb type under individually optimized conditions were mixed at optimal volumetric proportions of 2, 1, 2, and 5 μL for anti-CPAOZ, anti-CPAOZ, anti-CPAMOZ, and anti-CPAHD mAbs, respectively; these mixtures were adjusted to 150 μL with a resuspending solution. For the test lines, the optimal concentrations of the coating antigens were 20, 20, 25, and 60 μg/mL for CPAOZ–BSA, CPSEM–BSA, CPAMOZ–BSA, and CPAHD–jeffamine–BSA, respectively. The concentration of the goat anti-mouse IgG antibody was 0.5 mg/mL. Under these optimized conditions, the multicomponent strip sensor showed clear test lines and good sensitivity.
Validation of the multiplex strip
The aim of this study was to establish a rapid screening method to evaluate nitrofuran metabolites using a visual detection system. Considering the unreliable quantification of visual tests by different personnel, this assay was a more suitable semi-quantification test with a cutoff value, which is the minimal concentration of contamination causing the total inhibition of the test lines because these results can be read with the naked eye. Concurrently, the negative control strip has clearly visible lines. Sample solutions containing different concentrations of the four NP–analytes were serially diluted in PBS and used as standards. Different concentrations of the four analytes were mixed in PBS to generate standards to be analyzed with the multistrip. As shown in Fig. 4, the color intensity of the test lines decreased as the analyte concentration increased. Based on visual inspection, the cutoff levels for this method were 0.5 ng/mL for AOZ and 0.75 ng/mL for AHD, SEM, and AMOZ. All cutoff values conformed to the maximum residue limits in animal food products set by China, the USA, and the EU.
To ensure there was no interference in the simultaneous detection of the four analytes, the specificity of the strip sensor was evaluated by testing each analyte separately to exclude false-positive results. The results shown in Fig. 5 indicated that the analytes were specifically recognized by their corresponding antibodies and that there was no interference between them. The developed multi-ICT strips still had high sensitivity and specificity, even when the interferent concentration was high (200 ng/mL).
The specificity of the strip test was also determined by testing the parent nitrofuran compounds and various other veterinary drugs. Our results show that the multi-ICT strip exhibits negligible cross-reactivity with the parent nitrofurans; the color intensity of the corresponding test zone decreased slightly when the concentration of each nitrofuran was higher than 200 ng/mL. However, the parent nitrofuran compound did not accumulate in edible tissues, such as muscle. Furthermore, we found there was no cross-reactivity with other chemicals that are commonly used or banned in aquatic foods and potentially in other animal-derived foods, such as norfloxacin, enrofloxacin, quinocetone, oxytetracycline, chloramphenicol, diethylstilbestrol, and leucomalachite green. Moreover, our results show that even drugs containing amine groups that likely react with 4-NBA (i.e., oxytetracycline, sulfamethazine, oxicillin, and chloramphenicol) do not cross-react with the antibodies in our ICT strip.
The freeze-dried mAb–AuNP conjugate mixtures stored at 4 °C for 12 months or at RT for 9 months showed continued sensitivity, and the cutoff levels for the ICT strip were 0.5 ng/mL for AOZ and 0.75 ng/mL for AHD, SEM, and AMOZ, which were the same sensitivity levels of freshly produced strips. The specificity of the ICT strip for the detection of these four analytes was not influenced, as evident from the finding that no negative sample became a false positive, regardless of the storage conditions. Apparently, the period of validity of the multi-ICT strip was at least 12 months at 4 °C or 9 months at RT without losses in sensitivity and specificity for detection of the four analytes.
Comparison of the ICT strip and commercially available kits for detecting AMOZ, AOZ, SEM, and AHD spiked into fish samples
To demonstrate the applicability of the multi-ICT strip for the evaluation of levels of residual AMOZ, AOZ, SEM, and AHD in fish samples, fortified samples and incurred samples were simultaneously analyzed using the multi-ICT strip and commercially available ELISA kits. Generally, there was good correlation obtained using these techniques. As shown in Table 2, the strip sensor performed well when the threshold concentrations (cutoff value) were analyzed. The reproducibility tests of the analytes in the fish samples were based on 20 independent experiments. Fifty “blind” fish samples were analyzed by using the multi-ICT strips and commercial ELISA kits; no positive samples were detected with either method, which indicates that the data obtained using these two methods are well-correlated. Therefore, this comparison demonstrated the accuracy of our multi-ICT strips for detecting these four analytes in fish samples.
ELISA is widely considered as an effective tool for detecting nitrofuran metabolites (AMOZ, AOZ, SEM, or AHD) in regulatory, residual, and industrial laboratories. However, it requires a laboratory, skilled technicians, and special instruments. Moreover, measurements take approximately 3.5 h to complete, making rapid and on-site detection difficult. Recently, some time-saving assay has been introduced, for example, magnetic beads functionalized with antibody against nitrofuran derivative were used as both the extraction and color-developing media in lateral flow biosensor, and this assay not only simplified the extraction procedure but also increased the sensitivity [38]. Compared to ELISA, the multi-ICT strip developed in this study has the following advantages: expertise, experience, and special equipment are not required; it is faster (the results were obtained within 15 min with a paper-based sensor); and it is more economical. Moreover, these four nitrofuran metabolites are the most common contaminants of fish or other types of food and may also be present in foodstuffs [6, 20]. Thus, in recent years, the need to develop a technology for the simultaneous detection of multiple analytes in a single sample has increased. The multi-ICT strip developed in this study exhibited strong advantages, including a simpler pretreatment process, higher sensitivity, and multiple drug detection. Our newly developed assay is suitable for field analysis and the rapid monitoring of nitrofuran metabolites in fish samples.
Conclusions
In this study, a new multi-ICT strip system was successfully developed that enables the simultaneous and rapid screening of AMOZ, AOZ, SEM, and AHD residues in fish samples. It has the advantages of simplicity, rapidity, sensitivity, specificity, and cost-effectiveness; it also required no instrumentation. The test results of the multistrip sensor method in the spiked, incurred, and blind samples demonstrated that this technique is both reliable and applicable. This method was successfully used for the detection of the four nitrofuran metabolites in fish and can potentially be used in other types of food. The newly developed multi-ICT strip system represents a high-throughput and improved option for the rapid detection of these four analytes in food and will substantially advance research requiring on-site detection, allowing fast initial screening.
References
Vervoort J, Xavier BB, Stewardson A, et al. An in vitro deletion in ribE encoding lumazine synthase contributes to nitrofurantoin resistance in Escherichia coli. Antimicrob Agents Chemother. 2014;58(12):7225–33.
Wang Q, Liu YC, Chen YJ, et al. Development of a direct competitive chemiluminescent ELISA for the detection of nitrofurantoin metabolite 1-amino-hydantoin in fish and honey. Anal Methods. 2014;6(12):4414–20.
Zhang Z, Wu Y, Li X, et al. Multi-class method for the determination of nitroimidazoles, nitrofurans, and chloramphenicol in chicken muscle and egg by dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2017;217:182–90.
Du NN, Chen MM, Sheng LQ, et al. Determination of nitrofuran metabolites in shrimp by high performance liquid chromatography with fluorescence detection and liquid chromatography-tandem mass spectrometry using a new derivatization reagent. J Chromatogr. 2014;1327(1):90–6.
Kaufmann A, Butcher P, Maden K, et al. Determination of nitrofuran and chloramphenicol residues by high resolution mass spectrometry versus tandem quadrupole mass spectrometry. Anal Chim Acta. 2015;862:41–52.
Zhang Y, Qiao H, Chen C, et al. Determination of nitrofurans metabolites residues in aquatic products by ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2016;192:612–7.
Auro A, Sumano H, Ocampo L, et al. Evaluation of the carcinogenic effects of furazolidone and its metabolites in two fish species. Pharmacogenomics J. 2004;4(1):24–8.
Rodziewicz L, Chromatogr J, Analyt B. Determination of nitrofuran metabolites in milk by liquid chromatography-electrospray ionization tandem mass spectrometry. Technol Biomed Life Sci. 2008;864(1):156–60.
Williams EM, Triller DM. Recurrent acute nitrofurantoin-induced pulmonary toxicity. Pharmacotherapy. 2006;26(5):713–8.
Commission Regulation (EC) 1442/95 amending Annexes I, II, III and IV of Regulation (EEC) No 2377/90 laying down a Community Procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off. J. Eur. Commun. 1995; L143, 26–30.
Federal Register. Topical nitrofurans, extralabel animal drug use, and order of prohibition. 2002; 67:5470–71.
Regulation of Department of Agriculture of China. List of banned veterinary drugs and other compounds in food producing animals. 2002; no. 193.
European Commission. Commission Directive 96/23/EC measuring to monitor certain substances and residues thereof in live animals and animal products. Off. J. Eur. Commun. 1996; L125,10.
European Commission. Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results. Off. J. Eur. Commun. 2002; L221, 8.
European Commission. Commission decision 2003/182/EC amending decision 2002/657/EC as regards setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin. Off. J. Eur. Commun. 2003; L71, 17–18.
Xia X, Li X, Zhang S, et al. Simultaneous determination of 5-nitroimidazoles and nitrofurans in pork by high-performance liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2008;1208(1):101–8.
Zhang S, Guo Y, Yan Z, et al. A selective biomarker for confirming nitrofurazone residues in crab and shrimp using ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407(30):8971–7.
Leitner A, Zöllner P, Lindner W. Determination of the metabolites of nitrofuran antibiotics in animal tissue by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr. 2008;939(1):49–58.
Shendy AH, Al-Ghobashy MA, Gad Alla SA. Development and validation of a modified QuEChERS protocol coupled to LC-MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem. 2016;190:982–9.
Cooper KM, Samsonova JV, Plumpton L. Enzyme immunoassay for semicarbazide—the nitrofuran metabolite and food contaminant. Anal Chim Acta. 2007;592(1):64–71.
Liu YC, Jiang W, Chen YJ, et al. A novel chemiluminescent ELISA for detecting furaltadone metabolite, 3-amino-5-morpholinomethyl-2-oxazolidone (AMOZ) in fish, egg, honey and shrimp samples. J Immunol Methods. 2013;395(2):29–36.
Jester ELE, Abraham A, Wang Y. Performance evaluation of commercial ELISA kits for screening of furazolidone and furaltadone residues in fish. Food Chem. 2014;145(7):593–8.
Cooper KM, Caddell A, Elliott CT, Kennedy DG. Production and characterisation of polyclonal antibodies to a derivative of 3-amino-2-oxazolidinone, a metabolite of the nitrofuran furazolidone. Anal Chim Acta. 2004;520(1):79–86.
Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron. 2016;75:166–80.
Guo Y, Ngom B, Le T, Jinet X, Wang L, Shi D, et al. Utilizing three monoclonal antibodies in the development of an immunochromatographic assay for simultaneous detection of sulfamethazine, sulfadiazine, and sulfaquinoxaline residues in egg and chicken muscle. Anal Chem. 2010;82(18):7550–5.
Jia X, Song T, Liu Y, Meng L, Mao X. An immunochromatographic assay for carcinoembryonic antigen on cotton thread using a composite of carbon nanotubes and gold nanoparticles as reporters. Anal Chim Acta. 2017;969:57–62.
Li X, Li PW, Zhang Q, Li R, Zhang W, Zhang Z, et al. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens Bioelectron. 2013;49:426–32.
Kong D, Liu L, Song S, Suryoprabowo S, Li A, Wang L, et al. Gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nano. 2016;8(9):5245–53.
Xing C, Liu L, Song S, Feng M, Kuang H, Xu C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens Bioelectron. 2015;66:445–53.
Glupczynski Y, Jousset A, Evrard S, Bonnin RA, Huang TD, Dortet L, et al. Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J Antimicrob Chemother. 2017;72(7):1955–60.
Wang W, Su X, Ouyang H, Wang L, Fu Z. A novel immunochromatographic assay based on a time-resolved chemiluminescence strategy for the multiplexed detection of ractopamine and clenbuterol. Anal Chim Acta. 2016;917:79–84.
Jiang W, Liu Y, Chen Y, Yang Q, Chun P, Yao K, et al. A novel dynamic flow immunochromatographic test (DFICT) using gold nanoparticles for the serological detection of Toxoplasma gondii infection in dogs and cats. Biosens Bioelectron. 2015;72:133–9.
Kai M, Wenjing S, Peng Z, et al. Development of colloidal gold-based immunochromatographic assay for rapid detection of Mycoplasma suis in porcine plasma. Biosens Bioelectron. 2014;55:396–9.
Liu Y, Jiang W, Chen Y, Zeng P, Zhang M, Wang Q. Simultaneous detection of four nitrofuran metabolites in honey using high-throughput suspension array technology. Anal Methods. 2015;7(10):4097–103.
Gao AZ, Chen QL, Cheng L, Lei J, Zeng LW. Preparation of monoclonal antibodies against a derivative of semicarbazide as a metabolic target of nitrofurazone. Anal Chim Acta. 2007;592(1):58–63.
Pimpitak U, Putong S, Komolpis K, Petsom A, Palaga T. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for detection of the furaltadone metabolite, AMOZ, in fortified shrimp samples. Food Chem. 2009;116(3):785–91.
Regulation (EC) 470/2009. Laying down community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin. Off J Eur Commun. 2009;L152:11–22.
Lu X, Liang X, Dong J, Fang Z, Zeng L. Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Anal Bioanal Chem. 2016;408(24):6703–9.
Acknowledgments
We are thankful to Dr. Xiangan Han for expert advice and helping to edit the manuscript.
Funding
This work was funded by the Shanghai Science and Technology Standard Fund (Grant No. 17140900400) and the National Special Research Programs for Non-Profit Trades (Agriculture) (Grant No. 201303045).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were in accordance with the Institutional Animal Care and Use Committee (IACUS) guidelines of the Shanghai Veterinary Research Institute at the Chinese Academy of Agricultural Sciences (CAAS). The Ethics Committee of CAAS approves the use of fish for the present experiment.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Q., Liu, Y., Wang, M. et al. A multiplex immunochromatographic test using gold nanoparticles for the rapid and simultaneous detection of four nitrofuran metabolites in fish samples. Anal Bioanal Chem 410, 223–233 (2018). https://doi.org/10.1007/s00216-017-0714-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0714-y