Abstract
Direct analysis in real time mass spectrometry (DART-MS) was used to characterize commercial polyurethane (PUR) samples without sample pretreatment. More than 50 substances, such as catalysts, stabilizers, antioxidants, flame retardants, plasticizers, chain extenders, chain terminators, polyols, solvents, degradation products and contaminants, a few of them presumably toxic, were detected and identified in 18 PUR items. The identification of 16 compounds was further confirmed by DART MS/MS experiments. Catalysts were the largest class of compounds detected in the PURs by DART-MS. In each of the 18 PUR samples, at least one catalyst residue was identified. In addition, DART-MS was able to detect the migration of hazardous chemicals from the PURs to other objects. The collision-induced dissociation (CID) properties of two PUR catalysts, such as the protonated bis[2-(dimethylamino)ethyl] ether (DMAEE) and the protonated 2,2-dimorpholinodiethylether (DMDEE), as well as those of two PUR antioxidants (Antioxidant 1135 and Antioxidant 1076), were explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PURs) represent one of the most important and versatile groups of plastics. Polyurethanes can be used as flexible and rigid foams, elastomers, fiber composite materials, paints and coatings, adhesives [1]. Industrial PURs are produced by the reaction of di- or polyisocyanates with polyols in the presence of a catalyst and other additives. Additives, such as chain extenders, surfactants, blowing agents, flame retardants, antioxidants, light stabilizers, plasticizers, etc. affect the final physical and chemical properties of the polyurethanes. In addition, compounds that are not intended to be present in the final product can also be found in the plastic materials. Among these residues are solvents, catalysts, degradation products, contaminants, and so forth. The analysis of additives and other chemicals is obviously very important for quality control during the production process or for the characterization of the products. Furthermore, because of the potential toxicity of some chemicals present in the plastics (e.g. phthalate plasticizers, halogenated flame retardants) the analysis of additives in polymers has gained increasing interest in recent years [2,3,4,5]. Solvent extraction and liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS) are conventionally used for the analysis of polymer additives in the final products [2, 3, 6]. However, all these techniques are quite time-consuming and labor-intensive. In contrast, the ambient mass spectrometric techniques, e.g. direct analysis in real time mass spectrometry (DART-MS), enable the analysis of solid samples in the open environment without sample preparation [7], and can serve as alternative or complementary methods for the analysis of chemicals in plastics. Moreover, direct sampling techniques can also provide spatial information on the analyte distribution in 2- or 3-dimensions. Only a few papers have reported the application of DART-MS for the analysis of polymers [5, 8,9,10,11,12,13]. Lebeau and Ferry have characterized model and commercial PURs using atmospheric solid analysis probe mass spectrometry (ASAP-MS) [14], however, to the best of our knowledge, there have been no studies on the detection of additives and other compounds in PURs by DART-MS. Accordingly, the main objective of our work was the mass spectrometric analysis of a wide variety of chemicals, a few of them potentially toxic, in commercial PURs by DART ionization tandem mass spectrometry (MS/MS). In this paper, using DART-MS as a novel technique for direct analysis of commercial PURs, we showed that the rapid screening of additives and other chemicals in PURs is possible without any preliminary sample preparation.
Experimental
Polyurethane samples
18 commercially available PUR items with a variety of types and countries of origin were analyzed. The samples were new, purchased in local stores. Further details are listed in Table 1.
Quadrupole time-of-flight mass spectrometry
Measurements were performed with a MicroTOF-Q type Qq-TOF MS instrument from Bruker (Bruker Daltoniks, Bremen, Germany). For MS/MS experiments, nitrogen was used as the collision gas and the collision energies were varied between 8 and 35 eV (in the laboratory frame). The pressure in the collision cell was ~1.2 × 10−2 mbar. The precursor ions for MS/MS were selected with an isolation width of 4 m/z unit. All of the spectra were recorded by a digitizer at a sampling rate of 2 GHz and were evaluated by the DataAnalysis 3.4 software from Bruker. As it is published in our previous paper [15].
Ion-source for direct analysis in real time (DART)
A DART SVP source was purchased from IonSense (IonSense, Inc., Saugus, MA, USA). The DART system was operated in the positive mode at 350 °C with helium 5.0 (purity >99.999%). The solid samples were manually introduced into the DART gas stream, into the middle of the gap (lgap = 2.5 cm) between the ion source and the spectrometer inlet. As it is published in our previous paper [15].
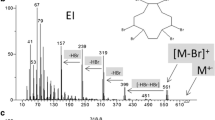
To assess the reproducibility of measurements and the heterogeneity of the samples 10 mass spectra were recorded from different spots (inside and outside too) of sample 3. The relative standard deviation of the absolute intensities of the two most intense peaks at m/z 113 and 161 (see Fig. 1c) were 37% and 58%, respectively.
Gas chromatography-mass spectrometry (GC-MS) analysis
Sample preparation
1 g of PUR sample was extracted for 12 h by soxhlet extraction using methanol as the extraction solvent, and subsequently filtered.
GC–MS analysis was performed on a Shimadzu GCMS-QP2010 Plus instrument (Shimadzu Corporation, Kyoto, Japan) equipped with a capillary column (Rxi-5MS, 30 m × 0.25 mm i.d., 0.25 μm film thickness). GC conditions: the flow rate for the helium carrier gas was 1.0 mL/min; injector temperature, 280 °C. 1 μl sample was injected in spitless mode. The temperature programme was 50 °C for 5 min, then 50–250 °C at a rate of 10 °C/min and subsequently held isothermal for 25 min. MS conditions: ionization energy 70 eV; ion source temperature 250 °C; scan mass range 25–600 m/z; solvent delay time 5 min. Mass spectral database: NIST05 (National Institute of Standards and Technology).
Results and discussion
Representative DART-MS spectra of the PUR items are shown in Fig. 1a-i, and additional spectra are given in Electronic Supplementary Material (ESM) Fig. S1a–i. The DART-MS mass spectra are rich in peaks indicating the presence of a number of additives and other chemicals in the PUR items, and that they can be effectively detected by DART-MS. All mass peaks labeled with the corresponding m/z values in Fig. 1 and ESM Fig. S1 were identified. The identification of the compounds was based on the exact masses derived from the molecular formulas obtained by the MolWeightToFormula utility of the DataAnalysis software from Bruker. The identified chemicals are compiled in Table 2. (The compounds by samples are summarized in Table 1.) The limit of detection (LOD) values in our DART-MS technique were estimated to be 450 ng/cm2, 1340 ng/cm2, and 1.6 ng/cm2 for aniline, diethanolamine, and dinonyl phthalate, respectively, as is published and detailed in our previous paper [15].
In the case of mass peaks with high or moderate intensities DART ionization tandem mass spectrometry experiments (DART-MS/MS) could also be performed. The proposed identification of several chemicals was confirmed by combining the MS/MS spectra with findings from the literature and databases of spectra of standard compounds, as it will be detailed later. The MS/MS spectra are shown in Fig. 2 (and in ESM Fig. S2), and the compounds subjected to tandem mass spectrometric experiments and their characteristic product ions are presented in Table 3.
DART-MS/MS spectrum of 1,4-diazabicyclo[2.2.2]octane (DABCO) (a) [M + H]+ (protonated molecule) detected from sample 3 recorded at a laboratory frame collision energy of 35 eV, diaminotoluene (b) [M + H]+ detected from sample 3 (20 eV), bis(2-dimethylaminoethyl)ether (c) [M + H]+ detected from sample 3 (20 eV), 1,4,7-trioxacyclotridecane-8,13-dione (d) [M + H]+ detected from sample 8 (20 eV), dibutyl phthalate (e) [M + H]+ detected from sample 2 (8 eV), tris (1-chloro-2-propyl) phosphate (TCPP) (f) [M + H]+ detected from sample 9 (10 eV), octyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (g) [M + NH4]+ detected from sample 18 (10 eV), bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate (Tinuvin 779) (h) [M + H]+ detected from sample 2 (35 eV), octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (Irganox 1076) (i) [M + NH4]+ detected from sample 1 (8 eV)
Additionally, the identification of the found compounds was confirmed by gas chromatography mass spectrometry (GC-MS) also. Four PUR sponge items (sample 3, 8, 10, and 18) were extracted and analyzed. The identification of the most remarkable compounds (e.g. 1,4-diazabicyclo[2.2.2]octane (DABCO), Bis(2-dimethylaminoethyl)ether (DMAEE), 1,4,7-trioxacyclotridecane-8,13-dione), altogether 9 compounds was confirmed using the NIST Mass Spectral Database. The results are presented in detail in ESM Table S1 and Fig. S3.
Catalysts
As seen in Table 2, catalysts are the largest class of compounds detected in the PURs by DART-MS based on the detection frequency. Basically, they are not intended to be present in the final product, and their rapid detection can be important, as will be detailed later. At least one catalyst residue was detected in each of the 18 PUR samples. For example, the peak at m/z 113.106 (see Fig. 1c) belongs to the elemental composition [C6H12N2 + H]+ with a theoretical m/z 113.107. As seen in Table 2, this compound was most frequently detected, it was found in 13 PUR samples. Interestingly, this was the only compound which was detected in all of the four yellow sponges from EU (sample 1, 3, 8, 18). The proposed structure for the chemical composition C6H12N2 is DABCO (1,4-diazabicyclo[2.2.2]octane or triethylenediamine), because it is one of the traditional base-catalysts for formation of polyurethanes [16]. To confirm the identification of C6H12N2 as DABCO, DART-MS/MS measurement was also performed by selecting the ion at m/z 113 as the precursor ion (see Fig. 2a and Table 3). The most abundant product ion at m/z 84 matches the characteristic ion formed from the protonated DABCO under electrospray ionization (ESI) MS/MS conditions [17]. Besides DABCO a number of other tertiary amine catalysts were found, such as dimethylethanolamine, N,N′-dimethylpiperazine, N-ethylmorpholine, methyldiethanolamine, N,N-dimethylbenzylamine, bis(2-dimethylaminoethyl)ether, dimethyldodecylamine, and 2,2-dimorpholinodiethylether. The undesirable degradation process in PURs may be initiated (among others) by catalyst residues, especially tertiary amines [18, 19]. Therefore the quick detection of the catalyst residues in the PUR products by DART-MS can have a great importance for quality control during the production process. On the other hand, most of the amine catalysts severely irritate the skin, eyes, and mucous membranes [20,21,22,23,24]. Moreover, bis(2-dimethylaminoethyl)ether (DMAEE) and dimethyldodecylamine (DMDA) are suspected to be toxic [23, 24]. The high intensity of the protonated DMAEE peak (m/z 161, see Fig. 1c) enabled us to perform tandem MS experiments. To our best knowledge no report on the collision-induced dissociation (CID) properties and fragmentation pathways of DMAEE has been published. As seen in Fig. 2c and Table 3, two product ions were formed at m/z 72 and 116. The proposed fragment structures for the protonated DMAEE are depicted in Scheme 1. The formation of these product ions confirms our identification for the m/z 161 peak as DMAEE.
The identification of DMDA was also confirmed by MS/MS experiments (see Fig. S2c). The characteristic fragment at m/z 46 formed by the loss of dodecene is in agreement with the main product ion found by Tsukatani and Tobiishi [24].
The mass peak of the protonated 2,2-dimorpholinodiethylether (DMDEE), a polyurethane blowing catalyst, at m/z 245 dominates the DART-MS spectra of the rigid PUR foam samples 5 and 14, as seen in the ESM in Fig. S1b and S1g, respectively. The MS/MS experiment of the protonated DMDEE reveals the formation of two well-defined product ions, as seen in Fig. S2d in the ESM. The proposed product ion structures are shown in Scheme 2 with a good agreement between the measured and calculated ion masses (m/z 114.090, 158.118, 245.184 and m/z 114.091, 158.118, 245.186, respectively). We can recommend the use of these product ions (m/z 114 and 158) for the detection or quantification of DMDEE in positive ion and selective reaction monitoring (SRM) MS/MS modes.
It must be noted, that some of the compounds identified as catalysts are possibly degradation or by-products and not intentionally added during the production.
Antioxidants, stabilizers
The second largest class of compounds detected in the PURs by DART-MS was that of the light stabilizers and antioxidants (11 substances, see Table 2). Octyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, commercial name Antioxidant 1135 (other names: Irganox 1135, Primanox 1135 or BNX 1135) was detected in 4 samples. It is a hindered phenolic antioxidant that is highly effective for the thermal stabilization of plastics. As Fig. 1i shows, both the protonated and the ammoniated adducts appear in the DART-MS spectrum at m/z 391.319 and 408.344, respectively, in good agreement with the calculated masses (m/z 391.321 and 408.347, respectively). The MS/MS spectrum of the m/z 408 ion (Fig. 2g) corresponds to the proposed fragmentation of octyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate presented in Scheme 3. A similar hindered phenolic antioxidant, octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, commercial name Antioxidant 1076 (other name: Irganox 1076) was recognized in one sample (sample 1, see Fig. 1a, [M + NH4]+ at m/z 548). The MS/MS spectra of this compound also show the C4H8 and C8H16 loss (see Fig. 2i, m/z 475 and 419, respectively), similarly to Antioxidant 1135 (see Scheme 3). Irganox 1076 is often used combined with the light stabilizer hexadecyl 3,5-bis-tert-butyl-4-hydroxybenzoate, commercial name Cyasorb 2908 (other name: UV-2908). Indeed, Cyasorb 2908 was also detected in sample 1 with low intensity (see Fig. 1a, at m/z 475).
Dioctyldiphenylamine and other alkylated diphenylamines with various alkyl chains were also detected in 3 PUR samples at m/z 226, 282, 338 and 394 (see Fig. 1a). They are commercially used antioxidants for polyurethanes (commercial name e.g. Irganox 5057, other name: Vanox 1081) [25].
Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate (commercial name e.g. Tinuvin 770) belongs to one of the most important classes of plastic photostabilizers, to the group of hindered amine light stabilizers (HALS). Tinuvin 770 was identified in one sample (Fig. 1b, m/z 481). The high intensity of the protonated Tinuvin 770 peak shows that DART-MS is capable of the detection of Tinuvin 770 very effectively from the PUR objects. It can be of great significance in the screening of Tinuvin 770 in pharmaceutical systems and devices, as this compound has a documented toxicological risk and it could leach from the plastic into a pharmaceutical product that is then administered to a patient in a clinical situation [26]. Moreover, not only the intact bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate compound, but two additional Tinuvin 770 related substances appeared in the DART-MS spectra at m/z 158 and 356 (see Fig. 1b), probably as impurities and decomposition products. The DART-MS/MS spectrum of Tinuvin 770 shows (see. Fig. 2h), that these two moieties were not produced by fragmentation during the DART-MS experiment, but were present in the PUR object probably as non intended substances. The product ions of Tinuvin 770 at m/z 342, 140, 123 and 58 shown in Fig. 2h agree with the characteristic ions of the literature spectrum recorded on an LC-MS/MS system [26].
Benzotriazole UV stabilizers are one of the most important families of light stabilizers, and their screening is important due to environmental and health concerns [27]. Benzotriazole was detected in sample 7 with remarkable intensity (see Fig. 1d, m/z 120), and confirmed by MS/MS. The characteristic product ions at m/z 92 and 65 (see ESM Fig. S2b) agree with those reported by Kannan et al. [27].
Flame retardants
As Table 2 shows, 6 flame retardant additives were detected by DART-MS. Ethyl, diethyl and triethyl phosphate were recognized in a foam filler (expanding fixing foam used e.g. for the installation of windows and doors; sample 14), as seen in the ESM Fig. S1g at m/z 127, 155 and 183, respectively. Melamine was identified with remarkable intensity in one PUR object (see Fig. 1f, m/z 127), and tris (2-chloroethyl) phosphate (TCEP) in two samples (see Fig. 1g, m/z 285). However, tris (1-chloro-2-propyl) phosphate (TCPP) was found in 8 samples, as seen e.g. in Fig. 1f with high abundance at m/z 327 as [M + H]+ and m/z 344 as [M + NH4]+. DART-MS/MS measurement was also performed by selecting the ion at m/z 327 as the precursor ion (see Fig. 2f). The product ions at m/z 251, 175 and 99 agree with the characteristic ions detected by atmospheric pressure photoionization (APPI)-quadrupole time-of-flight mass spectrometry [28]. To protect children from TCEP and TCPP, the European Commission set out specific limit value for these hazardous chemicals for toys intended for use by children under three years old or other toys intended to be placed in the mouth [29]. Therefore the rapid screening of TCEP and TCPP in the PUR products by DART-MS can have a great importance in terms of consumer safety.
Plasticizers
Four different phthalic acid esters (phthalates) and one adipate plasticizer were detected in the PUR samples by DART-MS. The phthalate plasticizers are not bound covalently to the polymer matrix, therefore, they are able to migrate into the environment (similar to the flame retardants). Due to the potential risks of phthalates related to the health and the environment, regulations of phthalic acid esters were issued by the European Parliament and the Council of the European Union [30] and by the US Congress [31]. As Table 2 shows, dibutyl phthalate (DBP) (Fig. 1d, m/z 279), bis(2-ethylhexyl) phthalate (DEHP) (Fig. 1b, m/z 391), dinonyl phthalate (DNP) (Fig. 1e, m/z 419), benzyl butyl phthalate (Fig. 1h, m/z 313), and bis(2-ethylhexyl) adipate (Fig. 1a, m/z 371) were detected in the PUR objects. DBP and DEHP were recognized in 7 and 4 samples, respectively. The collision induced dissociation of phthalic acid esters in tandem mass spectrometric experiments is well described [5], therefore our MS/MS measurement unambiguously identified DBP, DEHP and DNP on the basis of the characteristic product ions (see Table 3, Fig. 2e, ESM Fig. S2f, Fig. S2g, respectively). For example, ESM Fig. S2f shows the characteristic fragments of bis(2-ethylhexyl) phthalate at m/z 279, 261, 167, 149, and 113. A reliable indicator for the presence of phthalates in the sample is the appearance of the most specific product ion, the protonated phthalic anhydride at m/z 149 which is shown even in the DART MS spectra due to the low activation energy of dissociation of the phthalates (see e.g. in Fig. 1d).
Polyols, chain extenders, chain terminators
Polyurethanes are copolymers composed of alternating hard and soft segments, and chain extenders. Common soft segments are polyols, for example polypropylene glycol (PPG) or polytetrahydrofuran (PTHF) polyethers. PPG series with various length and end groups were detected in 8 PUR samples (see, e.g. in Fig. 1d at m/z 309, 1f at m/z 741, or 1g at m/z 540). Polyethylene glycol (PEG) (see, e.g. in ESM Fig. S1c at m/z 239) and PTHF segments (see, in ESM Fig. S1i at m/z 306) were detected in 4 and 1 PURs, respectively.
As seen in Table 2, DART-MS is capable of the detection of various chain extenders, such as di- and triethanolamine, di-, tri-, and tetraethylene glycol, and cyclohexanedimethanol. Diethanolamine and cyclohexanedimethanol appeared with rather high abundance in the spectrum of sample 11 (see Fig. 1g, m/z 106) and sample 17 (see ESM Fig. S1i, m/z 145), respectively. Diethylamine, acting as chain terminator or corrosion inhibitor in the PUR production was recognized with low intensity in 6 samples (see Fig. 1g at m/z 74), and caprolactam, a PUR blocking agent [32], was identified in 3 objects. The identification of caprolactam was confirmed by DART-MS/MS measurements (see ESM Fig. S2a and Table 3). The product ions at m/z 96, 79, 69, and 55 agree well with those present in the ESI quadrupole - Orbitrap MS/MS spectrum in the mass spectral database [33], and ESI quadrupole - linear ion trap MS/MS spectrum reported in the literature [34].
Non-intentionally added substances (NIAS)
Non-intentionally added substances are compounds that are present in a product but have not been added for a technical reason during the production process. NIAS can include degradation products, side products, impurities of starting materials or contaminants from recycling processes. Because of their health risk, primary aromatic amines (PAAs) stand out among the NIAS associated with PUR products. PAAs can be formed, for example by the reaction of residual isocyanates with water [35]. Many PAAs coming from polyurethane adhesives were found [36,37,38], which helped us in the identification. We indentified four hazardous PAAs by DART-MS, such as aniline (see Fig. 1h, m/z 94), o-toluidine (see Fig. 1f, m/z 108), diaminotoluene (see Fig. 1a, m/z 123), and o-anisidine (see ESM Fig. S1h, m/z 124). These compounds or its derivatives were also reported by Pezo et al. [36]. o-Toluidine has been classified Group 1: carcinogenic to humans; 2,4-diaminotoluene and o-anisidine have been classified Group 2B: possibly carcinogenic to humans by the International Agency for Research on Cancer. The Environmental Protection Agency (EPA) has classified aniline as a Group B2, probable human carcinogen. Diaminotoluene was detected in 6 PUR samples and the identification was confirmed by MS/MS. The characteristic product ions at m/z 108 and 106 (see Fig. 2b) agree the fragments reported by Mortensen et al. [39].
One of our key findings was the NIAS compound 1,4,7-trioxacyclotridecane-8,13-dione, which was detected in 5 PUR samples by DART-MS. This compound was found as volatile migrant by Felix et al. [37] from polyurethane adhesives. Probably, it is originated from the reaction of one adipate plasticizer or adipic acid with glycol [38]. As seen in Fig. 1e, two peaks appear with high intensity at m/z 217 and 234, corresponding to the protonated and ammoniated 1,4,7-trioxacyclotridecane-8,13-dione, respectively. Moreover, the H+ and NH4 + adducts of the dimer compound are also present at m/z 433 and 450, respectively. The MS/MS experiment confirmed the identification, the product ions at m/z 173, 155, and 111 (see Fig. 2d) agree with those reported by Felix et al. [37] and Isella et al. [38]. 1,4,7-trioxacyclotridecane-8,13-dione has high toxicity according to the Cramer rules (Cramer III) [38, 40], therefore its quick detection in the PUR products by DART-MS may have a great importance.
Out of the NIAS compounds listed in Table 2, pyridine (see Fig. 1g, m/z 80) and 1,4-dioxane (see ESM Fig. S1h, m/z 89) should be stressed, because they were detected in 11 and 4 PUR samples, respectively. Interestingly, a mold-release agent, stearamide was also recognized in the DART-MS spectrum of the PUR slippers (sample 2), as seen in Fig. 1b at m/z 284.
Migration of bis[2-(dimethylamino)ethyl]ether (DMAEE) from a PUR object
In further studies, we examined whether the hazardous chemicals could migrate from the PURs to other objects. As it was discussed previously, DMAEE, which is suspected to be toxic, was detected in PUR sponges used for dishwashing (see Fig. 1c, m/z 161). A porcelain plate was rubbed with the sponge sample 3, which was used for the first time. Then, the plate was introduced into the DART source. Seven independent experiments were performed (5 with dry sponge and 2 using water). Fig. 3 shows the DART-MS of the plate after subtracting the spectrum of the clean plate as background. As seen in Fig. 3, the protonated DMAEE appears at m/z 161.165 with considerable intensity (in line with the theoretical value m/z 161.165). In addition, the catalyst 1,4-diazabicyclo[2.2.2]octane (DABCO) is also present in the mass spectrum at m/z 113.105 (theoretical value: m/z 113.107). An additional spectrum is given as ESM Fig. S4. DMAEE could be identified with certainty in 4 dry and one wet experiment.
Conclusions
Our experiments show that DART-MS and DART-MS/MS can be efficiently applied for the detection of a wide range of chemicals, some of them hazardous, in polyurethane materials. More than 50 compounds were recognized in eighteen commercial PUR objects, and their identifications were confirmed by additional MS/MS experiments. The detected compounds revealed a great versatility in terms of catalyst residues, various additives, polyols, non-intentionally added substances, etc. The large number and diversity of the detected compounds proves unambiguously the effectiveness of DART-MS for the rapid identification of compounds in PURs without any sample preparation. We also demonstrated in this work that this method can be used for the quality control in the PUR industry, or for the fast screening of restricted or banned chemicals in the final product. Although it is difficult to obtain quantitative data by DART-MS, after the fast screening by DART-MS, traditional techniques (e.g. GC-MS, LC-MS) can be used for the quantitation of the found chemicals.
References
Engels HW, Pirkl HG, Albers R, Albach RW, Krause J, Hoffmann A, et al. Polyurethanes: versatile materials and sustainable problem solvers for today’s challenges. Angew Chem Int Ed. 2013;52:9422–41.
Ballesteros-Gómez A, Jonkers T, Covaci A, de Boer J. Screening of additives in plastics with high resolution time-of-flight mass spectrometry and different ionization sources: direct probe injection (DIP)-APCI, LC-APCI, and LC-ion booster ESI. Anal Bioanal Chem. 2016;408:2945–53.
Buchberger W, Stiftinger M. Analysis of polymer additives and impurities by liquid chromatography/mass spectrometry and capillary electrophoresis/mass spectrometry. Adv Polym Sci. 2012;248:39–68.
Paine MRL, Barker PJ, Blanksby SJ. Ambient ionisation mass spectrometry for the characterisation of polymers and polymer additives: a review. Anal Chim Acta. 2014;808:70–82.
Kuki Á, Nagy L, Zsuga M, Kéki S. Fast identification of phthalic acid esters in poly(vinyl chloride) samples by direct analysis in real time (DART) tandem mass spectrometry. Int J Mass Spectrom. 2011;303:225–8.
Crompton TR. Determination of additives in polymers and rubbers. Shawbury: Rapra Technology; 2007.
Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–302.
Ackerman LK, Noonan GO, Begley TH. Assessing direct analysis in real-time-mass spectrometry (DART-MS) for the rapid identification of additives in food packaging. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26:1611–8.
Mess A, Vietzke JP, Rapp C, Francke W. Qualitative analysis of tackifier resins in pressure sensitive adhesives using direct analysis in real time time-of-flight mass spectrometry. Anal Chem. 2011;83:7323–30.
Rothenbacher T, Schwack W. Rapid and nondestructive analysis of phthalic acid esters in toys made of poly(vinyl chloride) by direct analysis in real time single-quadrupole mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:2829–35.
Rothenbacher T, Schwack W. Rapid identification of additives in poly(vinyl chloride) lid gaskets by direct analysis in real time ionisation and single-quadrupole mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:21–9.
Haunschmidt M, Klampfl CW, Buchberger W, Hertsens R. Rapid identification of stabilisers in polypropylene using time-of-flight mass spectrometry and DART as ion source. Analyst. 2010;135:80–5.
Fouyer K, Lavastre O, Rondeau D. Direct monitoring of the role played by a stabilizer in a solid sample of polymer using direct analysis in real time mass spectrometry: the case of Irgafos 168 in polyethylene. Anal Chem. 2012;84:8642–9.
Lebeau D, Ferry M. Direct characterization of polyurethanes and additives by atmospheric solid analysis probe with time-of-flight mass spectrometry (ASAP-TOF-MS). Anal Bioanal Chem. 2015;407:7175–87.
Antal B, Kuki Á, Nagy L, Nagy T, Zsuga M, Kéki S. Rapid detection of hazardous chemicals in textiles by direct analysis in real-time mass spectrometry (DART-MS). Anal Bioanal Chem. 2016;408:5189–98.
Ashida K. Polyurethane and related foams: chemistry and technology. Boca Raton: Taylor & Francis Group; 2006.
Silva Santos L, Henrique Pavam C, Almeida WP, Coelho F, Eberlin MN. Probing the mechanism of the Baylis–Hillman reaction by electrospray ionization mass and tandem mass spectrometry. Angew Chem Int Ed. 2004;43:4330–3.
Abu-Zeid ME, Nofal EE. Effect of catalyst residues on the degradation of rigid foam polyurethane. J Appl Polym Sci. 1986;31:2407–15.
Dix LR, Gardiner DJ, Bradley JR. Studies of degradation in PVC caused by amine additives in polyurethane foam backing, catalysts in polyurethane foams, a one-day seminar. Shropshire: Rapra technology Limited; 1997.
Polyurethane amine catalysts: safe handling guidelines. American Chemistry Council. https://polyurethane.americanchemistry.com/resources-and-document-library/3852.pdf. 2011. Accessed 13 July 2017.
Pohanish RP. Sittig's handbook of toxic and hazardous chemicals and carcinogens 5th edition, volume I: A-H. Norwich: William Andrew Inc.; 2008.
National Center for Biotechnology Information. PubChem Compound Database; CID=8148 https://pubchem.ncbi.nlm.nih.gov/compound/8148. Accessed 13 July 2017.
Ballantyne B. The acute toxicity and irritancy of bis[2-(dimethylamino)ethyl]ether. Vet Hum Toxicol. 1997;39:290–5.
Tsukatani H, Tobiishi K. Determination of N,N-Dimethyldodecylamine and N,N-Dimethyloctadecylamine in river and sea water using liquid chromatography tandem mass spectrometry. Bull Environ Contam Toxicol. 2015;94:801–6.
Ash M, Ash I. Handbook of preservatives. Endicott: Synapse Information Resources Inc.; 2004.
Gill M, Garber MJ, Hua Y, Jenke D. Development and validation of an HPLC–MS–MS method for quantitating bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate (Tinuvin 770) and a related substance in aqueous extracts of plastic materials. J Chromatogr Sci. 2010;48:200–7.
Asimakopoulos AG, Bletsou AA, Wu Q, Thomaidis NS, Kannan K. Determination of benzotriazoles and benzothiazoles in human urine by liquid chromatography-tandem mass spectrometry. Anal Chem. 2013;85:441–8.
Matsukami H, Suzuki G, Takigami H. Compositional analysis of commercial oligomeric organophosphorus flame retardants used as alternatives for PBDEs: concentrations and potential environmental emissions of oligomers and impurities. Environ Sci Technol. 2015;49:12913–21.
European Commission Directive 2014/79/EU. Off J Eur Union. 2014. L 182/49.
European Communities. Directive 2005/84/EC of the European Parliament and of the Council. Off J Eur Union. 2005. L 344:40.
110th United States Congress. Consumer Product Safety Improvement Act (CPSIA). http://www.cpsc.gov/cpsia.pdf. 2008. Accessed 10 Feb 2017.
Camberlin Y, Michaud P, Pesando C, Pascault JP. Isocyanate blocking agents use in polyurethane processing. Macromol Symp. 1989;25:91–9.
"mzCloud.org™, Advanced Mass Spectral Database”, HighChem LLC, Slovakia. https://www.mzcloud.org. Accessed 10 Feb 2017.
Johnson V, Patel SJ, Shah D, Patel KA, Mehta MH. Caprolactam waste liquor degradation by various yeasts. World J Microbiol Biotechnol. 1994;10:524–6.
Brede C, Skjevrak I, Herikstad H. Determination of primary aromatic amines in water food stimulant using solid-phase analytical derivatization followed by gas chromatography coupled with mass spectrometry. J Chromatogr A. 2003;983:35–42.
Pezo D, Fedeli M, Bosetti O, Nerín C. Aromatic amines from polyurethane adhesives in food packaging: the challenge of identification and pattern recognition using quadrupole-time of flight-mass SpectrometryE. Anal Chim Acta. 2012;756:49–59.
Félix JS, Isella F, Bosetti O, Nerín C. Analytical tools for identification of non-intentionally added substances (NIAS) coming from polyurethane adhesives in multilayer packaging materials and their migration into food stimulants. Anal Bioanal Chem. 2012;403:2869–82.
Isella F, Canellas E, Bosetti O, Nerin C. Migration of non intentionally added substances from adhesives by UPLC–Q-TOF/MS and the role of EVOH to avoid migration in multilayer packaging materials. J Mass Spectrom. 2013;48:430–7.
Mortensen SK, Trier XT, Foverskov A, Petersen JH. Specific determination of 20 primary aromatic amines in aqueous food simulants by liquid chromatography–electrospray ionization-tandem mass spectrometry. J Chromatogr A. 2005;1091:40–50.
Cramer GM, Ford RA, Hall RL. Estimation of toxic hazard - a decision tree approach. Food Cosmet Toxicol. 1978;16:255–76.
Acknowledgements
The work was supported by the GINOP-2.3.2-15-2016-00041 project. The project was co-financed by the European Union and the European Regional Development Fund. Furthermore, this paper was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the grant K-116465, and supported through the New National Excellence Program of the Ministry of Human Capacities, ÚNKP-16-3 (T. Nagy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 523 kb)
Rights and permissions
About this article
Cite this article
Kuki, Á., Nagy, L., Nagy, T. et al. Screening of additives and other chemicals in polyurethanes by direct analysis in real time mass spectrometry (DART-MS). Anal Bioanal Chem 409, 6149–6162 (2017). https://doi.org/10.1007/s00216-017-0553-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0553-x