Abstract
Composting is widely used for recycling of kitchen waste to improve soil properties, which is mainly attributed to the nutrient and structural functions of compost-derived humic acids (HAs). However, the redox properties of compost-derived HAs are not fully explored. Here, a unique framework is employed to investigate the electron exchange capacity (EEC) of HAs during kitchen waste composting. Most components of compost-derived HAs hold EEC, but nearly two-thirds of them are found to be easily destroyed by Shewanella oneidensis MR-1 and thus result in an EEC lower than the electron - donating capacity in compost-derived HAs. Fortunately, a refractory component also existed within compost-derived HAs and could serve as a stable and effective electron shuttle to promote the MR-1 involved in Fe(III) reduction, and its EEC was significantly correlated with the aromaticity and the amount of quinones. Nevertheless, with the increase of composting time, the EEC of the refractory component did not show an increasing trend. These results implied that there was an optimal composting time to maximize the production of HAs with more refractory and redox molecules. Recognition of the heterogeneity of EEC of the compost-derived HAs enables an efficient utilization of the composts for a variety of environmental applications.

Microbial reduction of compost-derived HAs
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composting is an anoxic and staged oxic biological decomposition of organic solid substrates in which a large number of humic acids (HAs) are formed and termed compost-derived HAs [1–3]. Composts have potential functions such as animal feedstuff [4], for agronomic and horticultural use, and for the re-vegetation of brownfield and mineral waste sites [5, 6]. More recently, it was demonstrated that composts can provide a low-cost solution to the remediation of contaminated soils and waters and therefore play significant roles in both pollution remediation and biogeochemical process [7]. This is mainly attributed to the redox properties of compost-derived HAs, including the electron accepting capacity, electron donating capacity (EDC), and electron exchange capacity (EEC).
The EECs of natural HAs formed in soils [8], peats [9], and sediments [10] have been studied. Previous studies showed that the EECs of HAs were associated with several aspects, such as the molecular structures of HAs, the organic carbon precursors, the transformations that the organic carbon had undergone, and the environment of the isolated HAs [11]. Natural HAs were also demonstrated to effectively promote the reduction of redox metals such as Fe(III), Cr(VI), Mn(IV), and U(VI) [12] and chlorinated aliphatic and substituted nitrobenzene pollutants [13, 14] due to their EECs. Compared with natural HAs, compost-derived HAs have different structures, precursor transformation, and isolated environment [15]. Therefore, it is reasonable to assume that the EECs of compost-derived HAs are special, further affecting their potential use for the transformation of redox pollutants. Unfortunately, to date, the evolution of the EECs of compost-derived HAs during a composting process remains unclear.
In this study, a unique frame is employed for the investigation of the evolution of the EECs of kitchen waste compost-derived HAs. Given that the microbial reduction assay is an exclusive tool [16] that is able to reflect the real EECs of compost-derived HAs when applied in contaminated environments, which cannot be resolved by chemical [8] and electrochemical [17, 18] assays, the EECs of the compost-derived HA samples were determined using the microbial reduction assay in this work. Furthermore, both anaerobic and staged aerobic microbial reduction experiments were conducted to simulate the applied environments of composts, where temporarily anoxic conditions frequently occurred. Therefore, from the known strains of HS-reducing bacteria, we chose the facultative Shewanella oneidensis MR-1 because it is capable of metabolizing under both anoxic and oxic conditions [10]. Spectral methods were performed to analyze the molecular structures associated with the EECs of compost-derived HAs. The objectives of this work were to (1) investigate the microbial reduced EECs of compost-derived HAs and (2) identify the influences of the composition of compost-derived HAs on their EECs during composting.
Methods
Composting process and sample collection
Composting was conducted in an indoor composting reactor with a volume of 34 L and height × diameter of 400 × 330 mm. Ventilation was controlled at 0.5 L min−1 kg−1. Composting materials consisted of kitchen wastes (10.5 kg, from canteen), soil (9 kg), sawdust (0.23 kg), and a composite microbial system (1.6 kg). Composting continued for 47 days; the changes of temperature and pH are shown in Electronic supplementary material (ESM) Fig. S1. Samples were collected after 0, 3, 6, 8, 13, 19, 35, and 47 days of composting in depths of 5, 15, and 25 cm, respectively. They were immediately freeze-dried and preserved in a −20 °C freezer for HA extraction.
HA extraction
The isolation of compost-derived HAs was performed according to the IHSS standard assay [19]. In the isolation process, a 20-g sample was shaken for 24 h at room temperature in a 150-ml solution of 0.1 M NaOH and 0.1 M Na4P2O4 (1:1) in a 250-ml triangular flask. The residue (humic and other insoluble compounds) was separated from the supernatant by centrifugation (10 min, 8000 rpm). Then, the supernatant was acidified (6 M HCl, pH 2.0) such that the compost HAs were purified and precipitated [20] and were then stored in phosphate buffer (pH 7) at 4 °C before use.
Analytical technique
The dissolved organic carbon (DOC) of all samples was measured by a TOC automatic analyzer (MultinN/C2100TOC/TN) after being filtered by 0.22-μm mixed cellulose ester filter membranes. Fourier transform infrared absorption spectra (FT-IR) were recorded on a Hitachi EPI Infrared Spectrophotometer using the KBr disk method. The three-dimensional excitation–emission matrix (3DEEM) was recorded with a Hitachi model F-7000 luminescence spectrophotometer equipped with a 150-W xenon arc lamp as the excitation source. The slit widths of the excitation and emission monochromators were set at 5 nm, the voltage of the photomultiplier tube was adjusted to 700 V, and the emission (Em) wavelength was scanned from 280 to 550 nm by increasing the excitation (Ex) wavelength in 5-nm increments from 200 to 450 nm with a scan speed of 12,000 nm min−1. The EEM spectra of distilled water were obtained and were then subtracted from the EEM spectra of the compost-derived HAs [21]. Parallel factor (PARAFAC) analysis was applied to the three-dimensional data array by MATLAB 7.0 (Mathworks, Natick, MA) with the DOMFluor toolbox [22, 23]. F max values as scores in the score matrix were expressed and were used to evaluate the concentration of the fluorescence components determined through EEM–PARAFAC analysis [23].
UV–Vis spectroscopy was performed on a UNICO model UV-4802 double-beam spectrophotometer. Specific UV absorbance values SUVA254 (UV254 × 100/DOC) [24], SUVA280 (UV280 × 100/DOC) [25, 26], and SUVA290 (UV290 × 100/DOC) [8] were calculated by dividing the absorbance values 254, 280, and 290 nm by the corresponding DOC concentration. A 226–400 (226–400 nm) was calculated by integrating the UV absorbance from 226 to 400 nm. The absorption spectral slope ratio (S R) was obtained from the ratio of two distinct spectral slopes (S 275–295 and S 350–400) [27]. These parameters were selected to characterize the evolution of compost-derived HAs.
Solid-state CP/MAS 13C nuclear magnetic resonance (NMR) spectra were measured by a Bruker model AV-300 spectrometer at 12 kHz with a standard 4-mm double-bearing probe head. The recycle delay time, contact time, and pulse width time were set to 1 s, 2000 μs, and 2.4 μs, respectively [28]. The percentage of area regions 14–39, 50–60, 65–80, 110–140, and 164–185 ppm in the NMR spectra was calculated.
EEC and EDC measurements
The bacterium S. oneidensis MR-1 was grown under oxygen-limited conditions in Luria–Bertani liquid medium [28] (30 °C, 16 h; AQDS (1 mM) was the electron acceptor for MR-1), harvested by centrifugation (10 min, 8000 rpm) in the early stationary phase, and then washed two to three times with LM-lactate medium [29, 30]. Washed cells were resuspended with basal medium, which consisted of (amounts per liter of deionized water): 0.68 g NaH2PO4⋅2H2O, 0.25 g NH4Cl, 0.10 g KCl (50 mmol l−1) carbonate buffer, 1.59 g Na2CO3, 2.94 g NaHCO3 [23], 10 ml trace mineral solution, and 10 ml vitamin stock solution [31] to make cell suspension (1–5 × 107/106/105 CFU ml−1). The procedures of the microbial-reduced EEC of compost-derived HAs were as follows: First, 30 ml compost-derived HA (∼50 mg L−1, as electron shuttle; 30 ml PB was used instead of compost-derived HA in the control experiment), 35 ml cell suspension, and 2 ml sodium lactate (5 mM, as electron donator) were in turn injected into a 150-ml brown anaerobic bottle, then purged with 100 % N2 for 20 min, and immediately stoppered with butyl rubber bungs. Second, 5 ml microbial-reduced compost-derived HA was extracted by a 5-ml injector at 0, 8, 16, 24, 36, and 54 h and injected into (through a 0.22-um filter membrane) another 150-ml brown anaerobic bottle containing 5 ml ferric citrate (1 mM, as oxidant); the ferric citrate had been purged with 100 % N2 for 20 min before reaction with microbial-reduced compost-derived HAs. Then, the 150-ml brown anaerobic bottle containing 5 ml microbial-reduced HA and 5 ml ferric citrate was incubated on a horizontal shaker (150 rpm, 25 °C) in the dark for 24 h under 100 % N2 atmosphere. The EDC measurement was also conducted in a 150-ml brown anaerobic bottle, and 10 ml ferric citrate (1 mM) and 10 ml compost-derived HAs were added to the brown anaerobic bottle. The reaction solution was purged with 100 % N2 and air for 20 min in anoxic and oxic EDC measurements, respectively [8]. The amount of Fe2+ was quantified spectrophotometrically (ferrozine assay) to represent the EEC and EDC of compost-derived HAs after being divided by the DOC of the compost-derived HAs. All EEC and EDC measurements were conducted in an anoxic glove box (N2 atmosphere at 25 ± 1 °C, O2 < 0.1 ppm). All samples were run in triplicate.
Results and discussion
Characterization of the EEC of compost-derived HAs
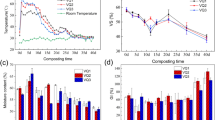
For most compost-derived HA samples, the Fe2+ concentrations in the anaerobic microbial reduction experiments (anaerobic experiments) with three cell densities of MR-1 (107, 106, and 105) were higher than those in the control experiments (without compost-derived HAs) after a 16-h reaction under anoxic conditions (see ESM Fig. S2a). This result indicated that most compost-derived HA samples were able to facilitate electron exchange between MR-1 and ferric citrate. The EDCs of the compost-derived HAs measured using the ferrozine assay showed the same order of magnitude as that of natural HAs (see ESM Fig. S2b) [10], demonstrating that electron donating groups also existed in the compost-derived HAs. In addition, lower EDCs were found for the compost-derived HAs under oxic conditions than those under anoxic conditions, indicating that among the electron donating groups within the compost-derived HAs, some were easily oxidized by oxygen, but others were antioxidants. The observation also implied that temporarily anoxic conditions favored the electron transfer mediated by HAs between the electron donators and acceptors.
In contrast to the increased EECs of the natural HAs with increasing reaction time in anaerobic experiments reported in previous studies [16], the EECs of eight compost-derived HA samples decreased during the reaction and became relatively stable after 48 h, with EECs of approximately one-third of their initial EECs (at 0 h of the reaction; Fig. 1a–c). One possible explanation for the unexpectedly low EECs of compost-derived HAs was that the compost-derived HAs were degraded by MR-1 during the reaction. To confirm this, the DOC of the reaction solution prior to and after the anaerobic experiments and of the compost-derived HAs in the initial reaction solution was calculated and measured, respectively. In addition, compost-derived HAs in the ultimate reaction, termed residual compost-derived HAs, were re-precipitated from the ultimate reaction solutions by acidifying the reaction solutions to pH 2 using dilute hydrochloric acid (6 M), then residual compost-derived HAs were re-dissolved by PB (pH 7) to the same volume as the ultimate reaction solutions where they re-precipitated, and the DOC of residual compost-derived HAs was measured again to represent the DOC of residual compost-derived HAs in ultimate reaction solutions. The DOC of the reaction solutions in the anaerobic experiments decreased after the reaction; more importantly, the DOC of the residual compost-derived HAs was also lower than the corresponding DOC of the compost-derived HAs in the initial reaction solutions (see ESM Fig. S3). In addition, some of the compost-derived HA samples had no residual compost-derived HAs re-precipitated, such as the 13-day compost-derived HA samples. These findings indicate the degradation of compost-derived HAs by MR-1 during the reaction. Furthermore, as shown in Fig. 1d–i, the EECs of the compost-derived HAs measured at different reaction times also changed during the reaction, indicating that the redox functional groups within the eight compost-derived HA samples were also changed during the reaction, which might also be due to the degradation of the compost-derived HAs by MR-1.
Electron exchange capacity (EEC) of compost-derived HAs under anoxic condition. a–c Changes of the EECs of compost-derived HAs in anaerobic microbial reduction experiments (anaerobic experiments) with 107, 106, and 105 cell densities of MR-1 during 54-h reaction, respectively. Data in (a)–(c) are the same as those in (d)–(i). d–i Changes of the EECs of compost-derived HAs at 0, 8, 16, 24, 36, and 54 h in anaerobic experiments with three kinds of cell densities of MR-1, respectively
Combining the findings mentioned above with the redox characteristics of natural HAs in previous research [16], two potential reasons were assumed to be responsible for the degradation of the compost-derived HAs by MR-1 during the reaction. First, the structures of the compost-derived HAs, which had both more aliphatic precursor carbon and far shorter transformation than those of natural HAs, were assumed to be simpler than those of natural HAs, such as soils, sediments, and aquatic HAs, so that compost-derived HAs might be used as a carbon source by MR-1 to maintain its metabolism and growth during the reaction [32], which resulted in the reduction of the DOC and EECs of the compost-derived HAs. Second, the forces between fragments within the compost-derived HAs were assumed to be weaker than those of natural HAs due to the differences between the physical and chemical conditions in the composting and natural environments where the HAs were formed [33].
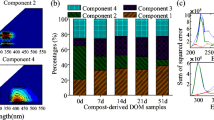
PARAFAC analysis, based on the 3DEEM spectral data of the compost-derived HAs, was performed to investigate the changes of the components within the compost-derived HAs prior to and after the anaerobic experiments. As shown in Fig. 2a, four components existed in the compost-derived HAs: component 1 (Ex/Em = 270/430) was related to humic-like organics [34]; component 2 (Ex/Em = 225, 275/340) was associated with simple aromatic proteins, such as tyrosine and soluble microbial by-product-like materials [35]; and components 3 and 4 (Ex/Em = 340/415 and 270, 375/485, respectively) were also related to humic-like organics and were assumed to be larger molecular weight components within the compost-derived HAs, which was supported by the lower S R of the later-staged compost-derived HAs (19–47 days) [36], dominated by components 3 and 4, than that of early-staged compost-derived HAs (0–6 days; see ESM Fig. S4). The data in Fig. 2b–e showed that the relative percent of both components 3 and 4 within the residual compost-derived HAs decreased compared with the compost-derived HAs; furthermore, except for the 6-day compost-derived HA sample in the 106 cell density, component 4 disappeared in the other residual compost-derived HA samples. These findings strongly confirmed that some humic-like organics formed during composting were degraded by MR-1 during the reaction.
PARAFAC analysis of compost-derived and residual HAs. a Four components of compost-derived HAs defined by PARAFAC analysis. b–d Relative percent of the F max values of four components within residual HAs re-precipitated from ultimate reaction solutions with 107, 106, and 105 cell densities, respectively. e Relative percent of the F max of four components within compost-derived HAs
To identify the redox functional components within the compost-derived HAs, correlation analysis between the EECs and EDCs of compost-derived HAs and the four components within the compost-derived and residual compost-derived HAs was conducted, respectively. The data in ESM Table S1 show that the correlation between component 4 and the EDCs of the compost-derived HAs under oxic conditions was significant (P < 0.05), indicating that component 4 is a major antioxidant component within the compost-derived HAs [9]. The correlation between component 3 and the microbial-reduced EECs of the compost-derived HAs under anoxic conditions was significant (P < 0.01), indicating that component 3 is a major microbial-reduced component within the compost-derived HAs. Differently, the correlations between the EDCs and EECs of the residual compost-derived HAs and component 1 within the residual compost-derived HAs were significant (P < 0.05; see ESM Table S2), indicating that component 1, whose peaks were similar to those of terrestrial HAs [35], was the most stable redox functional component within the compost-derived HAs. In addition, as shown in Fig. 3a–c, the EECs of the residual compost-derived HAs increased with increasing reaction time in the initial 48 h of anaerobic experiments, with 107 cell density of MR-1. Noteworthy is that the EDCs and EECs of the residual compost-derived HAs were nearly one to two and 4–25 times higher, respectively, than those of the corresponding compost-derived HA samples under the same conditions, further suggesting that component 1 was not only the most stable but also the most effective redox functional component within the compost-derived HAs.
EECs of residual compost-derived HAs measured using microbial reduction assay (anaerobic) with 107 cell densities of MR-1. a–c EECs of residual HAs re-precipitated from ultimate reaction solutions with 107, 106, and 105 cell densities, respectively. d–f EDCs of residual HAs re-precipitated from ultimate reaction solutions with 107, 106, and 105 cell densities, respectively
Influences of the structural composition and evolution of compost-derived HAs on their EECs
The EDCs of the compost-derived HAs decreased during the initial eight composting days and then increased from the 8th to the 47th day of composting under both anoxic and oxic conditions (see ESM Fig. S1b), indicating that the content of the electron donating groups within the compost-derived HAs changed during composting. Phenolic moieties have been confirmed as the major electron donating groups within natural HAs [9], and the content of phenolic moieties within compost-derived HAs increased during composting, as confirmed by the increase of the characteristic absorption band for phenolic moieties at 3300 cm−1 in the FT-IR spectrum (see ESM Fig.S5a). However, the correlations between the phenolic moieties and the EDCs of the compost-derived HAs were poor under both anoxic and oxic conditions (see ESM Table S3). This result indicated that the phenolic moieties might not be the only electron donating groups within the compost-derived HAs and that other redox functional groups, such as nitrogen- or sulfur-containing groups, within the compost-derived HAs might also act as electron donating groups [8]. In addition, in agreement with natural HAs, a poor correlation between the EDCs and the aromaticity of compost-derived HAs was also observed during composting. Comparing with the obviously increasing aromaticity of compost-derived HAs, confirmed by the increases of SUVA254, SUVA280, A 226–400, and the percent of the integral of the characteristic peaks at 110–140 ppm in the solid-state CP/MAS 13C NMR spectrum (see ESM Fig. S5b), the EDCs of compost-derived HAs increased slightly during composting. These findings suggested that abundant aromatic groups were formed in compost-derived HAs during composting, but most of them might not be redox. Another potential reason was that some electron donating groups within the compost-derived HAs had been oxidized by oxygen to lose the EDCs during the extraction of compost-derived HAs and parts of them might be aromatic.
The data in Fig. 1d–i showed that the micro-reduced EECs of all eight compost-derived HA samples were changed during the whole anaerobic experiments with three cell densities of MR-1. This result might be attributed to the degradation of compost-derived HAs by MR-1 during the reaction. Meanwhile, compared with the increasing quinone groups within the compost-derived HAs during composting, confirmed by the increase of the characteristic absorption band for quinone groups at 1650 cm−1 in the FT-IR (see ESM Fig. S5a) and the SUVA290 (Fig. S4) [8], the micro-reduced EECs of compost-derived HAs showed the poor correlation with the quinone groups of the compost-derived HAs (see ESM Table S3), though the quinone groups had been conformed to be the main redox functional groups in HAs and correlated well with the micro-reduced EECs of HAs [11]. One potential explanation for this phenomenon was that the quinone groups were not the only redox functional groups within the compost-derived HAs; other non-quinone redox functional groups might also exist in compost-derived HAs. Another reason was that the quinone groups within the compost-derived HAs might be encompassed by other fragments within the compost-derived HAs, which hindered the electron changes between the reduced quinone groups and ferric citrate and further reduced the EECs of compost-derived HAs. Differently, in contrast to the compost-derived HAs, the EDCs and EECs of the residual compost-derived HAs correlated well with their SUVA254 and SUVA290 (P < 0.01; Fig. 4), respectively. In addition, the specific UV absorbance values, SUVA254, of the residual compost-derived HAs were higher than those of the native compost-derived HAs (Fig. 4 and ESM Fig. S4), indicating that the aromaticity of residual compost-derived HAs was higher than that of native compost-derived HAs. Therefore, the residual compost-derived HAs were more refractory for MR-1 than native compost-derived HAs, for which they had the higher EEC than EDC (Fig. 3d–f). Furthermore, the SUVA290 of residual compost-derived HAs was also higher than that of native compost-derived HAs (Fig. 4 and ESM Fig. S4), demonstrating that the content of the quinone group within the residual compost-derived HAs was also higher than that of native compost-derived HAs, which was responsible for the higher EEC of residual compost-derived HAs than that of native compost-derived HAs. These results implied that, similar to natural HAs, quinone groups were the major redox functional groups within residual compost-derived HAs, and they were also the important redox functional groups within compost-derived HAs.
Based on the PARAFAC analysis, we observed that both components 3 and 4 of the compost-derived HAs increased during composting, and they correlated well with the micro-reduced EECs and EDCs of compost-derived HAs, respectively. This result indicated that some of the humic-like organics formed in the later stage of composting were redox reactive. However, as confirmed above, these organics were easily degraded by the bacterium S. oneidensis, although they had a higher aromaticity than the other components within the compost-derived HAs. Compared with components 3 and 4, component 1 decreased slightly during composting (see ESM Fig. S6), but it was the most stable and most effective redox functional component within the compost-derived HAs, indicating that some types of humic-like organics that were stable against bacterium S. oneidensis might also be decomposed or degraded by composting microbial communities during composting, further suggesting that the refractoriness of compost-derived HAs was relative and mainly depended on the environment where the compost-derived HAs were applied. Therefore, based on the evolutions of the EECs and the fluorescence components of the compost-derived HAs during composting, we implied that the major functions of compost-derived HAs extracted from different stages of composting would also be different. Given that temporarily anoxic conditions occur in natural environments, the staged aerobic microbial reduction experiments (staged aerobic experiments) were conducted to investigate the real EECs of compost-derived HAs when they were applied in temporarily anoxic environments. The staged aerobic experiment consisted of 16 h in 100 % N2 atmosphere, 8 h in air atmosphere, and another 24 h in 100 % N2 atmosphere. As shown in Fig. 5, the EECs of eight compost-derived HA samples in the staged aerobic experiments (with three cell densities of MR-1) were lower than their corresponding EECs in the anaerobic experiments after a 48-h reaction. These findings suggested that, compared with the anaerobic experiments, the degradation of compost-derived HAs by MR-1 in the staged aerobic experiments might be stronger, as confirmed by the larger reduction of the DOC of the ultimate reaction solutions in the staged aerobic experiments than that in the anaerobic experiments (see ESM Fig. S3b–d). In addition, in contrast to the anaerobic experiments, no residual compost-derived HA was precipitated from the reaction solutions in the staged aerobic experiments, further supporting the larger degradation of compost-derived HAs in the staged aerobic experiments. One potential reason responsible for this phenomenon was that oxygen facilitated the degradation of the compost-derived HAs by MR-1 in the staged aerobic experiments because MR-1 was a type of amphimicrobe, and their metabolism and growth were able to be facilitated by oxygen, resulting in the larger degradation of compost-derived HAs in the staged aerobic experiments. These findings implied that composting should be improved by forming more refractory redox functional components to increase the remediation effects of the contaminated matrix and that both raw materials and the technology of composting should also be improved according to the environment where the composting products will be used.
EECs of compost-derived HAs under staged oxic condition in 107, 106, and 105 cell densities of MR-1. a EECs of compost-derived HAs at 0-h reaction in N2 atmosphere. b EECs of compost-derived HAs at 16-h reaction in N2 atmosphere. c EECs of compost-derived HAs after 16 h in N2 atmosphere, 8 h in air, and another 24 h in N2 atmosphere
Conclusions
Compost-derived HAs facilitated the electron exchange between MR-1 and ferric citrate, and components 1, 3, and 4 within the compost-derived HA were all redox reactive. Compared with components 3 and 4, component 1 was more stable and effective. Accompanied by the changes of the components within the compost-derived HAs during composting, both the refractoriness and the redox properties (EDCs and EECs) of the compost-derived HAs changed. This study systematically presented the characteristics of the EECs of compost-derived HAs, suggesting that the EECs of compost-derived HAs were heterogeneous and that compost-derived HAs also had the potential to promote the transformation of redox organic pollutants and heavy metals in the contaminated matrix for their considerable EECs in different redox and microbial community conditions.
References
Bernal MP, Alburquerque JA, Moral R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol. 2009;100(22):5444–9.
Suhas, Carrott PJM, Carrott MMLR. Lignin—from natural adsorbent to activated carbon: a review. Bioresour Technol. 2007;98(12):2301–11.
Said-Pullicino D, Erriquens FG, Gigliotti G. Changes in the chemical characteristics of water-extractable organic matter during composting and their influence on compost stability and maturity. Bioresour Technol. 2007;98(9):1822–8.
Garcia AJ, Esteban MB, Marquez MC, Ramos P. Biodegradable municipal solid waste: characterization and potential use as animal feedstuffs. Waste Manage. 2005;25(8):780–8.
Farrell M, Jones DL. Critical evaluation of municipal solid waste composting and potential compost markets. Bioresour Technol. 2009;100(19):4301–9.
Silva M, Naik TR. Review of composting and anaerobic digestion of municipal solid waste and a methodological proposal for a mid-size city. Sustainable Construction Truction Materials and Technologies. 2007;7:631–3.
Paradelo R, Barral MT. Evaluation of the potential capacity as biosorbents of two MSW composts with different Cu, Pb and Zn concentrations. Bioresour Technol. 2012;104(1):810–3.
Ratasuk N, Nanny MA. Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol. 2007;41(22):7844–6.
Aeschbacher M, Graf C, Schwarzenbach RP, Sander M. Antioxidant properties of humic substances. Environ Sci Technol. 2012;46(9):4916–9.
Klüpfel L, Piepenbrock A, Kappler A, Sander M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci. 2014;7(3):195–200.
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32(19):2984–5.
Gu B, Chen J. Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions. Geochim Cosmochim Acta. 2003;67(19):3575–7.
Kappler A, Haderlein SB. Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol. 2003;37(12):2714–5.
Dunnivant FM, Schwarrenbach RP. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol. 1992;26(11):2133–8.
Silva MEF, Lemos LT, Nunes OC, Cunha-Queda AC. Influence of the composition of the initial mixtures on the chemical composition, physicochemical properties and humic-like substances content of composts. Waste Manage. 2014;34(1):21–6.
Jiang J, Kappler A. Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ Sci Technol. 2008;42(10):3563–6.
Aeschbacher M, Sander M, Schwarzenbach RP. Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol. 2009;44(44):87–93.
Yuan Y, Tao Y, Zhou S, Yuan T, Liu Q, He J. Electron transfer capacity as a rapid and simple maturity index for compost. Bioresour Technol. 2012;116(4):428–34.
Stevenson FJ. Humus chemistry: genesis, composition, reactions. New York: Wiley; 1983.
Wei Z, Xi B, Zhao Y, Wang S, Liu H, Jiang Y. Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid. Chemosphere. 2007;68(2):368–74.
He X, Xi B, Cui D, Liu Y, Tan W, Pan H, et al. Influence of chemical and structural evolution of dissolved organic matter on electron transfer capacity during composting. J Hazard Mater. 2014;268(3):256–7.
Stedmon CA, Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr-Methods. 2008;6(11):572–7.
Wu C, Zhuang L, Zhou S, Li F, He J. Corynebacterium humireducens sp. nov., an alkaliphilic, humic acid-reducing bacterium isolated from a microbial fuel cell. Int J Syst Evol Microbiol. 2011;61(Pt 4):882–7.
Nishijima W, Speitel GE. Fate of biodegradable dissolved organic carbon produced by ozonation on biological activated carbon. Chemosphere. 2004;56(2):113–6.
Chin Y, Aiken G, O’Loughlin E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol. 1994;28(11):1853–5.
Peuravuori J, Pihlaja K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal Chim Acta. 1997;337(2):133–49.
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr. 2008;53(5):955–69.
Qu X, Xie L, Lin Y, Bai Y, Zhu Y, Xie F, et al. Quantitative and qualitative characteristics of dissolved organic matter from eight dominant aquatic macrophytes in Lake Dianchi, China. Environ Sci Pollut Res. 2013;20(10):7413–23.
Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol. 2005;7(8):4414–26.
Myers CR, Myers JM. Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J Appl Microbiol. 1994;76(3):253–5.
Ma C, Zhuang L, Zhou SG, Yang GQ, Yuan Y, Xu RX. Alkaline extracellular reduction: isolation and characterization of an alkaliphilic and halotolerant bacterium, Bacillus pseudofirmus MC02. J Appl Microbiol. 2012;112(5):883–8.
Ding Y, Peng N, Du Y, Ji L, Cao B. Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr(VI) immobilization. Appl Environ Microbiol. 2014;80(4):1498–506.
Sutton R, Sposito G. Molecular structure in soil humic substances: the new view. Environ Sci Technol. 2005;39(23):9009–15.
Ahmad SR, Reynolds DM. Monitoring of water quality using fluorescence technique: prospect of on-line process control. Water Res. 1999;33(9):2069–74.
Coble PG. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar Chem. 1996;51(4):325–46.
Marhuenda-Egea FC, Martinez-Sabater E, Jorda J, Moral R, Bustamante MA, Paredes C, et al. Dissolved organic matter fractions formed during composting of winery and distillery residues: evaluation of the process by fluorescence excitation-emission matrix. Chemosphere. 2007;68(2):301–8.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (nos. 51408573 and 51325804).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no any actual or potential conflict of interest in this work, including any financial, personal, or other relationships with other people or organizations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 967 kb)
Rights and permissions
About this article
Cite this article
Yuan, Y., Tan, WB., He, XS. et al. Heterogeneity of the electron exchange capacity of kitchen waste compost-derived humic acids based on fluorescence components. Anal Bioanal Chem 408, 7825–7833 (2016). https://doi.org/10.1007/s00216-016-9885-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9885-1