Abstract
Infliximab (IFX) is a chimeric monoclonal antibody targeting tumor necrosis factor-alpha. It is currently approved for the treatment of certain rheumatic diseases or inflammatory bowel diseases. Clinical studies have suggested that monitoring IFX concentrations could improve treatment response. However, in most studies, IFX was quantified using ELISA assays, the resulting discrepancies of which raised concerns about their reliability. Here, we describe the development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for IFX quantification in human plasma. Full-length stable-isotope-labeled antibody (SIL-IFX) was added to plasma samples as internal standard. Samples were then prepared using Mass Spectrometry Immuno Assay (MSIA™) followed by trypsin digestion and submitted to multiple reaction monitoring (MRM) for quantification of IFX. The chromatographic run lasted 13 min. The range of quantification was 1 to 26 mg/L. For two internal quality controls spiked with 6 and 12 mg/L of IFX, the method was reproducible (coefficients of variation (CV%): 12.7 and 2.1), repeatable (intra-day CV%: 5.5 and 5.0), and accurate (inter-day and intra-day deviations from nominal values: +6.4 to +3.7 % and 5.5 to 9.2 %, respectively). There was no cross - contamination effect. Samples from 45 patients treated with IFX were retrospectively analyzed by LC-MS/MS and results were compared to those obtained with an in-house ELISA assay and the commercial Lisa Tracker® method. Good agreement was found between LC-MS/MS and in-house ELISA (mean underestimation of 13 % for in-house ELISA), but a significant bias was found with commercial ELISA (mean underestimation of 136 % for commercial ELISA). This method will make it possible to standardize IFX quantification between laboratories.

Interassay comparison of the three methods: LC-MS/MS vs inhouse ELISA assay or vs Lisa Tracker® ELISA assays, Passing & Bablok (a) and Bland & Altman (b) for the comparison of LC-MS/MS vs in-house ELISA assay; Passing & Bablok (c); Bland & Altman (d) for the comparison LC-MS/MS vs Lisa Tracker® ELISA assay
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infliximab (IFX) is a chimeric IgG1κ antibody targeting tumor necrosis factor (TNFα), currently approved for the treatment of certain rheumatic or inflammatory bowel diseases (IBD).
Compelling evidence suggests a relationship between IFX trough concentrations and clinical response, particularly in patients with IBD [1–3]. These observations have led to the recent emergence of therapeutic algorithms integrating IFX trough concentrations and scheduled infusions [3]. Data suggest that IFX therapeutic drug monitoring (TDM) may help to improve treatment response or mucosal healing. However, the different methods currently used to quantify IFX, i.e., ELISA assays [1, 3–5], fluid-phase radioimmunoassay, homogenous shift mobility assay [6], or reporter gene assay [7], presented discrepancies (differences in absolute concentration of IFX, false positives) [5] that could be of major clinical concern. Although most studies demonstrated higher IFX serum concentrations in patients with a clinical response compared to patients with clinical relapse, the reference therapeutic ranges have yet to be better determined at least in part to analytical limitations, i.e., poor concordance between analytical methods, interferences with circulating antidrug antibodies (ADA) [8]. To overcome this issue, some authors have suggested that IFX TDM should always be performed with the same analytical technique to avoid inadequate therapeutic changes [5, 7].
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is widely used in clinical pharmacology laboratories to detect and/or to quantify small drug molecules with optimal specificity [9, 10]. In contrast, LC-MS/MS-based quantification of therapeutic monoclonal antibodies has been poorly described and represents a new analytical challenge, in particular requiring the selection of signature peptides and an optimized internal standard strategy [11–15]. To our knowledge, only one study, published while we were developing our method, reported LC-MS/MS-based quantification of IFX in human plasma [16] by a LC-MS/MS method but used isotope-labeled peptides as internal standards, while here we have used a full-length stable-isotope-labeled antibody.
In this paper, we present the development and validation of an immunoaffinity LC-MS/MS method to quantify IFX in human plasma. A stable-isotope-labeled IFX was used as internal standard. After validation of the method with spiked samples, serum concentrations from IBD patients treated with IFX were measured by our LC-MS/MS method and compared to those obtained with an in-house ELISA method and the Lisa Tracker® enzymatic immunoassay.
Materials and methods
Chemicals and reagents
IFX, Remicade® (Johnson and Johnson, New Brunswick, NJ, USA), and its biosimilar Inflectra® (Hospira, Lake forest, IL, USA) were obtained from the institutional pharmacy and reconstituted in water to a concentration of 10 g/L in line with the instructions for use. Biotinylated human TNFα was obtained from Labomics (Nivelles, Belgium). Full-length stable-isotope-labeled IFX (Arginine 13C6-15N4 and Lysine 13C6-15N2) (SIL-IFX) was purchased from Promise Advanced Proteomics (Grenoble, France).
Sequencing-grade modified trypsin was purchased from Promega (WI, USA). BioXtra urea, ammonium bicarbonate, 30 % hydrogen peroxide solution (H2O2), and trishydroxymethylaminomethan (TRIS) Trizma® base were from Sigma Aldrich (MI, USA). LC-MS grade methanol (MeOH), acetonitrile (ACN), and formic acid were from Carlo Erba Reagents (Val de Reuil, France). Ammonia solution (25 %) was obtained from Merck (Darmstadt, Germany). Ultrapure water (H2O) with resistivity ≥18.0 MΩ.cm was produced using a Milli-Q Plus® system (Millipore, Molsheim, France). Dulbecco’s phosphate-buffered saline (PBS) solution and Mass Spectrometry Immunoassay (MSIA®) D.A.R.T.’S streptavidin tips were purchased from Thermo Scientific (MA, USA). Drug-free batches of human plasmas from volunteers were provided by the French National Blood Service (Grenoble, France). Skirted 96-well PCR plates and a Finnpipette Novus i Multichannel Electronic Pipette stand were kindly provided for use during tests by Thermo Scientific (MA, USA).
Infliximab proteomic study and preliminary workflow
Due to the high molecular weight of IFX (144,099.4 Da), a bottom-up proteomics approach was applied. With this approach, the protein was first digested to obtain peptides of masses compatible with the mass range and resolution of common bench-top tandem mass analyzers [17].
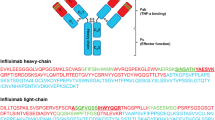
First we had to determine the amino acid sequence for IFX. Partial amino acid sequences for the variable regions of IFX heavy and light chains were available in the IMGT database (http://www.imgt.org/3Dstructure-DB). In silico trypsin digestion was used to allow selection of proteotypic or signature peptides. These peptides should have a unique, non-human sequence which is representative of the amino acid sequence of the monoclonal antibody. Uniqueness of the selected peptides was verified by performing online BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Once proteotypic peptides had been selected, proteolytic digests were analyzed on a Velos LC-ESI-Orbitrap (Thermo Scientific, MA, USA) to identify the most abundant peptides matching the in silico list, and to find the most abundant, specific, and sensitive MS/MS fragments for multiple reaction monitoring (MRM) analysis. MS/MS data were processed using Skyline 2.5 to select the best MRM transitions for each peptide selected. These MRM transitions were then exported into Analyst 1.5.2 Software for triple-quadrupole MS/MS quantification.
Preparation of working solutions, calibration standard, and quality control samples
Stock solution of IFX was prepared in water at 10 g/L and stored at +4 °C. Two batches of IFX stock solutions were made: one to use for calibration curves, and one for use as internal quality control (IQC) samples. Two batches of working solutions were then prepared by successive dilutions in drug-free plasma. Working solutions were prepared at 100 mg/L (WS1) and 10 mg/L (WS2).
A 50 mg/L stock solution of SIL-IFX was provided by Promise Advanced Proteomics.
Appropriate volumes of each stock solution were added to a series of low-binding Eppendorf centrifugation tubes. Drug-free plasma was added to obtain a final volume of 50 μL in each tube (see Table 1 for details). This process was repeated for both IQC samples.
Sample preparation
Labeled and unlabeled IFX were extracted from sample by immunoaffinity. Fifty microliters of plasma and 5 μL of SIL-IFX were added to each well of a PCR plate and mixed with a pipette for 30 s. After a 15-min equilibration period, 50 μL of 10 mM PBS was added. IFX and SIL-IFX were then extracted by applying a specific affinity sample purification protocol combining the use of biotinylated TNFα and streptavidin-coated MSIA tips according to the manufacturer’s instructions [18]. After the MSIA step, the dried samples were reconstituted with 5 μL of 2 M urea + 0.5 μL of 20 mM TRIS base + 45 μL of 25 mM ammonium bicarbonate. The pH of the resulting mixture was around 7, which is compatible with optimal trypsin digestion. Following reconstitution, 1 μg trypsin was added to achieve an enzyme/substrate ratio of 1/50. Samples were immediately vortexed and then centrifuged at 8000×g for 1 min. Trypsin digestion as allowed to proceed overnight (at least 12 h) in a 37 °C incubator.
Trypsin digestion was stopped by adding 5.5 μL of 10 % formic acid. After vortex and 1 min centrifugation (10,500×g), 15.6 μL of 30 % H2O2 was added followed by vortex and a 15-min equilibration period at room temperature. Samples were centrifuged for 1 min (10,500×g) before transferring the supernatant to microinsert polypropylene HPLC vials.
LC-MS/MS quantification of infliximab
Two-dimensional liquid chromatography configuration
The LC system was composed of an Ultimate 3000 RS quaternary pump (Pump A) and an Ultimate 3000 quaternary pump (Pump B), equipped with an Ultimate 3000 RS autosampler and a column compartment (Thermo Scientific, Germering, Germany). Online sample clean-up was performed on a μ-Precolumn (C18 PepMap100, 5 μm, 100 Å, Thermo Scientific, MA, USA) in two steps: first, 20 μL of extracted sample (at 4 °C thanks to the thermostat-controlled autosampler) was injected into the system and transferred onto the PepMap column. The peptides were retained, while potentially interfering matrix compounds [19] were washed directly into the waste by the mobile phase A (water/ACN 98/2 + 0.1 % formic acid) delivered at a flow rate of 0.05 mL/min over 4 min (Fig. 1A). Next, the six-port valve was switched. To obtain good chromatographic separation, the extract was eluted in backflush mode and transferred to the analytical column, a C18 analytical column (Phenomenex Kinetex, 2.6 μm, 2.1 × 100 mm, Aschaffenburg, Germany). Column temperature was maintained at 60 °C. Elution from this column was performed at 0.4 mL/min under the following conditions: mobile phase B (same composition as mobile phase A) and mobile phase C (water/ACN 20/80 + 0.1 % formic acid): 0–4 min, 8 % C; 4–9 min, 8–60 % C; 9–9.1 min, 60–90 % C; 9.1–10 min, 90 % C; 10–10.1 min, 90–8 % C; and 10.1–13 min, 8 % C (Fig. 1B). The valve was switched back to its original configuration at 10 min, allowing equilibration of the PepMap column with mobile phase A in preparation for the next run.
A supplemental diverter valve was set to the waste from 0 to 4 min, to the mass spectrometer from 4 to 10 min when the analytes were eluted, and then to the waste till the end of the run.
Mass spectrometric conditions
Measurements were performed on an API 4000 Tandem Mass Spectrometer (Sciex, Toronto, Canada) equipped with a Turbo V® ion source operated in positive electrospray ionization mode (ESI). Quantification was performed in MRM mode, monitoring two ion transitions per peptide (one for quantification and one for confirmation) and two ion transitions for all corresponding SIL-IFX peptides. The ion transitions used for quantification were doubly charged molecular ions [M+2H]2+ associated with singly charged fragment ions [M+H]+. The confirmation transitions were singly charged molecular ions [M+H]+ associated with singly charged fragment ions [M+H]+, including the mass contribution from the H2O2 treatment (during sample preparation) when peptides and fragments contained methionine residues. The source-dependent parameters were set as follows: ESI voltage, 5500 V; ion source temperature, 600 °C; turbo heater gas, 50 psi; nebulizer, 60 psi; and curtain gas, 10 psi. In preliminary experiments, six proteotypic peptides were studied. A repeatability test (n = 6) on the two levels of IQCs (6 and 12 μg/L) was then performed to select the two best peptides. Three performance indicators were evaluated on the most sensitive MRM ion transitions of each six peptides: bias, coefficient of variation (CV), and chromatographic peak surface. For each IQC level, a radar chart was done to plot these three performance indicators (Fig. 2). The highest values, the best performances. This resulted in the choice of the following peptides having the best sensitivity associated with acceptable bias and CV: SAVYLQM[2Ox]TDLR+2y8 and DILLTQSPAILSVSPGER+2y11 for IFX, SAVYLQM[2Ox]TDLR+2y8 and DILLTQSPAILSVSPGER+2y11 for SIL-IFX, the MRM settings of which are listed in Table 2. The final quantification of IFX was performed by calculating the mean of the concentrations for the quantification ion transitions for these two selected peptides.
Radar chart plotting three performance indicators for the six proteotypic peptides selected after the proteomic study. For bias and CV (coefficient of variation), the highest values, the best performances. Peak surface was used to express the sensitivity of the chromatographic peaks (the highest peak surface, the highest sensitivity). Data are expressed as arbitrary units. The two selected peptides are underlined
Method validation
The method was validated in line with the Food and Drug Administration’s (FDA) Guidelines for Industrial Bioanalytical Method Validation [20]. Selectivity was tested by analyzing six different batches of blank plasma samples, to which SIL-IFX had been added (blank sample) or not (double blank sample). This protocol was used to detect possible interference caused by SIL-IFX or endogenous compounds. The calibration model (linearity) was examined for each peptide by choosing a suitable regression model and the appropriate weighting factor to compensate for heteroscedasticity. Within- and between-day accuracy (bias) and precision were examined by performing replicate analyses (n = 6) for both IQC standards. Inter-day accuracy and precision were assessed with the same IQCs by repeated injection (n = 6) over 6 days and by having data analyzed by several analysts. Precision was calculated as the percentage deviation of the average calculated concentration from the nominal concentration, it was expressed as the CV%. The acceptance limits were <15 % for precision and within ±15 % of the nominal value for accuracy, except for the lower limit of quantification (LLOQ), for which a CV <20 % for precision and within ±20 % of the nominal value for accuracy were accepted. The upper limit of quantification (ULOQ) was detected and validated with the same criteria for precision and accuracy criteria as those applied for the two IQC samples. A dilution factor (fivefold) was tested by spiking blank plasma (n = 5) with IFX at concentrations above the ULOQ.
Inter-sample carryover was studied to measure the persistence of IFX from one injection to another: the highest calibrator was analyzed three times consecutively (H1, H2, H3), and the mean calculated (Ħ). The lowest calibrator (LLOQ) was then analyzed three times (B1, B2, B3). The percentages of contamination were calculated as follows:
Qualitative matrix effects were studied by analyzing ion suppression and enhancement phenomenon. Extracted double blank plasma samples were injected into the LC system while a trypsin-digested solution of IFX 20 mg/L was continuously post-column infused in the ionization source through a tee [21]. The stability of the prepared samples in the injection vials was evaluated for both levels of IQCs (n = 5) to test the conditions that are likely to be encountered during sample transfer, handling, and analysis. The effect of sample storage at 4 °C for 4 days was studied.
Finally, our method was tested for compatibility with quantification of IFX biosimilar (Inflectra®). A calibration curve was developed as indicated in Table 1 with drug-free plasma spiked with fixed concentrations of Inflectra® biosimilar IFX and assessed by comparison with IQC drug-free plasma spiked with known concentrations of Remicade® IFX.
Infliximab quantification in human plasma
Blood samples from 45 patients treated with IFX for IBD were collected immediately before infusion of a new IFX dose between July 2014 and June 2015 for TDM in a clinical setting. Samples were centrifuged at 2000×g for 10 min and stored at −80 °C in a biobank (DC-2013-1983) after measuring IFX concentration using the Lisa Tracker® immunoassay.
Clinical status information including age, gender, body weight, disease (Crohn’s disease or ulcerative colitis), IFX concentrations determined using Lisa Tracker® assay, and anti-IFX antibody (ADA) levels were collected from patient medical records completed by consultants when patients attended their outpatient appointment.
The concentrations of IFX obtained with our LC-MS/MS method were compared to those obtained with two ELISA methods (an in-house method and the Lisa Tracker® assay; see flow diagram in Fig. 3). The in-house ELISA method (quantification range 0.1–60 mg/L) was adapted from a previously published method [22] (see Electronic Supplementary Material (ESM) for details of the protocol). The Lisa Tracker® method (quantification range 0.1–8 mg/L) was used according to the manufacturer’s instruction [23].
Statistical analysis
The concentrations of IFX measured by LC-MS/MS were compared with those determined using the in-house ELISA method and the Lisa Tracker® assay using non-parametric Passing and Bablok analysis and Bland and Altman plotting to assess method agreement between the three methods [24]. Statistical tests were performed using MedCalc® software (version 12.5).
Results
Figure 4 shows representative chromatograms of six overlaid double blanks (4a), blanks (4b), the first level of the calibration curve (4c) which was set as the LLOQ at 1 mg/L, and a sample from a patient treated with IFX (4d) for which quantification gave a result of 8.8 mg/L.
Chromatograms illustrating the two selected prototypic peptides of infliximab. a Double blank revealing no interference on infliximab and heavy labeled infliximab (IS); b blank revealing no interference on infliximab MRM signals while monitoring heavy labeled infliximab (internal standard); c first point of the calibration curve measured at the LLOQ 1 mg/L; d sample from a patient treated by infliximab and quantified at 8.8 mg/L. (1) corresponds to the patient for whom both ELISA method quantified a very low IFX concentration (0.1 mg/L) compared to the 4.37 mg/L measured by LC-MS/MS
The signals measured in the double blanks (4a) showed no interference due to signals from endogenous compounds overlapping with the selected ion transitions for the peptides selected for SIL-IFX. The blanks (4b) also showed that, in drug-free serum spiked only with SIL-IFX, the MRM signals measured for the transitions corresponding to unlabeled peptides were at the level of the background noise. Thus, compared to the level of signal obtained at the LLOQ (4c), these levels of interference were not significant.
The within-day and between-day precision and accuracy for the two IQC are presented in Table 3. All calculated concentrations were within the ranges stipulated in the FDA guidelines: <15 % for CV and +/−15 % for bias.
The LLOQ and ULOQ were 1 mg/L (bias, −4 %; CV = 12 %; n = 6) and 26 mg/L (bias, 3 %; CV = 5 %; n = 6), respectively. The characteristics of both of these limits complied with the FDA guidelines.
The ratios of IFX peptide peak areas and SIL-IFX peptide peak areas (y-axis) were plotted against the ratios of IFX and SIL-IFX concentrations (x-axis), and calibration curves were calculated for a 1/x-weighted least-squares linear regression. The regressions for the ion transitions for the two quantification peptides were described by equations, the slope of which was 0.28 ± 0.09 (correlation coefficient, 0.9961 ± 0.0022; n = 6) for SAVYLQM[2Ox]TDLR+2y8 and 0.27 ± 0.08 (correlation coefficient, 0.9981 ± 0.0020; n = 6) for DILLTQSPAILSVSPGER+2y11.
The percent contamination was 0.01 %, suggesting that no inter-sample contamination occurred and excluding the possibility of an over-estimation of low IFX concentrations measured just after samples containing a very high concentration.
For the target concentration of 100 mg/L (n = 5), diluting samples 1/5 was associated with a bias of 6.3 % and a CV of 5.7 %.
A chromatogram obtained for plasma spiked with IFX Remicade® as compared to a chromatogram obtained from plasma spiked with IFX biosimilar Inflectra® (Fig. 5) showed no qualitative difference: the same MRM signal at the same retention times for the signature peptides and their corresponding internal standards, no additional chromatographic peaks were generated apart from those monitored, and none of the monitored peaks were missing.
Infliximab biosimilar comparison: chromatograms obtained from a a calibration point (8 mg/L) spiked with Inflectra® and B a calibration point (8 mg/L) spiked with Remicade®. In a and b, the peaks corresponded to the ion transitions of the peptide selected for quantitation: SAVYLQMTDLR (light and heavy) at the first retention time, DILLTQSPAILSVSPGER (low and heavy) at the second retention time
Quantification of IFX Remicade®-spiked drug-free plasma quality controls (6 and 12 mg/L) on the calibration curve developed with drug-free plasma spiked with IFX biosimilar Inflectra® showed that the concentrations measured for both IQC were between ±15 % of the target values (data not shown).
After storage of extracted samples in injection vials at 4 °C for 4 days, IFX concentrations were within 85 to 115 % of the original concentrations.
The characteristics of the 45 patients for whom serum IFX concentrations were measured are presented in Table 4.
The IFX concentrations determined by the LC-MS/MS method ranged from 1.0 to 37.9 mg/L. Over this concentration range, they showed a good agreement with the in-house ELISA. The Passing and Bablok regression revealed no significant deviation from linearity (Cusum test, P = 0.99) and the slope from the equation for the linear regression (1.13) indicated that the LC-MS/MS results overestimated concentrations by 13 % compared to the in-house ELISA results (Fig. 6a). The Bland and Altman plot showed that the mean bias of the two methods was 25.9 %, but revealed a notable discrepancy for one patient: 0.1 mg/L of IFX according to in-house ELISA compared to 4.4 mg/L according to LC-MS/MS (circled with a dotted line in Fig. 6b(1)). Interestingly, this patient was the only one presenting a high ADA concentration (1458 μg/L).
Interassay comparison of the three methods: LC-MS/MS vs in-house ELISA assay or vs Lisa Tracker® ELISA assays, Passing & Bablok (a) and Bland & Altman (b) for the comparison of LC-MS/MS vs in-house ELISA assay; Passing & Bablok (c); d Bland & Altman (d) for the comparison LC-MS/MS vs Lisa Tracker® ELISA assay
When compared to results obtained with the Lisa Tracker® ELISA assay (n = 32), the results for the LC-MS/MS method were about twofold higher. Thus, although the Passing and Bablok regression (Fig. 6c) revealed no significant deviation from linearity (Cusum test, P = 0.38), the slope of the regression line was 2.43, and the Bland and Altman plot (Fig. 6d) indicated that the mean bias between the two methods was −78.3 %. Both in LC-MS/MS and Lisa Tracker®, two samples had a concentration <1 mg/L and eight samples had a concentration >8 mg/L, which are beyond the limits of linearity for the Lisa Tracker® kit used.
The same outlier was observed with the Lisa Tracker® ELISA assay (Fig. 6d(1)) as with the in-house ELISA method, corresponding to the patient presenting very high concentration of ADA. Among the six remaining patients with ADA, five had ADA concentrations ranging from 10 to 94 μg/L and their IFX concentrations were measurable with the three analytical methods; the other patient had an IFX concentration below the LLOQ with all the three methods associated with an ADA concentration of 200 μg/L.
Discussion
The results presented here demonstrate that IFX can be quantified in human serum by an LC-MS/MS method. The LLOQ (1 mg/L), ULOQ (26 mg/L, validated up to 100 mg/L after 1/5 dilution), precision, and accuracy were consistent with use of this method for the TDM of IFX in patients presenting either IBD or inflammatory rheumatic diseases. For example, in IBD patients, a trough IFX concentration between 3 and 7 mg/L [25] in the maintenance phase of treatment has been proposed to be predictive of treatment efficacy, whereas a trough IFX concentration of 21 mg/L at week 2 has been associated with a favorable 14-week clinical activity index [26]. In rheumatoid arthritis, trough concentrations lower than 2.5 mg/L, associated or not with a clinical 28-day disease activity score >4.2 at week 6, are predictive for failing to achieve low disease activity [27].
The main challenges with the development of our method were (i) the selection of IFX signature peptides that could distinguish it from endogenous human immunoglobulins and (ii) the selection of the most specific and sensitive signature peptide MRM ion transitions. The selectivity of the method was confirmed by the analysis of blank human plasma samples. In these samples, no signal was detected which could be confused with the signature peptide ion transitions selected to monitor IFX concentration. This specificity was greatly enhanced by the immunoaffinity step during which most of the endogenous proteins except those bound to biotinylated TNFα were eliminated.
The within-day and between-day accuracies of our method were <15 %, meeting the FDA validation criteria. The CV for the within-day and between-day precisions of our LC-MS/MS method for a concentration of 6 mg/L was lower than those obtained with Lisa Tracker® and Promonitor ELISA assays for a concentration of 8 mg/L [28]. This level of precision was possible thanks to the use of the SIL-IFX that was added directly to the plasma. This addition of SIL-IFX at the first step of the extraction procedure represents the main advantage of our method over LC-MS/MS procedures based on the addition of isotope-labeled peptides after the trypsin digestion step [16] as whole-protein standards can compensate for matrix effects and technical problems occurring during the immonocapture and enzymatic digestion steps [13].
The analysis of drug-free plasma samples spiked with either IFX Remicade® or its biosimilar Inflectra® showed that the proteotypic peptides selected for quantification were present in both therapeutic antibodies. Therefore, our method is fully specific for IFX, whatever its origin. This finding is of major importance as the differences between two biosimilar therapeutic monoclonal antibodies are located at the level of the glycosylation patterns and/or of microvariations in the primary amino acid sequences [29, 30]. With respect to the MS/MS selectivity, any changes to amino acid sequences would change the mass/charge ratio of the peptides, making them undetectable. Thus, a biosimilarity study should be performed whenever a new biosimilar drug becomes available.
Storage of extracted samples in injection vials at 4 °C for 4 days induced no significant change of IFX concentration, suggesting that significant degradation of IFX during analysis appears unlikely.
The concentrations of IFX measured by LC-MS/MS correlated with those determined using an in-house ELISA (Cusum test, P = 0.99) and with the Lisa Tracker® results (Cusum test, P = 0.38). The LC-MS/MS showed good agreement with the in-house assay (slope of 1.13 and an intercept of 0.77). Good agreement between an LC-MS/MS-based method and ECLIA methods was also recently described with a slope of 0.967 and an intercept of 0.488 [16]. All these data suggest that LC-MS/MS is a suitable analytical approach for the quantification of IFX. Since the concentrations of IFX measured by LC-MS/MS are similar to those measured with the in-house ELISA assay, it is possible that the therapeutic ranges proposed by clinical studies in which an in-house ELISA assay was used could be transposable to IFX TDM with an LC-MS/MS method [25, 31]. Obviously, further clinical studies will be required to better determine the therapeutic index for IFX when quantified by an LC-MS/MS method.
Unexpectedly based on comparisons with the in-house ELISA, when results from our LC-MS/MS method were compared to those obtained with a Lisa Tracker® assay, a systematic bias was found, indicating a twofold difference in plasma concentration between the two methods. A similar degree of underestimation (slope of 0.61 and intercept of −0.44) was reported when the Lisa Tracker® assay was compared with the Sanquin biological level assay [5] and this bias was confirmed with the in-house assay used here (slope of 0.45). Discrepancies between ELISA kit assays have been widely reported [5, 32–34] and could be partly explained by non-specific binding. In this study, the underestimation of the concentration by the Lisa Tracker® assay could be due either to the presence of interfering proteins or to an orientation of IFX that would prevent the anti-human Fc antibody from binding. However, the anti-human IgG used as secondary antibody to reveal the presence of IFX in ELISA assays is the most probable cause of the discrepancies reported here as LC-MS/MS-based assays can directly detect IFX bound to TNF without requiring a secondary antibody.
Sterically hindered IFX, inappropriate orientation of the IFX Fc domain, or binding of ADA could all partly explain the discrepancy observed for the patient presenting very high ADA concentration (1458 μg/L) (see Fig. 4b(1) and D(1)). In the serum from this patient, the IFX concentration was less than 0.1 mg/L when measured by both ELISA assays, whereas the LC-MS/MS assay indicated a concentration of 4.37 mg/L. This type of case caused by the presence of high-affinity ADA interferences [8] can occur frequently in a clinical setting; it reveals another weakness of immunoassays due to their lack of specificity, as discussed above.
In the present study, the LC-MS/MS and in-house measurements were performed around 12 months later than the Lisa Tracker assays. Although it is unlikely that IFX was degraded during storage at −80 °C, we acknowledge that this interval between assays could be a limitation in the comparison with the Lisa Tracker results.
The present study validated the proof of concept that IFX could be reliably quantified in human plasma by an LC-MS/MS method using a SIL-IFX. The precision and accuracy of our method is consistent with its use for IFX TDM. Since LC-MS/MS has become a cornerstone technology in clinical pharmacology laboratories, the proposed method could help to standardize IFX quantification.
References
Afif W, Loftus EV, Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi:10.1038/ajg.2010.9.
Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–307.e5. doi:10.1053/j.gastro.2014.08.035.
Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–307.e3. doi:10.1053/j.gastro.2015.02.031.
Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi:10.1136/gut.2009.183095.
van Bezooijen JS, Koch BCP, van Doorn MBA, Prens EP, van Gelder T, Schreurs MWJ. Comparison of three assays to quantify infliximab, adalimumab, and etanercept serum concentrations. Ther Drug Monit. 2016;38:432–8. doi:10.1097/FTD.0000000000000310.
Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118–24.e3. doi:10.1016/j.cgh.2014.12.026.
Steenholdt C, Ainsworth MA, Tovey M, Klausen TW, Thomsen OØ, Brynskov J, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohnʼs disease. Ther Drug Monit. 2013;35:530–8. doi:10.1097/FTD.0b013e31828d23c3.
Lee MWM, Connor S, Ng W, Toong CM-L. Comparison of infliximab drug measurement across three commercially available ELISA kits. Pathology. 2016;48:608–12. doi:10.1016/j.pathol.2016.07.001.
Wu AHB, French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin Chim Acta. 2013;420:4–10. doi:10.1016/j.cca.2012.10.026.
Grebe SKG, Singh RJ. LC-MS/MS in the clinical laboratory—where to from here? Clin Biochem Rev. 2011;32:5–31.
Rauh M. LC–MS/MS for protein and peptide quantification in clinical chemistry. J Chromatogr B. 2012;883–884:59–67. doi:10.1016/j.jchromb.2011.09.030.
Xu K, Liu L, Maia M, Li J, Lowe J, Song A, et al. A multiplexed hybrid LC–MS/MS pharmacokinetic assay to measure two co-administered monoclonal antibodies in a clinical study. Bioanalysis. 2014;6:1781–94. doi:10.4155/bio.14.142.
Lebert D, Picard G, Beau-Larvor C, Troncy L, Lacheny C, Maynadier B, et al. Absolute and multiplex quantification of antibodies in serum using PSAQ™ standards and LC-MS/MS. Bioanalysis. 2015;7:1237–51. doi:10.4155/bio.15.56.
Ramagiri S, Moore I. Hybridizing LBA with LC–MS/MS: the new norm for biologics quantification. Bioanalysis. 2016;8:483–6. doi:10.4155/bio.16.9.
Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F. Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography−tandem mass spectrometry. Anal Chem. 2008;80:1290–6. doi:10.1021/ac702115b.
Willrich MAV, Murray DL, Barnidge DR, Ladwig PM, Snyder MR. Quantitation of infliximab using clonotypic peptides and selective reaction monitoring by LC–MS/MS. Int Immunopharmacol. 2015;28:513–20. doi:10.1016/j.intimp.2015.07.007.
Grebe SKG, Singh RJ. Clinical peptide and protein quantification by mass spectrometry (MS). TrAC Trends Anal Chem. 2016;1–13. doi:10.1016/j.trac.2016.01.026.
Inc. TFS. Thermo Scientific MSIA Streptavidin D.A.R.T.’S. 2013. https://tools.thermofisher.com/content/sfs/manuals/D21140~.pdf.
Qian W-J, Jacobs JM, Liu T, Camp DG, Smith RD. Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol Cell Proteomics. 2006;5:1727–44. doi:10.1074/mcp.M600162-MCP200.
U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry bioanalytical method validation guidance for industry bioanalytical method validation. 2001. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–4.
Ternant D, Mulleman D, Degenne D, Willot S, Guillaumin J-M, Watier H, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit. 2006;28:169–74. doi:10.1097/01.ftd.0000189901.08684.4b.
Theradiag Lisa-Tracker—Monitoring of patients under biotherapies. http://www.theradiag.com/en/files/2016/04/6-pages-LISA-TRACKERv01-2016-UK-planches.pdf.
Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7.
Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9.e3. doi:10.1053/j.gastro.2015.02.031.
Kobayashi T, Suzuki Y, Motoya S, Hirai F, Ogata H, Ito H, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51:241–51. doi:10.1007/s00535-015-1102-z.
van den Bemt BJF, den Broeder AA, Wolbink G-J, van den Maas A, Hekster YA, van Riel PLCM, et al. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol. 2013;76:939–45. doi:10.1111/bcp.12142.
Schmitz EMH, van de Kerkhof D, Hamann D, van Dongen JLJ, Kuijper PHM, Brunsveld L, et al. Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin Chem Lab Med. 2016. doi:10.1515/cclm-2015-0987.
Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe. MAbs. 2013;5:621–3. doi:10.4161/mabs.25864.
Beck A, Debaene F, Diemer H, Wagner-Rousset E, Colas O, Van Dorsselaer A, et al. Cutting-edge mass spectrometry characterization of originator, biosimilar and biobetter antibodies. J Mass Spectrom. 2015;50:285–97. doi:10.1002/jms.3554.
Papamichael K, Van Stappen T, Vande Casteele N, Gils A, Billiet T, Tops S, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–9. doi:10.1016/j.cgh.2015.11.014.
Vande Casteele N, Buurman DJ, Sturkenboom MGG, Kleibeuker JH, Vermeire S, Rispens T, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–71. doi:10.1111/apt.12030.
Guiotto C, Daperno M, Frigerio F, Vizzini M, Cerruti R, Ercole E, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis. 2016;48:138–43. doi:10.1016/j.dld.2015.10.023.
Bults P, van de Merbel NC, Bischoff R. Quantification of biopharmaceuticals and biomarkers in complex biological matrices: a comparison of liquid chromatography coupled to tandem mass spectrometry and ligand binding assays. Expert Rev Proteomics. 2015;12:355–74. doi:10.1586/14789450.2015.1050384.
Acknowledgments
We thank Ravindra Chaudhari from Thermo-Fisher for advice and technical assistance with the MSIA procedure. We are also indebted to Karine Scalabrino for excellent relevant technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the Grenoble University Hospital ethics committee IRB6705 and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 53 kb)
Rights and permissions
About this article
Cite this article
Jourdil, JF., Lebert, D., Gautier-Veyret, E. et al. Infliximab quantitation in human plasma by liquid chromatography-tandem mass spectrometry: towards a standardization of the methods?. Anal Bioanal Chem 409, 1195–1205 (2017). https://doi.org/10.1007/s00216-016-0045-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0045-4