Abstract
This work describes the characterization of a solid-phase extraction (SPE) and liquid-chromatography-tandem mass spectrometry-based method for the analysis of acrylamide (AA) in complex environmental waters. The method involved the SPE of AA using activated carbon, and the AA was detected with tandem mass spectrometry after separating on an ion exclusion high-performance liquid chromatography column. The method incorporated two labeled AA standards for quantification using isotope dilution and to assess absolute extraction recovery. The method was evaluated for inter- and intra-day precision and accuracy. The method was both accurate (i.e., <30 % error) and precise (i.e., <20 % relative standard deviation), with absolute extraction recoveries averaging 37 %. The mass spectrometry provided excellent sensitivity, with instrumental limits of detection and quantitation values of 23 and 75 pg, respectively. The method detection limit was determined to be 0.021 μg/L. The analysis of AA was successfully performed in real-world samples that contained total dissolved solids concentrations ranging from 23,600 to 297,000 mg/L and AA concentrations ranging from 0.082 to 1.0 μg/L.

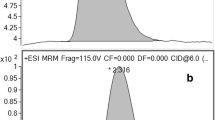
Product ion spectra of, from top to bottom, acrylamide, acrylamide-1-13C, and acrylamide-2,3,3-d3. The predominant peak in each spectrum was used for quantitation
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acrylamide (AA) is the monomer that forms the widely used polyacrylamide, which is utilized extensively to aid water clarification at wastewater treatment plants (WWTPs) and as a grouting agent in wells [1, 2]. Water treatment and industrial operations, especially from plastics and resins, are the main sources of AA in the environment [1, 3]. AA has been classified as probably carcinogenic to humans by the International Agency for Research on Cancer [4], who subsequently considered it a high-priority agent in 2008 due to the discovery that AA is also a dietary contaminant [5, 6]. According to the US Environmental Protection Agency’s (EPA) Toxic Release Inventory database, AA releases from industry to land and water totaled over 7 million lbs in 2010 in the US, with >98 % of the total released AA being injected into underground injection class I wells [7]. The US EPA has set a maximum contaminant level goal for AA of zero and requires water suppliers to demonstrate that the AA monomer is present at concentrations less than 0.5 μg/L [3].
AA is a small, polar, and hydrophilic molecule, which makes it difficult to analyze using conventional techniques, especially in complex aqueous matrixes. Methods for the detection and quantitation of AA have typically involved chromatographic separation followed by detection using spectrophotometry or mass spectrometry. For example, EPA method 8316 utilizes high-performance liquid chromatography (HPLC) coupled to UV absorbance (195 nm) detection to monitor AA [8]. However, the reversed-phase C18 column used in this method does not retain AA, and the detection limit (10 μg/L) is too high for regulatory purposes. Similarly, Weideborg et al. used an HPLC-UV method with a detection limit of 5 μg/L to monitor AA in drainage water contaminated from grouting agents [9]. EPA Method 8032A utilizes the Hashimoto method [10], which involves the bromination of AA, followed by gas chromatography-electron capture detector (GC-ECD) analysis [11]. This method provides much lower detection limits (0.03 μg/L); however, the derivatization process is time-consuming, and the AA may suffer from interferences and poor extraction efficiencies in complex matrixes. Large-volume injection (LVI) liquid chromatography-mass spectrometry (LC-MS-) and LC-tandem mass spectrometry (MS/MS)-based methods that do not utilize extraction or derivatization have also been developed for the determination of AA [12, 13]. However, the LVI-LC-MS method suffered from high detection limits [12], and the LVI-LC-MS/MS method required the use of the Ion Sabre APCI ionization source to attain acceptable limits of detection (0.03 μg/L) [13]. The recent discovery that AA is a common food contaminant in many starchy foods [6] has spurred the development of methods that use solid-phase extraction (SPE) techniques prior to LC-MS/MS analysis [14, 15]. Because environmental aqueous samples are quite different from food samples; however, different types of extraction sorbents are required.
Activated carbon has been used for the SPE of AA, first in conjunction with GC-MS [16], then with LC-MS/MS [17]. This sorbent has been shown to effectively retain AA from large volumes (i.e., >250 mL) of aqueous samples. However, differences in the manufacturing of the cartridges (i.e., in-house construction versus commercially made SPE cartridges) and differences in the extraction and analysis conditions have led to a lack of overall characterization of the SPE-based method. In this work, we characterize the LC-MS/MS-based method and incorporate both a surrogate and internal standard to improve the method. We also demonstrate for the first time the method’s effectiveness in complex environmental samples that contain total dissolved solids (TDS) at concentrations well in excess of 20,000 mg/L.
Materials and methods
Chemicals and reagents
AA (CAS# 79-06-1), acrylamide-2,3,3-d3 (98 atom % D, AA-d3, CAS# 122775-19-3), and hydroquinone were from Sigma-Aldrich (St. Louis, MO). Acrylamide-1-13C (99 atom % 13C, AA-13C, CAS# 287399-24-0) was from Isotec (Miamisburg, OH). Methanol was from Fox Scientific (Alvarado, TX). Acetonitrile was from Burdick and Jackson (Honeywell International, Muskegon, MI), and formic acid (FA, +96 %) was from EK Industries (Joliet, IL). Water was generated in-house from a Barnstead NANOpure water purification system. Stock solutions (0.5–1 mg/mL) of individual standards and standard mixtures were prepared by dissolving accurate amounts of the standard compounds in methanol. The stock solutions contained 0.1 mg/mL hydroquinone to prevent polymerization of the AA monomer. Working standard solutions were obtained by further dilution of the stock solutions with methanol and were stored at 4 °C in the dark.
Samples
The method was initially validated using samples consisting of AA spiked into ultrapure water at various concentrations. Various environmental samples were also used to assess the method for the determination of AA. The samples included discharge from a wastewater treatment plant, pit water (i.e., flowback water from a horizontal well that was stored for at least 2 months in a pit), and two different samples of produced water from a horizontal well. The environmental water samples were analyzed for AA and were also spiked with AA to determine matrix effects and spike recovery (i.e., laboratory-fortified matrix). Sample volume permitting, the sample analyses were performed in duplicate. Field and laboratory blanks were also analyzed to ensure that the analytical method and laboratory equipment were free from contamination. The quality control (QC) samples analyzed in parallel with the environmental samples included the abovementioned blanks and replicates, instrument blanks, laboratory-fortified blanks, and continuing calibration verification samples. The QC samples were run to ensure no analyte was detected in any blanks, that replicate measurements had sufficient precision (i.e., relative standard deviation [RSD] or relative percent difference [RPD] in the case of duplicates <30 %), and that fortified samples had sufficient recoveries (i.e., 70–130 %). Samples with visible suspended solids were filtered prior to extraction with type 934-AH glass microfiber paper (Whatman). The TDS and total suspended solids (TSS) were determined according to EPA methods 160.1 and 160.2, respectively [18, 19], except that 50 mL sample volumes were used instead of 100 mL. The pH values of the samples were determined using type CF pH indicator paper (Whatman).
SPE extraction of acrylamide
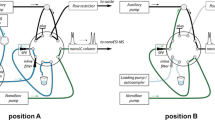
A 500-mL volume of each aqueous sample was generally used for each extraction, and 100 ng of AA-d3 was added as a surrogate standard prior to extraction. An exception to the extraction volume included one of the flowback water samples in which the measured concentration of AA was greater than the range of the calibration curve, and only 250 mL was used for extractions. The samples were extracted using an Autotrace SPE Workstation (Dionex, Sunnyvale, CA). The SPE cartridges used for the extraction of AA from water were Isolute-activated carbon cartridges (6 cc, 500 mg) from Biotage (Charlotte, NC). The cartridges were first conditioned with 5 mL methanol and 5 mL water at a flow rate of 5 mL/min. After conditioning, 500 mL of sample was passed through the cartridges at 5 mL/min. The sample flasks were then rinsed with 50 mL water, and the rinsate was also loaded onto the cartridges. The SPE cartridges were then rinsed with 2 mL water before drying with N2 gas for 30 min. The samples were eluted off the cartridges with 10 mL methanol at 1 mL/min. The eluate was then concentrated and solvent exchanged with a TurboVap Concentrator (Biotage) to 0.5 mL in methanol and transferred to HPLC sample vials for analysis. Following concentration, 100 ng of AA-13C was added to each sample as an internal standard.
HPLC-MS/MS
Samples were analyzed using an AB Sciex 4000 Q Trap MS interfaced with a Shimadzu HPLC. Detection was performed using the AB Sciex 4000 Q Trap MS in the triple quadrupole mode. The MS was equipped with a Turbo V Ion Source (TIS), which utilized the TIS source probe for positive-mode electrospray ionization (ESI+). The HPLC system consisted of LC-20 AD pumps, an SIL-20 AC HT autosampler, and a CTO-20A column oven. The injection volume was set at 30 μL. The column was an IonPac ICE-AS1 ion exclusion column from Dionex (7.5 μm, 4 × 250 mm), which was connected to an IonPac ICE-AS1 guard column (4 × 50 mm). Mobile phase A was 0.1 % FA in water, and mobile phase B was 0.1 % FA in acetonitrile. The AA was eluted off of the ion exclusion column under isocratic conditions at 50:50 mobile phase A/mobile phase B at a flow rate of 0.18 mL/min. The column temperature was maintained at 30 °C.
The AB Sciex software Analyst version 1.5.2 was used for data acquisition and analysis. The MS parameters for each compound were optimized to ensure the most favorable ionization and ion transfer conditions and attain optimum signal of both the precursor and fragment ions by infusing the analytes into 50 % B at 0.18 mL/min and manually tuning the parameters. The source parameters were identical for all of the analytes: curtain gas, 40 psi (N2); ion spray voltage, 5,500 V; source temperature, 400 °C; ion source gas 1 (nebulizer gas) 40 psi (N2); ion source gas 2 (auxiliary gas), 60 psi (N2); and the interface heater was on. The ESI probe y-axis was set to 2.5 mm, and the x-axis was positioned at 5.0 mm. The collision gas (N2) was set to a value of 5. The ion-specific multiple reaction monitoring (MRM) parameters are shown in Table 1.
The instrumental limit of detection (LOD) was defined as the minimum amount of compound analyzed in the LC-MS/MS that produced a signal-to-noise (S/N) ratio of 3. The instrumental limit of quantitation (LOQ) was defined as the minimum amount of compound that produced a S/N ratio of 10. The method detection limit (MDL), defined as “the minimum concentration of a substance that can be identified, measured, and reported with 99 % confidence that the analyte concentration is greater than zero,” was determined from seven replicate analyses of 0.05 μg/L AA in ultrapure water according to Glaser et al. [20], following the method described above for extraction and LC-MS/MS analysis. The MDL was calculated according to Eq. 1:
where t (n-1, 1-α=0.99) is the Student’s t -value that is approximate to a 99 % confidence level and a standard deviation estimate with (n − 1) degrees of freedom, and S is the standard deviation of the replicate analyses.
Quantitation
The MRM transitions corresponding to the loss of ammonia from the protonated AA were used for quantitation (i.e., m/z 72 > 55 for AA, m/z 73 > 56 for AA-13C, and m/z 75 > 58 for AA-d3). Additionally, the transitions corresponding to the loss of C2H4 were monitored as confirmation ions (i.e., m/z 72 > 44 for AA, m/z 73 > 45 for AA-13C, and m/z 75 > 44 for AA-d3). Quantitation was performed using isotope dilution by utilizing the deuterated surrogate AA-d3 according to the following equation:
where C AA and A AA are the concentration of AA and the integrated area of AA, respectively, C d and A d are the concentration of the deuterated surrogate and the integrated area of the deuterated surrogate, respectively, and \( \overline{RR} \) is the mean relative response of the deuterated surrogate to the native AA. The \( \overline{RR} \) was calculated by averaging the individual RRs from each of eight calibration standards, which ranged from 10 to 800 μg/L AA and contained constant concentrations of 200 μg/L AA-d3 and 200 μg/L AA-13C. The RR of each calibration standard was determined according to Eq. 3:
The extraction recovery was determined by calculating the recovery of the deuterated surrogate through the use of the 13C-labeled AA internal standard. The mean response factor (RF) of the AA-13C relative to AA-d3 was first calculated from the calibration standards analogously to Eq. 3, substituting the variables associated with AA with AA-d3 (e.g., substituting A AA with A d) and substituting the variables associated with AA-d3 with AA-13C. The mean RF was then used in Eq. 2 after appropriate substitutions of variables to calculate the concentration of AA-d3 in each sample. The extraction recovery was then calculated as the ratio of the measured concentration of AA-d3 to the spiked concentration of AA-d3.
Results and discussion
Selection of extraction conditions
The search for sorbents that can effectively extract AA has been a familiar challenge associated with AA analysis [14, 21]. Because AA is extremely polar and hydrophilic, it is not easily retained on SPE cartridges when loading aqueous samples. The SPE cartridges we initially tested included ENVI-Carb (Supelco), a graphitized carbon black cartridge; Isolute ENV+, containing a hydroxylated styrene divinyl benzene polymer and used frequently for AA analysis in food samples; and Isolute-activated carbon. However, the breakthrough volume of the ENV+ and ENVI-Carb columns were too low, and no AA was recovered from either type of cartridge. Rosén et al. [14] conducted a nice study of 14 different silica-, polymer-, and carbon-based SPE columns with regard to AA recovery as a function of volume of water loaded through the column. They determined that most of the tested columns retained less than 50 % AA within the first 10 mL water loaded, that very few columns retained any AA at volumes of water loaded greater than 30 mL, and that none of the tested columns retained any AA after 50 mL water was passed through the column. This low breakthrough volume is not typically a significant issue during the analysis of food samples; however, the volumes associated with aqueous environmental samples are often much higher due to the low concentrations of analytes. Therefore, most conventional SPE cartridges are not suitable for the SPE of AA. The use of activated carbon has previously been used for the extraction of AA from aqueous matrixes [16, 17], and indeed, the activated carbon was able to effectively extract AA from water samples. The absolute extraction recovery from the activated carbon cartridges is discussed below. We did not observe any increase in extraction recovery by adjusting the pH of the samples to basic or acidic conditions; therefore, a pH of 7 was maintained in all future extractions.
LC-MS/MS optimization
A triple quadrupole MS was used for quantitative purposes. The mass spectra of the AA and labeled standards showed that the [M+H]+ ions were the dominant species, which was beneficial for MS/MS purposes. A polypropylene glycol standard had been used to mass calibrate the MS, but the lowest calibrant possessed a m/z of 77. Therefore, some of the observed m/z values were off by 0.1 Da. The product ion spectra of AA, AA-13C, and AA-d3 are shown in Fig. 1. The major product ion in each spectrum, used for quantitation, resulted from the loss of NH3, which corresponded to m/z values of 55, 56, and 58 for AA, AA-13C, and AA-d3, respectively. The only other significant product ions were used as confirmation ions and occurred with the loss of C2H4 (or C2 1H2H3 in the case of AA-d3), resulting in the [CONH2]+ species at m/z 44 for AA and AA-d3 and [13CONH2]+ at m/z 45 for AA-13C. However, as shown in Fig. 1c for AA-d3, a peak at m/z 43 was observed to be more intense than m/z 44, potentially caused by hydrogen abstraction to form an odd-electron species. Additionally, there was considerably more “peak noise” in the vicinity of the confirmation ions of AA-13C and AA-d3 (Fig. 1b, c, respectively) than the single peak at m/z 44 for AA (Fig. 1a), which may have partially been due to rearrangements with the isotopic labeling or impurities in the isotopically labeled compounds. The relative intensity of the confirmation ion in each case was approximately 10 % of the quantitation ion. This observation made confirmation of the analyte at low concentrations difficult, which has previously been noted [13].
For chromatography, we first investigated the use of reversed-phase C18 and Hypercarb porous graphitic carbon (Thermofisher) HPLC columns, both of which have been used for the analysis of AA [14, 21–23]. Because of the polar, hydrophilic nature of AA, the analyte was not retained at all and eluted off both columns with the dead volume. Because the reproducible retention of AA was desired, these columns were not optimal for the analysis of AA. We next investigated the use of the ICE-AS1 ion exclusion column and found that it retained the AA quite well (see Fig. 2), as has been demonstrated previously [12, 17]. A smaller-circumference 4 mm column was used here, as opposed to the 9 mm column used by Lucentini et al. [17] that required a 1:10 split of the column eluate prior to MS analysis. The smaller size column was selected for its higher compatibility with the MS interface.
Method validation
The inter- and intra-day accuracy and precision of the method were assessed by spiking AA into five samples of ultrapure water each day for 3 days at concentrations of 50, 200, and 1,000 ng/L (see Table 2), following the guidance provided by ICH Q2(R1) [24]. The intra- and inter-day accuracies ranged from 102 to 115 % and 105–123 %, respectively. The intra- and inter-day precisions, measured as the RSD, ranged from 3.4 to 6.6 % and 6.3–13.0 %, respectively. Therefore, the accuracy and precision were sufficient for low-concentration measurements of AA in a laboratory-generated matrix. No AA was ever detected in any of the method or instrument blanks.
The instrumental LOD of AA was 23 pg injected on-column, and the LOQ was 75 pg. The MDL was determined to be 0.021 μg/L, which is lower than the reported detection limits using EPA GC-ECD method 8032A [11] (0.03 μg/L). The detection limit here was the same as that reported by Kawata et al. [16], which, to the best of our knowledge, is the lowest detection limit for AA analysis in aqueous samples.
The absolute recovery of AA, determined from the labeled standards AA-d3 and AA-13C, from the clean ultrapure water matrixes using the activated carbon SPE cartridges averaged 37 ± 8 % (mean ± standard deviation).
Analysis of AA in complex environmental samples
Various complex matrixes, including WWTP effluent, pit water, and two produced water samples, were chosen to demonstrate the method’s applicability to environmental samples. The pit water and produced water samples contained high levels of suspended sediments and oils, which were filtered prior to extraction, and the TDS in the pit water and produced water samples ranged from 23,600–297,000 mg/L (see Table 3). Therefore, three of the four samples contained extremely high TDS and TSS values. The samples and sample spikes were analyzed in duplicate, sample volume permitting. The analysis of the environmental samples in triplicate would have been preferred; however, the available volume of the samples only allowed for the analyses to be performed in duplicate. No AA was detected in the wastewater effluent; however, AA was detected in the three high-TDS samples, with the produced water sample #1 measuring as high as 1.0 μg/L. The produced water sample #2 and the pit water sample contained AA concentrations of 0.082 and 0.091 μg/L, respectively, as shown in Table 3. The spike recoveries of the four samples ranged from 98 to 104 %, with the exception of the produced water sample #1, which measured a spike recovery of 143 %. This sample contained the highest levels of TDS and TSS, and it also measured the lowest absolute recovery of the surrogate standard at 23 %. Therefore, it is likely that this sample experienced some matrix effects as a result of the nearly 300,000 mg/L dissolved solids that passed through the activated carbon simultaneously with AA, much of that likely being co-extracted and then co-eluted with LC. An additional clean-up step, such as a liquid–liquid extraction or an additional alternative SPE sorbent, would most likely reduce the matrix effects by removing interfering contaminants that coelute with AA during HPLC, which should be considered for samples containing extremely high TDS. The absolute recoveries of AA in the environmental samples ranged from 23 to 29 %, which were slightly lower than the recoveries obtained from the ultrapure water.
Though there are now a small number of LC-MS methods for the analysis of AA in aqueous and food samples, very few reported methods have presented data on absolute recoveries of AA from the sample matrixes. Hoenicke et al. [25] reported extraction recoveries of AA ranging from 20 to 116 % in various food matrixes. However, their methods consisted of liquid–liquid extractions of a variety of homogenized food samples, which are extremely different than aqueous environmental samples, followed by GC-MS/MS or LC-MS/MS. Chu and Metcalfe [21], using a coevaporation sample preparation approach followed by LC-MS/MS, reported recoveries from agricultural runoff samples ranging from 28 to 54 %. Kawata et al. [16] reported that the use of 1.5 g activated carbon was more effective in extraction recovery than 0.5 g, and that by increasing the SPE sorbent from 0.5 to 1.5 g, the recovery increased from 40 % to 80 % in groundwater and river water. Therefore, the use of 1.5 g of sorbent should improve the recovery of AA. It is worthwhile noting, however, that through the use of appropriate standards and the sensitivity of the MS instrument, the low recoveries did not affect the results reported here, as all QC samples analyzed alongside the samples passed the relevant QC criteria, except for the spiked produced water sample #1 (300,000 mg/L TDS) that measured a 143 % spike recovery.
Conclusions
We successfully characterized an activated carbon SPE and LC-MS/MS-based method for the analysis of AA. The method incorporated AA-d3 for quantification using the isotope dilution method and AA-13C to gauge extraction recovery. The method was both accurate (i.e., <30 % error) and precise (i.e., <20 % RSD), with extraction recoveries averaging 37 %. The instrumental LOD and LOQ of AA were 23 and 75 pg, respectively, and the MDL was 0.021 μg/L. The analysis of AA was successfully performed in real-world samples that contained TDS concentrations ranging from 23,600 to 297,000 mg/L, and AA concentrations ranged from 0.082 to 1.0 μg/L. This method was demonstrated to effectively analyze AA in high-TDS samples.
Abbreviations
- AA:
-
Acrylamide
- AA-13C:
-
Acrylamide-1-13C
- AA-d3 :
-
Acrylamide-2,3,3-d3
- HPLC:
-
High-performance liquid chromatography
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- MDL:
-
Method detection limit
- MRM:
-
Multiple reaction monitoring
- SPE:
-
Solid-phase extraction
- TDS:
-
Total dissolved solids
- TSS:
-
Total suspended solids
References
IPCS INCHEM (1985) International programme on chemical safety, environmental health criteria. http://www.inchem.org/documents/ehc/ehc/ehc49.htm. Accessed 18 Sept 2012
WHO (2011) Acrylamide in drinking-water: background document for development of WHO guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/dwq/chemicals/acrylamide.pdf. Accessed 19 Sept 2012 2012
US EPA Consumer factsheet on: acrylamide. http://www.epa.gov/ogwdw/pdfs/factsheets/soc/acrylamide.pdf. Accessed 18 Sept 2012
IARC (1994) Monographs on the evaluation of carcinogenic risks to humans, vol 60. International Agency for Research on Cancer, Lyon, France
Report of the advisory group to recommend priorities for IARC Monographs during 2010-2014, Internal report 08/001. (2008) International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Publications/internrep/08-001.pdf. Accessed 19 Sept 2012
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
US EPA (2012) Toxic release inventory (TRI) program. http://www.epa.gov/tri/. Accessed 19 Sept 2012
US EPA (1994) Method 8316: Acrylamide, acrylonitrile and acrolein by high performance liquid chromatography (HPLC). US Environmental Protection Agency. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/8316.pdf. Accessed 19 Sept 2012
Weideborg M, Källqvist T, Odegård KE, Sverdrup LE, Vik EA (2001) Environmental risk assessment of acrylamide and methylolacrylamide from a grouting agent used in the tunnel construction of Romeriksporten, Norway. Water Res 35:2645–2652
Hashimoto A (1976) Improved method for the determination of acrylamide monomer in water by means of gas–liquid chromatography with an electron-capture detector. Analyst 101:932–938
US EPA (1996) Method 8032A: acrylamide by gas chromatography. US Environmental Protection Agency. http://www.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/8032a.pdf. Accessed 19 Sept 2012
Cavalli S, Polesello S, Saccani G (2004) Determination of acrylamide in drinking water by large-volume direct injection and ion-exclusion chromatography-mass spectrometry. J Chromatogr A 1039:155–159
Marín JM, Pozo OJ, Sancho JV, Pitarch E, López FJ, Hernández F (2006) Study of different atmospheric-pressure interfaces for LC-MS/MS determination of acrylamide in water at sub-ppb levels. J Mass Spectrom 41:1041–1048. doi:10.1002/jms.1063
Rosén J, Nyman A, Hellenäs KE (2007) Retention studies of acrylamide for the design of a robust liquid chromatography-tandem mass spectrometry method for food analysis. J Chromatogr A 1172:19–24
Bermudo E, Moyano E, Puignou L, Galceran MT (2008) Liquid chromatography coupled to tandem mass spectrometry for the analysis of acrylamide in typical Spanish products. Talanta 76:389–394
Kawata K, Ibaraki T, Tanabe A, Yagoh H, Shinoda A, Suzuki H, Yasuhara A (2001) Gas chromatographic-mass spectrometric determination of hydrophilic compounds in environmental water by solid-phase extraction with activated carbon fiber felt. J Chromatogr A 911:75–83
Lucentini L, Ferretti E, Veschetti E, Achene L, Turrio-Baldassarri L, Ottaviani M, Bogialli S (2009) Determination of low-level acrylamide in drinking water by liquid chromatography/tandem mass spectrometry. J AOAC Int 92:263–270
US EPA (1971) Method 160.1: residue, filterable (gravimetric, dried at 180 °C). Methods for the chemical analysis of water and wastes (MCAWW) (EPA/600/4-79/020)
US EPA (1971) Method 160.2: residue, non-filterable (gravimetric, dried at 103–105 °C). Methods for the chemical analysis of water and wastes (MCAWW) (EPA/600/4-79/020)
Glaser JA, Foerst DL, McKee GD, Quave SA, Budde WL (1981) Trace analyses for wastewaters. Environ Sci Technol 15:1426–1435
Chu S, Metcalfe CD (2007) Analysis of acrylamide in water using a coevaporation preparative step and isotope dilution liquid chromatography tandem mass spectrometry. Anal Chem 79:5093–5096
Zhang Y, Ren Y, Jiao J, Li D, Zhang Y (2011) Ultra high-performance liquid chromatography-tandem mass spectrometry for the simultaneous analysis of asparagine, sugars, and acrylamide in Maillard reactions. Anal Chem 83:3297–3304
Mastovska K, Lehotay SJ (2006) Rapid sample preparation method for LC-MS/MS or GC-MS analysis of acrylamide in various food matrices. J Agric Food Chem 54:7001–7008
ICH (1996) Q2(R1) Validation of analytical procedures: methodology. (CPMP/ICH281/95)
Hoenicke K, Gatermann R, Harder W, Hartig L (2004) Analysis of acrylamide in different foodstuffs using liquid chromatography-tandem mass spectrometry and gas chromatography-tandem mass spectrometry. Anal Chim Acta 520:207–215
Acknowledgments
The United States Environmental Protection Agency, through its Office of Research and Development, funded and managed the research described here. It has been subjected to the Agency’s peer and administrative review and has been approved for publication. Mention of trade names or commercial products in this paper does not constitute endorsement or recommendation by the EPA.
This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
The authors thank Matt Landis (EPA) for collecting the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DeArmond, P.D., DiGoregorio, A.L. Characterization of liquid chromatography-tandem mass spectrometry method for the determination of acrylamide in complex environmental samples. Anal Bioanal Chem 405, 4159–4166 (2013). https://doi.org/10.1007/s00216-013-6822-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6822-4