Abstract
In this study, the chemical composition of sidestream smoke (SSS) emissions of cigarettes are characterised using a laser-based single-photon ionisation time-of-flight mass spectrometer. SSS is generated from various cigarette types (2R4F research cigarette; Burley, Oriental and Virginia single-tobacco-type cigarettes) smoked on a single-port smoking machine and collected using a so-called fishtail chimney device. Using this setup, a puff-resolved quantification of several SSS components was performed. Investigations of the dynamics of SSS emissions show that concentration profiles of various substances can be categorised into several groups, either depending on the occurrence of a puff or uninfluenced by the changes in the burning zone during puffing. The SSS emissions occurring directly after a puff strongly resemble the composition of mainstream smoke (MSS). In the smouldering phase, clear differences between MSS and SSS are observed. The changed chemical profiles of SSS and MSS might be also of importance on environmental tobacco smoke which is largely determined by SSS. Additionally, the chemical composition of the SSS is strongly affected by the tobacco type. Hence, the higher nitrogen content of Burley tobacco leads to the detection of increased amounts of nitrogen-containing substances in SSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, environmental tobacco smoke (ETS) and second-hand smoke is under intense investigation, especially aiming at its possible health risks for non-smokers. ETS is a highly complex mixture of particles, droplets and gases resulting from a burning cigarette and is composed mainly of exhaled mainstream smoke (MSS) and sidestream smoke (SSS), the latter a sum of all emissions emerging from the cigarette between puffs. Approximately 15 to 43 % of the particulate matter of ETS and 13 % of the vapour phase constituents can be traced back to exhaled MSS [1]. After diffusion into the atmosphere from the cigarette, both MSS and SSS become significantly diluted, resulting in a large variety of physical and chemical changes. Levels of ETS components in a room are dependent on air circulation, room ventilation and deposition onto surfaces. The effects of air exchange rates on the level of selected components were investigated by Kotzias et al. [2] and several studies have been published on aerosol composition in areas such as pubs and bars, where high levels of ETS can typically be found [3]. As a consequence, there is a problem of associating possible health effects directly to SSS as addressed by Reasor in the late 1980s [4]. Nevertheless, many studies have been carried out dealing with the effects of exposure to SSS on health [5–25]. People are not directly exposed to pure SSS but rather a mixture of SSS and exhaled MSS which has undergone significant effects of aging. In the last years, several North American and European authorities have reacted with regulations to protect non-smokers from second hand smoke in offices and public places.
In general, the composition of SSS is similar to MSS. The quantities of substances, however, can differ significantly between MSS and SSS. As a consequence, the toxicological impact of MSS and SSS probably differ, too. There is a considerable large amount of data available on the various substances found in MSS and SSS as well as their respective SSS/MSS ratios for different cigarette types [26–37]. Baker [38] gives a comprehensive overview of MSS/SSS ratios for many substances. With a few exceptions, most substances are present in larger amounts in SSS, among them especially carcinogenic polycyclic aromatic hydrocarbons as well as a huge number of potentially health-relevant nitrogen-containing compounds and a selection of unsaturated hydrocarbons. On the other side, organic acids containing more carbon atoms than acetic acid as well as the inorganic compounds carbonyl sulphide and hydrogen cyanide show higher amounts in MSS. As SSS is one of the prominent sources of ETS, a deeper understanding of the chemical composition and in particular of the chemical dynamics in SSS formation during and between puffs is of interest.
Real-time or on-line analytical techniques have proven very useful in terms of a better understanding of the dynamic of smoke formation processes. Such studies have been reported applying quantum-cascade infrared laser spectroscopy [39–42], dual infrared tuneable diode lasers [43–45] and gas chromatography/mass spectrometry utilising a puff-resolved trapping mechanism [46–49]. Recently several studies dealing with online analysis of mainstream tobacco smoke-utilising photo ionisation mass spectrometry (SPI-MS), namely single-photon ionisation (SPI) and resonance-enhanced multi-photon ionisation (REMPI) time-of-flight mass spectrometry (TOFMS) have been published [50–57]. In contrast to electron ionisation where molecules are heavily fragmented by the excess energy of the electrons, PI methods result in soft ionisation, which, in general, only lead to the generation of the mother ion. This enables the interpretation of mass spectra of complex mixtures, such as tobacco smoke, at the expense of a loss of structural information of the substances resulting from fragmentations patterns. The puff-resolved dynamics and behaviour of regular cigarettes, as well as single tobacco grade cigarettes were investigated [52–54, 58]. In doing this, SPI proved to be a valuable tool for the investigation of MSS.
There are much fewer examples of real-time analysis of SSS. Particles in SSS have been measured by light scattering [59]. Cole et al. reported Fourier-transform infrared spectrometry (FTIR) determination of five compounds [60]. Quantum-cascade infrared laser spectroscopy has been applied to MSS and SSS analysis. Carbon monoxide and nitric oxide were monitored by FTIR as well [61].
The main objective of this study is the characterisation of SSS, i.e., both, gas phase and whole smoke in real time by laser-based single-photo ionisation TOFMS. Different trends of various substances should be devised and the interaction between MSS and SSS and its influence of the product pattern should be elaborated. Thereby, the effect of distinct tobacco types is of special interest.
Experimental
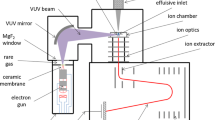
An overview of the experimental setup of the sampling can be seen in Fig. 1a. SSS is generated from various cigarette types (2R4F research cigarette; Burley, Oriental and Virginia single tobacco type cigarettes) smoked under ISO conditions on a Borgwaldt single-port smoking machine and collected using a fishtail chimney device introduced by Proctor et al. [62]. Note that the fishtail chimney-based sampling is a standard approach in tobacco science. Therefore the device was used as described for comparability with the literature (e.g., [29, 33, 35]). However, as the device is not heated it is restricted for fast response measurements to VOC and the more volatile SVOC. The membrane vacuum pump (KNF Neuberger, Germany) adjusted to an ISO flow rate of 2.0 L/min is used to transport the smoke through the fishtail chimney to the sampling point at the end of the chimney. A filter is used to prevent contamination of the vacuum pump and an additional filter pad can optionally be installed right before the sampling port to allow gas phase measurements.
a Schematic instrumental setup and photograph of the smoke generation and the so called fishtail sampling device for the SSS SPI-TOF on-line monitoring experiment. b SPI-TOFMS mass spectra of the single aromatic core region (m/z 76–98) of SSS (top) and exhaled MSS (bottom), demonstrating the large SSS/MSS differences in particular in the nitrogen containing species (odd m/z values in soft SPI mass spectra)
The smoke is injected into the ionisation chamber of a SPI/REMPI-TOFMS system by a 0.32-mm (i.d.) deactivated quartz capillary tube with a length of ∼2.0 m. The instrument has been extensively explained and characterised in previous studies on the investigation of tobacco smoke, waste incineration and pyrolysis [53, 63–65]. Therefore, only a brief description is given here. The fundamental of a Nd/YAG laser with 1,064 nm is frequency tripled by a second harmonics and a third harmonics generator β-barium borate crystal. The resulting 355-nm laser beam with an energy of ∼220 mW is guided through a gas cell filled with ∼10 mbar of Xenon. In this medium, a second frequency tripling step occurs. However, the conversion efficiency is quite low and therefore a separation of the 355-nm pump and the resulting 118-nm (10.49 eV) vacuum ultraviolet (VUV) beam is necessary. This is done by using the different refraction indices of 118 and 355 nm: by of axis irradiation of MgF2-focussing lens, the VUV and UV beams are spatially separated. While the VUV laser beam is focused underneath the inlet capillary, the 355 nm beam is blocked by a beam dump. Molecules exhibiting an ionisation energy below 10.49 eV can be ionised by absorbing a single photon. Since the ionisation energies of many organic substances are in the range of 8–11 eV, usually the excess energy that is transferred to the molecules upon ionisation is small. This results in little or no fragmentation of the molecular ions. The ions formed by SPI are detected by a Wiley–McLaren 800-mm reflectron mass spectrometer (Kaesdorf Instruments, Munich, Germany) and the signal is recorded with wave converter boards (Acqiris, Switzerland, 250 MHz, 1GS, signal resolution at 8 bits) and a National Instruments LabView based acquisition software.
Identification and assignment of the compounds based on mass spectra with nominal mass resolution is based on GC-MS measurements [66], on-line fast gas chromatography SPI-MS results [56], previous SPI and REMPI mass spectrometry results [50, 53, 67, 68] and literature data (see citations above). Note that assignment with a molecular structure/formula means that more than 80 % of the peak intensity is evaluated to be originated by the indicated compound. In Fig. 1b, an exemplary comparison of soft SPI mass spectra of SSS and exhaled MSS in the mass region of benzoid aromatic (m/z 76–98) is depicted. The obvious differences in the pattern motivate the following, more thorough study on SSS emission.
Quantification of selected substances is done by external calibration with reference gas mixtures. In this paper, quantification was performed by a 10-ppm mixture of benzene, toluene and m-xylene in nitrogen. Calibration gas mixtures were purchased from Linde AG, Germany. This reference mixture was measured with the same instrument parameters, such as inlet temperature and flow into the instrument before and after the respective SSS analyses. Therefore, the measured signal integral can be used as a direct reference for concentration and to calculate total amounts. Note, that also other compounds can be quantified as well, if the respective relative SPI cross sections are available [69].
A set of filter cigarettes made of a non-blended single tobacco sort was used. These research cigarettes each are exhibiting the same physical smoking parameters (pressure drop and ventilation) as the 2R4F standard cigarette manufactured by the University of Kentucky. All cigarettes were smoked five times, the generated whole SSS gas phase was analysed three times whereas the combined particulate and gas phase was analysed twice. SSS gas phase was measured by incorporating a Cambridge filter pad [29] in the on-line sampling train to remove the particulate phase.
Results
In Fig. 2, the comparison of typical SSS and MSS whole smoke SPI-MS mass spectra (averaged spectra of a single puff out of 100 single-mass spectra each) is presented, whereas a three-dimensional representation of a SSS on-line measurement over a full cigarette smoking cycle is depicted in Fig. 3. The MSS and SSS mass spectra show the standard deviation as determined from the averaging. Although there are many similarities in both spectra, as pointed out before; overall composition of MSS and SSS shows also obvious striking differences, such as the large peak of ammonia in the SPI mass spectrum of SSS. It is well known from literature that the ammonia content in SSS is higher up to a factor of 150 compared with MSS [38].
Comparison of SSS (bottom) and MSS (top) SPI-MS mass spectra of a single puff in bar graph representation (i.e. mass integrated for each m/z bin). The data for five full cigarette measurements were averaged (equals 100 single mass spectra), respectively. The standard variation of the obtained mass spectral profile is indicated (error margins)
Three dimensional representation of an on-line SPI-TOFMS cigarette measurement (full smoking cycle, ISO conditions). a MSS measurement. The sharply structured appearance of the profile is due to the fact that MSS is only formed during the 2-s puff duration. One puff/min was taken by the smoking machine (ISO). b MSS measurement. SSS smoke is emitted during the whole smoking cycle. The puffing structure however is still visible
Another unique attribute of the substance pattern in SSS consists of the elevated peaks of some odd m/z values, which are due to the intensified occurrence of nitrogen containing compounds. This is especially noticeable for m/z = 79, 93, 105 and 107. These signals can be assigned to pyridine, aniline, vinylpyridine and toluidines, respectively. The fraction of these odd mass number signals caused by the 13C1-isotopomer contribution of the even mass numbered signal from the aromatic hydrocarbons benzene, toluene, styrene and xylenes, respectively lies in the region of 10 % of the observed relative signal of the pure 12C-isotopomer peaks at m/z 78, 92, 104 and 106. As the signal intensities of the odd m/z values of 79, 93, 105 and 107 in SSS are almost as high as those of the preceding even m/z ones the 13C1-isotopomer signals could be neglected for the qualitative statement that high concentrations of nitrogen containing aromatics are present in SSS.
In the MSS mass spectrum, the situation is different. Here, almost no additional intensity exceeding the expected 12C1-isotopomers to the presence of additional species at these m/z values remains. Phenolic compounds, such as phenol itself (m/z = 94), cresols (m/z = 108) and dihydroxybenzenes (m/z = 110) are in the same intensity range in both MSS and SSS or even higher in MSS. These findings are all in good agreement with established data using conventional gas chromatography/mass spectrometry analysis techniques for determination of MSS/SSS compounds concentration ratios [38]. On the other hand, there are some peaks, most noticeably m/z = 30 (NO), 44 (acetaldehyde), 58 (acetone), 68 (isoprene), 78 (benzene), 92 (toluene) and 162 (nicotine), whose behaviour deviates from literature data insofar, as their SSS concentrations should be much higher as in MSS.
However, for evaluation of the impact of the emitted compounds on health, the yield and finally the second-hand smoke or ETS concentration is a by far more important property. Thus, if the whole cigarette is regarded, the SSS yield is considerably higher than the MSS yield. For example, the SSS yields of benzene and toluene could be directly quantified utilising a standard gas mixture (see above) for external calibration. The respective SSS yields were determined to be 275 (benzene) and 602 μg (toluene). Comparison with MSS yields measured with the same method [52] reveals that SSS yields are higher by a factor of 5 (benzene) and 7 (toluene). A more detailed discussion of these quantified values follows below.
In the three-dimensional overview of a whole cigarette MSS and SSS time profile measurement, it is clearly visible that in contrast to the MSS emissions (Fig. 3a) the release of many SSS compounds show an elevated, rather constant level between the individual puffs (Fig. 3b). Although many species show a certain dependency on cigarette puffing, these puff-related emission exhibit a much broader time structure if compared with the sharp, 2 s lasting MSS puff emission pulses (see Fig. 3a). If the emission yields are analysed as a function of the puff-number for MSS components, several unique time profiles are observed. While many species show a steadily increasing yield puff-by-puff, other species show different behaviours. For example, some unsaturated compounds, such as butadiene, show the so called “first-puff-high” behaviour, characterised by an exceptional high yield in the first puff. The puff number—yield dependencies were thoroughly investigated, described and quantified by Adam et al. [52, 58].
In contrast to MSS the SSS emissions show a larger variety of puff behaviours and a constant emission component also between the individual puffs. In Fig. 4, the time profiles of selected masses of unfiltered SSS, namely m/z = 93 (b), 58 (c), 30 (d), 17 (e), 105 (f), 78 (g), 92 (h) and 106 (i) are plotted in comparison to a typical MSS profile, exemplarily shown at m/z = 44 (acetaldehyde) (a). In general, these masses represent the five different time behaviours observed during the measurements. The m/z = 93 (methylpyridine/aniline, Fig. 4b) shows a constant increase after ignition (first puff) within 30–40 s. The succeeding level is constant and no dependency on MSS puffs can be observed. Another substance exhibiting no significant puff dependence is m/z = 30 (NO) (Fig. 4d). However, in contrast to m/z = 93 (methylpyridine/aniline), a large concentration peak associated with the first puff can be observed. Another compound, where in the conjunction with the first puff relatively high emissions can be seen is acetone (m/z = 58, Fig. 4c). It increases in concentration right after ignition. In contrast to m/z = 30 (NO), however, here a puff-dependency can be seen for all of the puffs, including the first. The overall peak level within the puffs seems to decrease with the puff-number. This is visible on m/z = 17 (ammonia) (Fig. 4e) as well. However, there are no strong emissions during the first puff, instead the level increases constantly during the first 30–40 s after ignition. The concentration peaks following immediately after a puff are not as high compared with acetone; however a much higher decrease in concentration during the puff occurs (see also Fig. 5 on these effects). A linear increase over the whole cigarette can be found at m/z = 105 (vinylpyridine) (Fig. 4f), which exhibits an increase in concentration from the ignition to the end of the cigarette without significant effects of the occurrence of the puffs.
Different characteristic SPI-MS time profiles of selected main- (MSS) and sidestream smoke (SSS) compounds. a The MSS emission behaviour exemplarily demonstrated by acetaldehyde (m/z 44). The following traces show the temporal behaviour of SSS compounds: b aniline, methylpyridine (m/z 93), c acetone (m/z 58), d nitrogen monoxide (m/z 30), e) ammonia (m/z 17), f) vinyl-pyridine (m/z 105) g) benzene (m/z 78), h toluene (m/z 92) and the C2-alkylated benzenes (m/z 106). It is obvious that the observed emission consists of a constant and—for some compounds—a puff-driven component
The different time profiles can be explained by the change of conditions of the combustion processes within the cigarette. The average temperature during a puff is much higher than during smouldering and higher temperatures can be found in much larger areas of the burning zone. Previous investigations by Baker [70] show that the high temperature distribution shortly after the end of the puffing phase still consists inside the glowing tip. As previously illustrated by Adam et al. [52, 71] in investigations of pyrolysis gases of tobacco with single-photon ionisation time-of-flight mass spectrometry (SPI-TOFMS), various substances are formed and decomposed at different temperatures. Therefore, they can be classified into primary (formed directly from tobacco ingredients), secondary (formed at higher temperatures from primary compounds) and tertiary products, which are formed from secondary products at very high temperatures. For SSS this explains the chemical shifts resulting from the rapid temperature increase. Additionally, as air is drawn through the tip, a constant source of fresh oxygen is provided, which lacks during the smouldering. Substances such as m/z = 93 (methylpyridine/aniline), 30 (nitric oxide) and 105 (vinylpyridine) are not or hardly affected by the higher temperatures and oxygen content right after a MSS puff is drawn. In contrast, due to more extreme burning conditions a large amount of acetone is formed from combustion of various carbohydrates in the hot burning zone. After the puff suction ends, it diffuses into SSS. The relatively constant level of emissions between all puffs suggests a state of steady smouldering conditions between the puffs, after re-equilibrating from the “disturbance” of a MSS puff. The time profiles of the three aromatic compounds benzene (Fig. 4g), toluene (Fig. 4h) and the mixture of C2-alkylated benzenes (Fig. 4i) can also be categorized into the different emission characteristics shown earlier. Benzene and toluene exhibit the same characteristics as acetone. This suggests that large amounts of benzene and toluene, which are typical products of incomplete combustion, are emitted right after the end of a puff because of lack of oxygen in the burning zone. However, the C2-alkylated benzenes show different behaviour, similar to Fig. 3b, suggesting a different mechanism of formation less sensitive to changes in temperature and level of oxygen. Although also the standard, non-heated fishtail chimney SSS sampling system may play a role in blurring the profile for the higher boiling compounds, the observation fits well to the fact that the less alkylated aromatics and in particular the carbon-skeletons itself are rather combustion products (i.e. formed predominately during the puff) while the higher alkylated species rather are formed under low-temperature pyrolysis (i.e. smouldering).
To visualise the dynamics of SSS emissions in more detail, the fifth and sixth puff of the SSS emissions of m/z = 17 (ammonia) in unfiltered SSS are presented in Fig. 5. The decrease in concentration during the puff and the following increase for a period of ∼10 s, which is representative for all compounds with puff dependency, is in consistence with observations made by Dittmann et al. [72] who performed measurements of different aerosol concentrations in SSS. This decrease in concentration occurs simultaneously with the drawing of a MSS puff and lasts ∼2 s, which equals the duration of a standard puff and can therefore be utilised to correlate corresponding SSS and MSS data. It results from the air flow through the cigarette into the MSS. Additionally, with higher oxygen content and temperature within the burning zones the chemical composition of the emissions change significantly, e.g. more NO will be formed from nitrogen-containing compounds instead of ammonia.
As an example of puff resolved quantification of SSS the data of two 2R4F research cigarettes measured without a Cambridge filter pad is depicted in Fig. 6. The summed values of benzene (Fig. 6a) and toluene (Fig. 6b) for a whole cigarette are well within the range of previously published work. The total amount of xylenes (Fig. 6c) is calculated based on the assumption that the whole signal on m/z = 106 originates from m-xylene. Since the different isomeric C2-alkylated benzenes might have slightly different photo ionisation cross sections and, additionally, benzaldehyde and ethylbenzene may also contribute to the ion signal, the calculated values only represent an estimate. However, it becomes obvious that the values emitted between puffs are many times higher than the corresponding MSS values. During smouldering more tobacco is burned than in the two second puff duration. Additionally, different burning conditions may also contribute. The values of the different puffs show great variations without clear tendencies within any of the substances. However, a slightly reduced amount can be seen during the first puff, which could be a result of inhomogeneous lighting of the tobacco during ignition. A puff resolved analysis including benzene and toluene has previously been done by Brunnemann et al. [32, 73] and achieved slightly higher values. However, these studies were published prior to the general introduction of the fishtail chimney or an ISO standard for SSS sampling and various cigarettes including the 1R4F research cigarette were used. The different sampling conditions and cigarette types make a comparison of absolute values difficult.
Puff resolved quantification of benzene (a), toluene (b) and the isobaric xylenes and ethylbenzene (c) in SSS of a Kentucky 2R4F research cigarette smoked under ISO-conditions. For comparison, the value of MSS evaluated by Adam et al. [52] is also shown for a and b.
For a deeper insight into the puff-resolved chemical signatures of SSS—also in comparison to MSS—a multivariate statistical data treatment was performed, using the principal component analysis (PCA) methodology. Data pre-treatment and normalisation was done as described in earlier studies [52].
In Fig. 7, the results of the PCA of puff-resolved Cambridge-pad filtered SSS (gas phase, Fig. 7a, b) and unfiltered SSS (whole smoke, Fig. 7c, d), respectively, compared with puff-resolved MSS data are depicted. The explained variance of the data set for the first principal component is 67 and 73 % and for the second principal component 22 and 21 %, respectively. No significant changes between the gas phase and the combined gas and particulate phase data are observed. In the score plot (Fig. 7a, c), MSS and SSS are clearly separated according to the first principal component. Evaluating the loading plots (Fig. 7b, d), it becomes obvious that MSS is characterised, e.g. by sulphur-containing substances, such as m/z = 34 (H2S) and 48 (methanthiole) as well as by major MSS compounds, such as m/z = 44 (acetaldehyde). In contrast, SSS is represented by various odd m/z values, corresponding to nitrogen containing species such as ammonia, pyridine, aniline, benzonitrile, vinylpyridine and indole. Some well-known tobacco smoke substances do not show a clear association for either SSS or MSS smoke; this implies butadiene, isoprene, benzene and phenol, albeit they are tending more to the SSS. The second principal component describes the changes of the puff sequence of the MSS. These effects have been observed and discussed in detail previously [52]. However, interestingly the SSS does not show this behaviour. Here, the concentrations of most species remain rather constant over the whole smoking cycle. This suggests a state of rather steady burning conditions between the puffs.
Principal component analysis of puff resolved data with score (a, c) and loading (b, d) plots of MSS in comparison to filtered (a, b) and unfiltered (c, d) SSS emissions of a 2R4F research cigarette. MSS and SSS are separated according to principal component 1. Principal component 2 depicts the puff dependency of MSS which is absent for SSS. Dots in the loading plot depict different m/z values
However, an interesting question arises from looking at Fig. 5, whether the composition of the smoke, which is emitted immediately after a MSS puff, has more similarities with MSS. Figure 8 depicts the result of a PCA analysis of MSS, inter- and post-puff gas phase SSS (filtered, Fig. 8a, b) and unfiltered SSS (combined gas and particulate, Fig. 8c, d) of a 2R4F research cigarette. The integration time frames for the respective regions (inter- and post-puff SSS) are shown in Fig. 5. The explained variance within the first principal component is 86 and 81 %, respectively. The second principal component describes in both cases 11 % of the variance in the data set and can be interpreted as the temperature axis. Temperature while a puff is taken is much higher than during smouldering. Compounds whose formations are favoured by higher temperatures start to diffuse into SSS when the puff has ended as already explained before. Thus in the first phase immediately after the puff SSS shows still some similarities to MSS. After a short time, SSS compound distribution tends to return to steady burning conditions again. Hence, the basic chemical properties of MSS and SSS are visible in these plots while there are no significant changes between the gas phase and the combined gas and particulate phase. Additionally, the SSS post-puff emissions are slightly shifted towards the MSS measurements. This emphasises the earlier observations of the puff-resolved analysis of inter-puff and post-puff SSS emissions, where typical MSS masses are found in post-puff emissions. Examples for this are butadiene, propyne (m/z = 40) and 1-butene-3-yne (m/z = 52).
Principal component analysis with score (a, c) and loading (b, d) plots of MSS, inter- and post-puff-filtered SSS (gas phase, a, b) and unfiltered SSS (c, d) of a 2R4F research cigarette. MSS and SSS are separated according to principal component 1. Principal component 2 depicts the temperature influence on SSS in various time intervals. Dots in the loading plot depict different m/z values
Motivated by earlier studies which depicted that the chemical fingerprint of MSS is significantly different for cigarettes made from different tobacco sorts [71], it was investigated if the same is true for SSS as well. Model cigarettes made of pure Burley, Oriental and Virginia tobacco were investigated. In Fig. 9, a PCA of SSS from these single-tobacco-type cigarettes with identical physical parameters (Burley, green; Oriental, red; and Virginia, black) smoked under ISO conditions is portrayed. The same holds for SSS, for Burley and Oriental could clearly be separated on principal component 1, whereas Virginia is additionally separated on principal component 2. The observed chemical variations can be seen in both, gas phase and combined gas and particulate phase SSS emissions. Mainly, the nitrogen enriched Burley tobacco is characterised by compounds such as m/z = 17 (ammonia), 30 (nitric oxide), 79 (pyridine) and 93 (aniline). In contrast, Oriental, a tobacco enriched with oxygen containing compounds, is for example represented by species, such as dihydroxybenzenes (m/z = 110), carbonyl compounds (m/z = 86, 100) and furfural/dimethylfuran (m/z = 96).
Principal component analysis with score (a) and loading (b) plots of SPI-MS data of SSS measurements of single tobacco-type cigarettes with identical physical parameters. The cigarettes were smoked under ISO conditions. The SSS data of three cigarettes per type were individually averaged for the analysis (Burley, green; Oriental, red; and Virginia, black). Dots in the loading plot depict different m/z values
Conclusions
Modern on-line mass spectrometric techniques can provide deep insights in the time dependence and dynamics of the chemical signature of fast processes, such as SSS and MSS generation during cigarette smoking. Even a puff-resolved quantification of selected compounds is possible and summed values are clearly within the expected ranges. However, the highly dynamic behaviour, which for many compounds was observed for the first time, needs to be further investigated in the future.
References
Baker RR, Proctor CJ (1990) The origins and properties of environmental tobacco smoke. Environ Int 16(3):231–245
Kotzias D, Giess O, Leva P, Bellinanti A, Arvanitidis A, Kephalopoulos S (2004) Impact of various air exchange rates on the levels of environmental tobacco smoke (ETS) components. Fresenius Environ Bull 13(12b):1536–1549
Morrical BD, Zenobi R (2002) Determination of aromatic tracer compounds for environmental tobacco smoke aerosol by two step laser mass spectrometry. Atmos Environ 36(5):801–811
Reasor MJ (1987) The composition and dynamics of environmental tobacco smoke. J Environ Health 50(1):20–24
Harke HP (1974) Air pollution in smoke filled rooms. Rev Environ Health 1(4):305–326
Hoffmann D, Haley NJ, Adams JD, Brunnemann KD (1984) Tobacco sidestream smoke: uptake by nonsmokers. Prev Med 13(6):608–617
Lesmes GR, Donofrio KH (1992) Passive smoking: the medical and economic issues. Am J Med 93(1 suppl 1):S38–S42
Joseph PM, Witten ML, Burke CH, Hales CA (1996) The effects of chronic sidestream cigarette smoke exposure on eicosanoid production by tracheal epithelium. Exp Lung Research 22(3):317–335
Gebremichael A, Tullis K, Denison MS, Cheek JM, Pinkerton KE (1996) Ah-receptor-dependent modulation of gene expression by aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol 141(1):76–83
Aufderheide M, Ritter D, Knebel JW, Scherer G (2001) A method for in vitro analysis of the biological activity of complex mixtures such as sidestream cigarette smoke. Exp Toxicol Pathol 53(2–3):141–152
Ejaz S, Seok KB, Woong LC (2005) Toxicological screening for the effects of short-term exposure of sidestream cigarette smoke on angiogenesis. Drug Chem Toxicol 28(4):447–465
Schick S, Glantz SA (2006) Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob Control 15(6):424–429
Zou N, Hong J, Dai Q-Y (2009) Passive cigarette smoking induces inflammatory injury in human arterial walls. Chin Med J 122(4):444–448
Bernert JT, Gordon SM, Jain RB, Brinkman MC, Sosnoff CS, Seyler TH, Xia Y, McGuffey JE, Ashley DL, Pirkle JL, Sampson EJ (2009) Increases in tobacco exposure biomarkers measured in non-smokers exposed to sidestream cigarette smoke under controlled conditions. Biomarkers 14(2):82–93
Van Hemelrijck MJJ, Michaud DS, Connolly GN, Kabir Z (2009) Secondhand smoking, 4-aminobiphenyl, and bladder cancer: two meta-analyses. Cancer Epidemiol Biomarkers Prev 18(4):1312–1320
Yuan H, Shyy JY-J, Martins-Green M (2009) Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol 51(3):535–547
Reynolds P, Goldberg D, Hurley S, Nelson DO, Largent J, Henderson KD, Bernstein L (2009) Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev 18(12):3389–3398
Larsson L, Pehrson C, Dechen T, Crane-Godreau M (2012) Microbiological components in mainstream and sidestream cigarette smoke. Tob Induc Dis 10(1)
Lee HL, Hsieh DPH, Li LA (2011) Polycyclic aromatic hydrocarbons in cigarette sidestream smoke particulates from a Taiwanese brand and their carcinogenic relevance. Chemosphere 82(3):477–482
Marchetti F, Rowan-Carroll A, Williams A, Polyzos A, Berndt-Weis ML, Yauk CL (2011) Sidestream tobacco smoke is a male germ cell mutagen. Proc Natl Acad Sci U S A 108(31):12811–12814
Sadeu JC, Foster WG (2011) Effect of in vitro exposure to benzo[a]pyrene, a component of cigarette smoke, on folliculogenesis, steroidogenesis and oocyte nuclear maturation. Reprod Toxicol 31(4):402–408
Schramm S, Carré V, Scheffler JL, Aubriet F (2011) Analysis of mainstream and sidestream cigarette smoke particulate matter by laser desorption mass spectrometry. Anal Chem 83(1):133–142
Valenti VE, Abreu LC, Saldiva PH, Carvalho TD, Ferreira C (2011) Effects of sidestream cigarette smoke exposure on baroreflex components in spontaneously hypertensive rats. Int J Environ Health Res 20(6):431–437
Yu B, Jing Y, Liu J, Li H, Zhang X, Qu X, Lei J, Zhu J (2011) Determination of benzopyrene in mainstream and sidestream smoke. Applied Mechanics and Materials 29–34
Zhao J, Hopke PK (2012) Concentration of reactive oxygen species (ROS) in mainstream and sidestream cigarette smoke. Aerosol Sci Technol 46(2):191–197
Stedman RL (1968) The chemical composition of tobacco and tobacco smoke. Chem Rev 68:153–207
Johnson WR, Hale RW, Nedlock JW, Grubbs HJ,Powell DH (1973) The distribution of products between mainstream and sidestream smoke. Tob Sci 17(141–144)
Klus H (1990) Distribution of mainstream and sidestream cigarette smoke components. Rec Adv Tob Sci 16:189–232
Dube MF, Green CR (1982) Methods of collection of smoke for analytical purposes. Rec Adv Tob Sci 8:42–102
Browne CL, Keith CH, Allen RE (1980) The effect of filter ventilation on the yield and composition of mainstream and sidestream smoke. Beitr Tabakforsch Int 10(2):81–90
Sakuma H, Kusama M, Yamaguchi K, Sugawara S (1984) The distribution of cigarette smoke components between mainstream and sidestream smoke: III. Middle and higher boiling components. Beitr Tabakforsch Int 12(5):251–258
Brunnemann KD, Kagan MR, Cox JE, Hoffmann D (1990) Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis 11(10):1863–1868
Risner CH, Martin P (1994) Quantitation of formaldehyde, acetaldehyde, and acetone in sidestream cigarette smoke by high-performance liquid chromatography. J Chromatogr Sci 32(3):76–82
Lodovici M, Akpan V, Evangelisti C, Dolara P (2004) Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J Appl Toxicol 24(4):277–281
Borgerding M, Klus H (2005) Analysis of complex mixtures—cigarette smoke. Exp Toxicol Pathol 57(suppl 1):43–73
Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S (2008) A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 21(2):494–502
Pieraccini G, Furlanetto S, Orlandini S, Bartolucci G, Giannini I, Pinzauti S, Moneti G (2008) Identification and determination of mainstream and sidestream smoke components in different brands and types of cigarettes by means of solid-phase microextraction-gas chromatography–mass spectrometry. J Chromatogr A 1180(1–2):138–150
Baker RR (1999) Smoke Chemistry. In: Davis LD, Nielsen MT (eds) Tobacco: production, chemistry, and technology. Blackwell, Oxford, pp 398–439
Baren RE, Parrish ME, Shafer KH, Harward CN, Shi Q, Nelson DD, MacManus JB, Zahniser MS (2004) Quad quantum cascade laser spectrometer with dual gas cells for the simultaneous analysis of mainstream and sidestream cigarette smoke. Spectrochim Acta A 60:3437–3447
Crawford DR, Parrish ME, Gee DL, Harward CN (2007) Intra-puff CO and CO2 measurements of cigarettes with iron oxide cigarette paper using quantum cascade laser spectroscopy. Spectrochim Acta A 67(1):4–15
Shi Q, Nelson DD, McManus JB, Zahniser MS, Parrish ME, Baren RE, Shafer KH, Harward CN (2003) Quantum cascade infrared laser spectroscopy for real-time cigarette smoke analysis. Anal Chem 75:5180–5190
Thweatt WD, Harward CN Sr, Parrish ME (2007) Measurement of acrolein and 1,3-butadiene in a single puff of cigarette smoke using lead-salt tunable diode laser infrared spectroscopy. Spectrochim Acta A 67(1):16–24
Parrish ME, Harward CN (2000) Measurement of formaldehyde in a single puff of cigarette smoke using tunable diode laser infrared spectroscopy. Appl Spectrosc 54(11):1665–1677
Plunkett S, Parrish ME, Shafer KH, Nelson D, Shorter J, Zahniser M (2001) Time-resolved analysis of cigarette combustion gases using a dual infrared tunable diode laser system. Vib Spectrosc 27:53–63
Plunkett S, Parrish ME, Shafer KH, Shorter JH, Nelson DD, Zahniser MS (2002) Hydrazine detection limits in the cigarette smoke matrix using infrared tunable diode laser absorption spectroscopy. Spectrochim Acta A 58:2505–2517
Li S, Olegario RM, Banyasz JL, Shafer KH (2003) Gas chromatography–mass spectrometry analysis of polycyclic aromatic hydrocarbons in single puff of cigarette smoke. J Anal Appl Pyrol 66:156–163
Takanami Y, Chida M, Hasebe H, Sone Y, Suhara S (2003) Analysis of cigarette smoke by an online thermal desorption system and multidimensional GC-MS. J Chromatogr Sci 41(6):317–322
Thomas CE, Koller KB (2001) Puff-by-puff mainstream smoke analysis by multiplex gas chromatography–mass spectrometry. Beitr Tabakforsch Int 19(7):345–351
Wagner KA, Higby R, Stutt K (2005) Puff-by-puff analysis of selected mainstream smoke constituents in the Kentucky Reference 2R4F Cigarette. Beitr Tabakforsch Int 21(5):273–279
Adam T, Baker RR, Zimmermann R (2007) Characterization of puff-by-puff resolved cigarette mainstream smoke by single photon ionization-time-of-flight mass spectrometry and principal component analysis. J Agric Food Chem 55(6):2055–2061
Adam T, Baker RR, Zimmermann R (2007) Investigation, by single photon ionisation (SPI)-time-of-flight mass spectrometry (TOFMS), of the effect of different cigarette-lighting devices on the chemical composition of the first cigarette puff. Anal Bioanal Chem 387(2):575–584
Adam T, Mitschke S, Streibel T, Baker RR, Zimmermann R (2006) Quantitative puff-by-puff resolved characterization of selected toxic compounds in cigarette mainstream smoke. Chem Res Toxicol 19(4):511–520
Mitschke S, Adam T, Streibel T, Baker RR, Zimmermann R (2005) Application of time-of-flight mass spectrometry with laser-based photo-ionization methods for time-resolved on-line analysis of mainstream cigarette smoke. Anal Chem 77(8):2288–2296
Mühlberger F, Saraji-Borogzad M, Gonin M, Fuhrer K, Zimmermann R (2007) Compact ultra fast orthogonal acceleration time-of-flight mass spectrometer for on-line gas analysis by electron impact ionization and soft photon ionization using an electron beam pumped rare gas excimer lamp as VUV-light source. Anal Chem 79(21):8118–8124
Busch C, Streibel T, Liu C, McAdam K, Zimmermann R (2012) Pyrolysis and combustion of tobacco in a cigarette smoking simulator under air and nitrogen atmosphere. Anal Bioanal Chem 403:419–430
Eschner M, Selmani I, Gröger T, Zimmermann R (2011) On-line comprehensive two-dimensional characterization of puff-by-puff resolved cigarette smoke by hyphenation of fast gas chromatography to single-photon ionization time-of-flight mass spectrometry: quantification of hazardous volatile organic compounds. Anal Chem 83(17):6619–6627
Hertz R, Streibel T, Liu C, McAdam K, Zimmermann R (2012) Microprobe sampling—photo ionization-time-of-flight mass spectrometry for in situ chemical analysis of pyrolysis and combustion gases: examination of the thermo-chemical processes within a burning cigarette. Anal Chim Acta 714:104–113
Adam T, Mitschke S, Streibel T, Baker RR, Zimmermann R (2006) Puff-by-puff resolved characterisation of cigarette mainstream smoke by single photon ionisation (SPI)-time-of-flight mass spectrometry (TOFMS): comparison of the 2R4F research cigarette and pure Burley, Virginia, Oriental and Maryland tobacco cigarettes. Anal Chim Acta 572(2):219–229
Chung I-P, Dunn-Rankin D (1996) In situ light scattering measurements of mainstream and sidestream cigarette smoke. Aerosol Sci Technol 24(2):85–101
Cole SK, Martin P (1996) Determination of gas-phase sidestream cigarette smoke components using Fourier transform infrared spectrometry. Analyst 121(4):495–500
Thompson BT, Mizaikoff B (2006) Real-time Fourier transform-infrared analysis of carbon monoxide and nitric oxide in sidestream cigarette smoke. Appl Spectrosc 60(3):272–278
Proctor CJ, Martin C, Beven JL, Dymond HF (1988) Evaluation of an apparatus designed for the collection of sidestream tobacco smoke. Analyst 113(10):1509–1513
Mühlberger F, Hafner K, Kaesdorf S, Ferge T, Zimmermann R (2004) Comprehensive on-line characterization of complex gas mixtures by quasi-simultaneous resonance enhanced multiphoton ionization, vacuum-UV single photon ionization and electron impact ionization in a time-of-flight mass spectrometer: setup and instrument characterization. Anal Chem 76(22):6753–6764
Streibel T, Hafner K, Mühlberger F, Adam T, Warnecke R, Zimmermann R (2005) Investigation of NO x precursor compounds and other combustion by-products in the primary combustion zone of a waste incineration plant using on-line, real time mass spectrometry and Fourier-transform infrared spectrometry (FT-IR). Anal Bioanal Chem 384:1096–1106
Streibel T, Weh J, Mitschke S, Zimmermann R (2006) Thermal desorption/pyrolysis coupled with photo ionization time-of-flight mass spectrometry for the analysis of molecular organic compounds and oligomeric and polygomeric fractions in urban particulate matter. Anal Chem 78(15):5354–5361
Welthagen W, Mitschke S, Mühlberger F, Zimmermann R (2007) One-dimensional and comprehensive two-dimensional gas chromatography coupled to soft photo ionization time-of-flight mass spectrometry: a two- and three-dimensional separation approach. J Chromatogr A 1150:54–61
Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R (2009) Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal Bioanal Chem 394(4):1193–1203
Mitschke S, Welthagen W, Zimmermann R (2006) Comprehensive gas chromatography-time-of-flight mass spectrometry using soft and selective photoionization techniques. Anal Chem 78(18):6364–6375
Adam T, Zimmermann R (2007) Determination of single photon ionization cross sections for quantitative analysis of complex organic mixtures. Anal Bioanal Chem 389:1941–1951
Baker RR (1975) Temperature variation within a cigarette combustion coal during the smoking cycle. High Temp Sci 7:236–247
Adam T, Ferge T, Mitschke S, Streibel T, Baker RR, Zimmermann R (2005) Discrimination of three tobacco types (Burley, Virginia and Oriental) by pyrolysis single photon ionisation (SPI)/time-of-flight mass spectrometry and advanced statistical methods. Anal Bioanal Chem 381(2):487–499
Dittmann R, Feld HJ, Mueller BH, Schneider W (1992) Time resolved emission of sidestream smoke particles. Beitr Tabakforsch Int 15(2):53–57
Brunnemann KD, Kagan MR, Cox JE, Hoffmann D (1989) Determination of benzene, toluene and 1,3-butadiene in cigarette smoke by GC-MSD. Exp Pathol 37(1–4):108–113
Acknowledgments
The authors would like to thank Photonion GmbH, Schwerin, Germany and Borgwaldt KC, Hamburg, Germany for instrumental support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Photo Ionisation in Mass Spectrometry with guest editor Ralf Zimmermann.
Rights and permissions
About this article
Cite this article
Streibel, T., Mitschke, S., Adam, T. et al. Time-resolved analysis of the emission of sidestream smoke (SSS) from cigarettes during smoking by photo ionisation/time-of-flight mass spectrometry (PI-TOFMS): towards a better description of environmental tobacco smoke. Anal Bioanal Chem 405, 7071–7082 (2013). https://doi.org/10.1007/s00216-013-6739-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6739-y