Abstract
A method for analysis of diethofencarb and pyrimethanil in apple pulp and peel was developed by using dispersive liquid–liquid microextraction based on solidification of a floating organic droplet (DLLME-SFO) and high-performance liquid chromatography with diode-array detection (HPLC–DAD). Acetonitrile was used as the solvent to extract the two fungicides from apple pulp and peel, assisted by microwave irradiation. When the extraction process was finished, the target analytes in the extraction solvent were rapidly transferred from the acetonitrile extract to another extraction solvent (1-undecanol) by using DLLME-SFO. Because of the lower density of 1-undecanol than that of water, the finely dispersed droplets of 1-undecanol collected on the top of aqueous sample and solidified at low temperature. Meanwhile, the tiny particles of apple cooled and precipitated. Recovery was tested for a concentration of 8 μg kg−1. Recovery of diethofencarb and pyrimethanil from apple pulp and peel was in the range 83.5–101.3%. The repeatability of the method, expressed as relative standard deviation, varied between 4.8 and 8.3% (n = 6). Detection limits of the method for apple pulp and peel varied from 1.2–1.6 μg kg−1 for the two fungicides. Compared with conventional sample preparation, the method has the advantage of rapid speed and simple operation, and has high enrichment factors and low consumption of organic solvent.

Chromatogram of apple pulp (a) and spiked apple pulp (b) at the concentration level of 0.4 μg g−1 for two fungicides obtained by using DLLME-SFO combined with HPLC-DAD. Peak identification: 1-Diethofencarb, 2- Pyrimethanil
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two systemic fungicides, diethofencarb and pyrimethanil have recently been mixed to improve the control of grey mould on fruit and vegetables because of their different mechanism of action. Diethofencarb (isopropyl 3,4-diethoxyphenylcarbamate), is a phenylcarbamate; its mechanism of action is based on inhibition of phospholipid and fatty acid biosynthesis. Pyrimethanil (N-(4,6-dimethylpyrimidin-2-yl)aniline), is a novel anilinopyrimidine which inhibits protease secretion by the pathogen [1, 2].
Although the use of the two fungicides is continuously increasing, because of their attractive properties, there have been only a few studies of their analysis in plant tissue matrices. Pyrimethanil has been determined in green groceries, grapes, and water by gas chromatography (GC) or high-performance liquid chromatography (HPLC) with diode-array detection (DAD) or mass spectrometry (MS) [3–6]. Diethofencarb residues have been mainly determined in water and fruit juice by HPLC with diode-array detection (DAD) or MS [7–9].
Apples are one of the most widely grown fruit in China. Apple planting area and output has been ranked first in the world in recent years. Apples continue to show growth trends in China, but the quality and safety of the apples, particularly the adverse situation of pesticide residues, has affected China world market share. It is urgent to develop sensitive, accurate, rapid, and simple methods for analysis of pesticide residues on apples, to meet the maximum residue limits established by some countries owning advanced technology.
In general, for analysis of pesticide residues in complex matrices such as food matrices, Soxhlet extraction (SE) [10], liquid–liquid extraction [LLE] [11], and solid-phase extraction (SPE) [12] have been used as conventional sample preparation methods. However, these methods are generally time-consuming and a large amount of glassware and organic solvents are often required. On the other hand, alternative extraction approaches reducing solvent usage have been developed and commercialised since 1990, including accelerated solvent extraction (ASE) [13] and microwave-assisted extraction (MAE) [14]. ASE and MAE do not provide enough selectivity (extracts require more clean up than liquid-based extraction at room temperature) [15]. During the last decade, efforts have been made to minimize the time, reduce the volume of extract, and simplify sample preparation steps by miniaturizing extraction methods [16–20]. Consequently, dispersive liquid–liquid microextraction (DLLME) was developed by Assadi and his co-workers [21] and has become a very popular technique for preconcentration [22–26]. The consumption of organic solvent and extraction time are greatly reduced. But the extraction solvents used in this method are often chlorinated solvents that are toxic and the extraction phase often settled to the bottom of the test tube after centrifugation because of the higher density of chlorinated solvents than that of water. Recently, by using extraction solvents with low density and appropriate melting points, DLLME based on the solidification of a floating organic droplet (DLLME-SFO) was developed by Leong and Huang [27]. The extract, which forms a layer on the top of aqueous sample, can be collected by solidifying it at low temperature. Meantime, very tiny particles in the system settle without interfering with the target analytes. So this method is more sensitive and accurate. It has been successfully used for extraction and preconcentration of many trace substances from water samples [28–33].
As far as we are aware, only one paper has reported application of DLLME-SFO for the simultaneous determination of diethofencarb and pyrimethanil in water samples [34]. However, no data are available on the determination of diethofencarb and pyrimethanil in apple samples by DLLME-SFO. In this work, DLLME-SFO combined with DAD was investigated for analysis of the two fungicides in apple pulp and peel. Before DLLME-SFO, microwave irradiation was used to extract the target analytes and the effect of matrices was reduced effectively. Further extraction and clean up was carried out by DLLME-SFO. The effect of various experimental conditions on extraction of the two fungicides from apple pulp and peel was studied in detail.
Experimental
Materials and samples
Diethofencarb (98.0%) and pyrimethanil (98.0%) were obtained from Sigma–Aldrich (St Louis, MO, USA). Analytical grade n-hexadecane, 1-dodecanol, and 1-undecanol were purchased from Tokyo Chemical Industry (Tokyo, Japan). HPLC-grade acetonitrile, methanol, and acetone were purchased from Tedia (Fairfield, OH, USA). Sodium chloride used in the experiments was obtained from Zhanyun Chemical (Shanghai, China). Deionized water was purified by means of a Milli-Q water-purification system (Millipore, Billerica, MA, USA).
A stock solution (20 μg mL−1) was prepared by dissolving each fungicide in methanol, and stored in a refrigerator at 4 °C. Working standard solutions were freshly prepared by dilution of stock solution with distilled water to the required concentrations.
Apple samples were purchased from a supermarket in Wuhan, China. First, the apples were cleaned, peeled, and cored. The apple pulp (the peel was treated for the DLLME-SFO procedure in the same way) was cut into pieces, and then put into a stainless steel blender to be homogenized. After vacuum freeze drying, the apple pulp was powdered, sieved through a 40-mesh sieve, and stored in a refrigerator at 4 °C before analysis. For recovery studies, spiked pulp samples were prepared by adding specific volumes of standard solution to 0.5 g pulp. The mixture was equilibrated by shaking for 15 min and then left to stand for more than 4 h at room temperature before analysis.
Instrumentation
Chromatographic analysis was carried out on an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a manual injector and a diode-array detector (DAD). The analytes were separated on an Agilent Zorbax SB-C18 column (250 mm × 4.6 mm, i.d. 5 μm). The mobile phase was methanol–water 70:30 (v/v) and the flow rate was 0.8 mL min−1. The detection wavelength was 254 nm and the column temperature was 40 °C. The injection volume was 5 μL. The centrifugation was performed with an 80-2 centrifuge (Changzhou Guohua Electric Appliance, Jiangsu, China). A blender (Midea Group, Guangdong China), a vacuum freeze dryer (Songyuan Huaxing Technology Development, Beijing, China) and a SmithCreator Microwave oven (Personal Chemistry, Uppsala, Sweden) were used for sample preparation.
Extraction procedures
Acetonitrile (5 mL) was added to 0.5 g spiked apple samples in a microwave tube. The sealed tube was placed in a focused SmithCreator Microwave oven with irradiation at 80 °C for 30 min. The acetonitrile extract (0.4 mL) was removed from the sealed test tube by use of a 1.0 mL syringe and injected rapidly into a 10-mL screw-cap test tube with conical bottom containing 5 mL double-distilled water. Next, 1.4 g sodium chloride was added to the solution and 10 μL 1-undecanol was also immediately injected into the aqueous solution by use of a 50-μL syringe. The tube was then shaken gently by hand for several seconds. A cloudy solution (water, acetonitrile, and 1-undecanol) was formed in the glass test tube and the fungicides were extracted into the droplets of 1-undecanol. The mixture was then centrifuged for 2 min at 4,000 rpm, the 1-undecanol phase rose to the surface of the aqueous solution because its density was lower than that of water. The test tube was cooled in an ice bath for a few minutes. When the liquid organic drop had frozen, it was removed with small medical forceps and placed in a 200-μL polychloroprene rubber tube at room temperature. The solid organic drop melted quickly and 5 μL was collected with a syringe and injected for HPLC analysis.
Results and discussion
The possibility of combination of DLLME-SFO with HPLC–DAD for preconcentration and analysis of diethofencarb and pyrimethanil in apple samples was investigated. Several conditions affect DLLME-SFO performance, including type of extraction solvent used for apple sample, type of extraction and disperser solvent, and their volume in the DLLME-SFO procedure, salt addition, and extraction time. We selected two fungicides usually spraying on apples as target analytes and studied the extraction efficiency of the method.
Selection of organic solvent for extraction of diethofencarb and pyrimethanil from apple pulp and peel
The extraction solvent for apple samples with MAE (step 1) should be miscible with water, be a good extractor, and have a high boiling point. Among four solvents (acetonitrile, acetone, methanol) with the property of miscibility with organic and aqueous phases, acetonitrile was chosen because it has the highest boiling point as an extraction solvent for apple pulp and peel (step 1) and as a disperser solvent in DLLME-SFO (step 2).
Selection of the type and volume of extraction solvent for DLLME-SFO
For DLLME-SFO (step 2), the extraction solvent should have a melting point close to or below room temperature and the density must be lower than that of water. It should also be possible to extract the analytes, and its chromatographic peak could be easily distinguished from that of the target compounds. In this work three different organic solvents were evaluated, n-hexadecane, 1-dodecanol, and 1-undecanol. For hexadecane (melting point 18 °C) the hydrophobicity was so strong that it could not be dissolved in the common dispersive solvent. Good extraction results were obtained when 1-dodecanol (melting point 24 °C) and 1-undecanol (melting point 11 °C) were used as extraction solvents because of the suitable melting point and good extraction efficiency for diethofencarb and pyrimethanil. Extraction efficiency was greatest with 1-undecanol so this was selected as the extraction solvent for further studies.
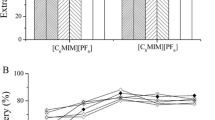
The volume of the floating phase increases with increasing extraction volume; however, the analytes are diluted as a result of the increase in the volume of the floating phase. To find the most suitable extraction volume, different volumes (8, 10, 15, 20, 30, 40, and 50 μL) were evaluated for the same DLLME-SFO procedure. As shown in Fig. 1, use of 10 μL of extraction solvent resulted in better peak area for both analytes. Therefore, 10 μL of extraction solvent was used in subsequent experiments.
Effect of extraction solvent (1-undecanol) volumes on extraction efficiency of fungicides by DLLME-SFO. Extraction conditions: sample volume, 5.0 mL; disperser solvent volume, 0.5 mL (containing different volumes of extraction solvent); room temperature and without salt addition; concentration of each fungicide, 0.4 μg mL-1. Error bars represent the standard deviation of the mean peak area for n = 3 replicates
Effect of volume of disperser solvent
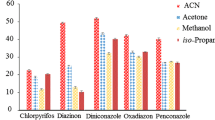
After selecting acetonitrile as the disperser solvent, its volume should be optimized. At low volume, acetonitrile cannot disperse the extraction solvent properly and a cloudy solution is not formed. However, at high volume, the solubility of fungicides in water increases, which will result in reduced extraction efficiency. To optimize the volume of acetonitrile, the effect of its volume on the extraction efficiency was investigated in the range of 0.2–1.0 mL (with the volume of 1-undecanol fixed as 10 μL). As shown in Fig. 2, extraction efficiency increased with increasing volume of acetonitrile when it was less than 0.4 mL. Reduced extraction efficiency was observed when the volume of acetonitrile exceeded 0.4 mL. On the basis of these results, 0.4 mL was chosen as the optimum volume of the disperser solvent.
Effect of disperser solvent (acetonitrile) volumes on extraction efficiency of fungicides by DLLME-SFO. Extraction conditions: sample volume, 5.0 mL; extraction solvent volume, 10 μL; room temperature and without salt addition; concentration of each fungicide, 0.4 μg mL-1. Error bars represent the standard deviation of the mean peak area for n = 3 replicates
Salt effect and effect of extraction time
In general, addition of sodium chloride to an aqueous solution increases its ionic strength, which reduces the solubility of the analytes in the sample solution and improves the extraction efficiency. Different amounts of sodium chloride in the range 0–28% (w/v) were investigated in DLLME-SFO procedure. The results showed that increasing the amount of salt from 0% to 8% resulted in a decrease of extraction efficiency. However, visible growth of extraction efficiency was observed when larger amounts were added. Consequently, 1.4 g (28%) sodium chloride (close to saturation concentration) was used in subsequent experiments.
The effect of extraction time on the extraction efficiency was examined in the range of 0–20 min, but extraction time had no significant effect on the extraction efficiency. This may be because the large contact surface area between the extraction solvent and aqueous phase results in very rapid transport of analytes from the aqueous phase to the extraction solvent. Therefore, the analytes were extracted into the fine droplets of 1-undecanol in a few seconds and the extraction equilibrium was reached quickly. To achieve high extraction efficiency and short analysis time, 3 min was chosen as the optimum extraction time (between injecting the disperser and extraction solvent in aqueous samples and before centrifuging).
Quantitative aspects
Under the optimized conditions, the proposed method was applied to a series of spiked apple samples containing different concentrations of the two fungicides in order to obtain calibration curves. (Separate calibration curves were constructed for apple pulp and peel.) The characteristics of the calibration curves are summarized in Table 1. For apple pulp, linearity was observed in the concentration range 8 to 800 μg kg−1 for diethofencarb, and 8 to 400 μg kg−1 for pyrimethanil. Correlation coefficients (r) were 0.9916 for diethofencarb and 0.9991 for pyrimethanil. The limits of detection (LODs) for diethofencarb and pyrimethanil were 1.6 and 1.4 μg kg−1. The reproducibility of the proposed method was determined by evaluating the intra-day and inter-day precision. The relative standard deviations (RSDs, n = 6) were below 8.3%. Quite similar results were obtained for apple peel.
Application of the method
The proposed method was used to determine the concentrations of the two fungicides in apple pulp and peel samples. As shown in Fig. 3, the results indicated the apple peel sample was free from the target analytes, i.e. the concentration of the analytes in the real sample was below the LOD. To study the effect of the sample matrix on the accuracy of the DLLME-SFO method, recovery experiments were carried out after spiking apple pulp and peel samples with both target analytes (8.0 μg kg−1). Recoveries of the analytes were 83.5–101.3% (Table 2). This demonstrated that DLLME-SFO assisted by microwave radiation was not significantly affected by the sample matrices and could be used for trace analysis of the two fungicide residues in apple pulp and peel samples.
Comparison of DLLME-SFO with other published methods
Table 3 summarizes the LODs, the volumes of organic solvent required, and extraction times in different methods for extraction and analysis of the two fungicides in fruits or vegetables. It is observed that DLLME-SFO has an LOD comparable with those of the other analytical methods. Moreover, DLLME-SFO has the advantage of lower organic solvent consumption and shorter analysis time than any other method.
Conclusion
DLLME-SFO assisted by MAE then HPLC–DAD have been used for analysis of two fungicides in apple pulp and peel samples. The DLLME-SFO procedure is a good alternative to conventional pretreatment techniques such as SE, LLE, and SPE, because it substantially reduces consumption of organic solvents, and operation is uncomplicated. Moreover, in DLLME-SFO procedure, an extraction solvent (1-undecanol) that is less toxic than the solvents used in DLLME is employed. Compared with previous studies, the proposed method gives comparable results but offers good extraction efficiency and reproducibility within a short time. Especially, this method can be used for extraction of fungicides in solid fruit matrices (apple pulp and peel). So far there is no report of the application of DLLME-SFO for the analysis of fungicides in solid matrices. In conclusion, DLLME-SFO–HPLC–DAD is a simple, rapid, sensitive, accurate and reproducible method for trace analysis of diethofencarb and pyrimethanil in apple pulp and peel.
References
Rial OR, Yagüe RC, Cancho-Grande B, Simal-Gándara J (2002) J Chromatogr A 942:41–52
Cui SH, Wan KY, Qian JL, Zhang LD, Liu JF, Xu ML (2010) Chin J Pestic Sci 12:195–200
Navalón A, Prieto A, Araujo L, Vίlchez JL (2002) J Chromatogr A 975:355–360
Juan-García A, Mañes J, Font G, Picó Y (2004) J Chromatogr A 1050:119–127
Sagratini G, Mañes J, Giardiná D, Damiani P, Picó Y (2007) J Chromatogr A 1147:135–143
Rial OR, Cancho-Grande B, Simal-Gándara J (2003) J Chromatogr A 992:121–123
Fernández M, Picó Y, Mañes J (2000) J Chromatogr A 871:43–56
Lian YJ, Pang GF, Shu HR, Fan CL, Liu YM, Feng J, Wu YP, Chang QY (2010) J Agric Food Chem 58:9428–9453
Wu QH, Chang QY, Wu CX, Rao H, Zeng X, Wang C, Wang Z (2010) J Chromatogr A 1217:1773–1778
Eskilsson CS, Bjorklund E (2000) J Chromatogr A 902:227–250
Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt LE, Haubruge E, De Pauw E, Focant JF (2007) J Chromatogr A 1152:116–123
López-Blanco MC, Reboreda-Rodríguez B, Cancho-Grande B, Simal-Gándara J (2002) J Chromatogr A 976:293–299
Lehotay SJ, Lee CH (1997) J Chromatogr A 785:313–327
Pylipiw HM, Arsenault TL, Thetford CM, Mattina MJI (1997) J Agric Food Chem 45:3522–3528
Anastassiades M, Lehotay SJ, Stajnbaner D, Schenck FJ (2003) J AOAC Int 86:412–431
Oliva J, Barta A, Vela N, Melendreras F, Navarro S (2000) J Chromatogr A 882:213–220
Jose LT, Sanchez-Brunete C, Albero B, Conzalez L (2004) Crit Rev Anal Chem 34:121–131
López-Blanco MC, Blanco-Cid S, Cancho-Grande B, Simal-Gάndara J (2003) J Chromatogr A 984:245–252
López-Blanco C, Gómez-Álvarez S, Rey-Garrote M, Cancho-Grande B, Simal-Gándara J (2005) Anal Bioanal Chem 383:557–561
López-Blanco C, Gómez-Álvarez S, Rey-Garrote M, Cancho-Grande B, Simal-Gándara J (2006) Anal Bioanal Chem 384:1002–1006
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1–9
Yazdi AS, Razavi N, Yazdinejad SR (2008) Talanta 75:1293–1299
Zhao XN, Fu LY, Hu J, Li JW, Wang HL, Huang CJ, Wang XD (2009) Chromatographia 69:11–12
Saraji M, Marzban M (2010) Anal Bioanal Chem 396:2685–2693
Zhao RS, Diao CP, Wang X, Jiang T, Yuan JP (2008) Anal Bioanal Chem 391:2915–2921
Yao C, Anderson JL (2009) Anal Bioanal Chem 395:1491–1502
Leong MI, Huang SD (2008) J Chromatogr A 1211:8–12
Dai LP, Cheng J, Matsadiqa G, Liu L, Li JK (2010) Anal Chim Acta 674:201–205
Liu L, Cheng J, Matsadiq G, Zhou HB, Li JK (2010) Chromatographia 72:1017–1020
Wu YL, Dai LP, Cheng J, Guo F, Li JK (2010) Chromatographia 72:695–699
Leong MI, Huang SD (2009) J Chromatogr A 1216:7645–7650
Chang CC, Huang SD (2010) Anal Chim Acta 662:39–42
Xu H, Ding ZQ, Lv LL, Song DD, Feng YQ (2009) Anal Chim Acta 636:28–33
Cheng J, Xiao J, Zhou YW, Xia YT, Guo F, Li JK (2010) Dispersive liquid-liquid microextraction based on solidification of floating organic droplet method for the determination of diethofencarb and pyrimethanil in aqueous samples. Microchim Acta. doi:10.1007/s00604-010-0458-2
Acknowledgement
The authors would like to thank the National Natural Science Foundation of China (30971948) and Wuhan’s program for tackling key problems in science and technology (200760423155) for financial support for this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
YiWen Zhou and LinTao Han are co-first authors.
Rights and permissions
About this article
Cite this article
Zhou, Y., Han, L., Cheng, J. et al. Dispersive liquid–liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Anal Bioanal Chem 399, 1901–1906 (2011). https://doi.org/10.1007/s00216-010-4567-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4567-x